Abstract
Background and Purpose:
Clinical methods have incomplete diagnostic for early diagnosis of acute stroke and large vessel occlusion (LVO). Electroencephalography (EEG) is rapidly sensitive to brain ischemia. This study examined the diagnostic utility of EEG for acute stroke/transient ischemic attack (TIA) and for LVO.
Methods:
Patients (n=100) with suspected acute stroke in an Emergency Department (ED) underwent clinical exam then an EEG using a dry-electrode system. Four models classified patients, first as acute stroke/TIA or not, then as acute stroke with LVO or not: [1] clinical data, [2] EEG data, [3] clinical+EEG data using logistic regression, and [4] clinical+EEG data using a deep learning neural network. Each model used a training set of 60 randomly selected patients, then was validated in an independent cohort of 40 new patients.
Results:
Of 100 patients, 63 had a stroke (43 ischemic/7 hemorrhagic) or TIA (13). For classifying patients as stroke/TIA or not, the clinical data model had AUC=62.3, while clinical+EEG using deep learning neural network model had AUC=87.8. Results were comparable for classifying patients as stroke with LVO or not.
Conclusions:
Adding EEG data to clinical measures improves diagnosis of acute stroke/TIA, and of acute stroke with LVO. Rapid acquisition of dry-lead EEG is feasible in the ED and merits prehospital evaluation.
Introduction
Even small improvements in time to stroke diagnosis and treatment can significantly improve patient outcomes. Improving tools for early identification of stroke and LVO in the prehospital setting is a key strategy.
Clinical assessments for prehospital diagnosis of stroke or LVO have good diagnostic value but have been criticized for having inconsistent/incomplete diagnostic performance or being too elaborate for some emergency medical service providers1. Given these limitations, non-invasive brain monitoring devices, including electroencephalography (EEG), are under study to identify stroke and LVO. EEG immediately detects changes in brain function following onset of brain ischemia, prior to cell death2--an advantage for early prehospital stroke diagnosis--and has long-established sensitivity to early stroke in humans. To date, EEG has had limited clinical application due to the technical expertise and long times needed to apply gel-electrodes. However, advances in EEG technology, including rapidly applied dry-electrodes3, suggest feasibility of prehospital EEG recordings.
The long-term goal is to improve prehospital stroke diagnosis using EEG. Towards this goal, we examined the utility of EEG to diagnose (1) acute stroke/TIA and (2) acute stroke with LVO in 100 patients with suspected acute stroke in the ED. We hypothesized that clinical and EEG measures each perform well, and that combining the two increases diagnostic accuracy.
Methods
Additional details appear in the Supplement (https://www.ahajournals.org/journal/str). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients
Patients with suspected/definite acute stroke were recruited from the ED of a single comprehensive stroke center. Ethics approval was obtained from the local IRB and written informed consent was obtained from all enrollees or surrogates. Entry criteria targeted suspected acute stroke.
EEG acquisition
The Quick-20 (Cognionics, Inc., San Diego, CA; Figure 1A) EEG system3 utilizes dry-electrodes (no gel/skin preparation), enabling rapid application and data collection in an acute care setting. Each dry-electrode is supported by a local active amplifier plus Faraday cage, enabling high quality signal acquisition despite higher electrode impedances encountered with dry skin contact. Three-minutes of eyes-open, resting-state brain activity was recorded at bedside.
Figure 1.
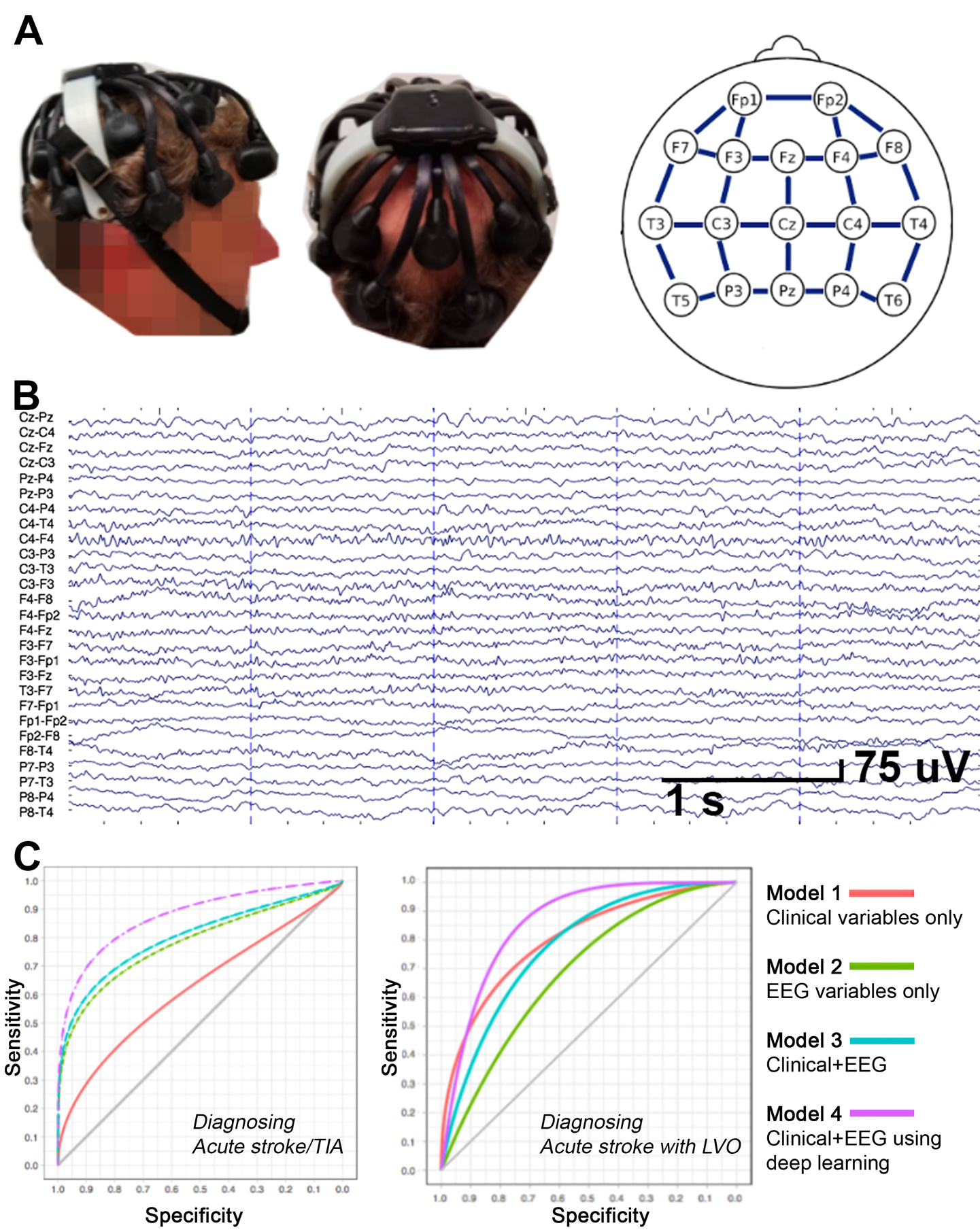
[A]. The Quick-20 dry-lead Cognionics headset and the EEG montage, having 17 leads and 27 bipolar lead-pairs (blue lines). [B]. EEG from a 69 year-old male 8.5 hours after stroke onset with right thalamocapsular infarct and NIHSS=9. [C] ROC curves for each model. The model combining clinical and EEG data using deep learning showed best diagnostic performance for both acute stroke/TIA (left; AUC=87.8) and for acute stroke with LVO (right; AUC=86.4).
EEG processing
EEG data were exported to MATLAB for offline analysis, including filtering and removal of noise. Each lead was re-referenced, creating a bipolar montage of 27 bipolar lead-pairs (Figure 1A&B). Spectral power was examined within each of the 27 bipolar lead-pairs, across five frequency bands: delta (1–3 Hz), theta (4–6 Hz), alpha (7–12 Hz), low beta (13–19 Hz), and high beta (20–30 Hz), using odd numbers (Fp1-T5) for ipsilesional, and even numbers (Fp2-T6) for contralesional, leads.
Statistical Analyses
Receiver operating characteristic (ROC) curve analysis was used to test and validate predictive performance of clinical and EEG variables, with higher area-under-the-curve (AUC) values indicating better prediction. All models used a 60–40 split; training on the same randomly selected 60 patients and testing on an independent validation cohort of the same 40 new patients.
Given the high-dimensionality of the EEG data, Lasso regression modeling was used to select a subset of EEG variables.
Four predictor models were evaluated and validated, using acute stroke/TIA (or not) as the dependent measure: [1] clinical data only, using 4 measures that would be available to an Emergency Medical Technician (age, sex, time from last-known-well (LKW) to EEG, and Rapid Arterial oCclusion Evaluation (RACE) score4), using logistic regression modeling; [2] EEG data only, using the Lasso-selected 4 EEG lead-band pairs (F8-T4 alpha, C3-F3 low beta, Cz-C3 high beta, and C4-F4 high beta band), using logistic regression modeling; [3] combined clinical and EEG data using logistic regression, using the most significant clinical predictor from model [1] and RACE score, plus the 4 Lasso-selected EEG lead-band pairs; and [4] combined clinical and EEG data using a deep learning neural network model, using the same six variables as model [3].
The same four models were again examined, instead using acute stroke with LVO (or not) as the dependent measure. Clinical variables were as above; EEG variables were the two identified by Lasso procedure for LVO (C3-F3 theta band, and T3-F7 alpha band).
Results
Subjects
Among 100 enrollees (Table 1), discharge diagnosis was acute stroke/TIA in 63, (43 ischemic stroke, 7 intracerebral hemorrhage, and 13 TIA). Infarcts were deep+cortical (n=31), deep only (n=17), and posterior fossa (n=2). Of the 43 with ischemic stroke, seven had an LVO (all M1 occlusion) and 14 received IV tPA (median 8.1 hours before EEG).
Table 1:
Subject characteristics and procedures timeline
| All patients | Acute Stroke and TIA | Acute stroke with LVO | |
|---|---|---|---|
| Number | 100 | 63 | 7 |
| Demographics/medical history | |||
| Age | 64.5±15.8 | 64.8±16.7 | 68.9±12.54 |
| Sex | 53M/47F | 38M/25F | 4M/3F |
| Race | |||
| White | 52 | 34 | |
| Hispanic | 30 | 18 | |
| Asian | 14 | 9 | |
| Black | 4 | 2 | |
| Clinical Scales | |||
| NIHSS score^ | 4.4±5.6 | 5.0±6.3 | 12.4±7.7 |
| RACE score^ | 1.6±2.3 | 1.8±2.4 | 5.6±3.6 |
| Timeline relative to ED presentation and EEG acquisition | |||
| LKW-ED arrival (h:m) | 3:22 [00:11–20:25] | 3:50 [00:27–20:25] | 3:22 [00:45–12:58] |
| LKW-EEG Acquisition (h:m) | 9:27 [00:55–22:50] | 11:49 [00:55–22:42] | 14:15 [3:30–19:05] |
| ED admit-EEG (h:m) | 3:47 [00:36–19:28] | 4:02 [00:45–19:21] | 4:33 [1:38–18:20] |
| Time from consent-start EEG recording | 00:09 [00:00:36–23:00] | 00:09 [00:00:36–23:00] | 00:10 [00:02–00:23] |
| Brain Injury | |||
| Infarct volume (cc) | n/a | 19.4±41* | 100.3±69.0 |
| Lesion side | n/a | 23L/27R* | 2L/5R |
Data are mean±SD or median [range].
Injury data provided for the 50 patients with stroke. Infarct volume range=0–206.7 cc.
NIHSS scores ranged from 0–27; RACE scores, from 0–9.
Median time from LKW to EEG was 9.4 hours; from ED arrival to EEG, 3.7 hours. Median time from start of EEG preparation to EEG recording (including preparing the EEG system, placing EEG leads, making any lead adjustments, and starting EEG) was 9 minutes, and with practice, as brief as 36 seconds; this time shortened during the study (r=−0.57, p<0.0001, Supplement).
Prediction of acute stroke/TIA or not (Table 2, Figure 1C):
Table 2:
Comparison of the Four Diagnostic Models
| Model | Identifying Acute Stroke/TIA | Identifying Acute Stroke with LVO | ||
|---|---|---|---|---|
| AUC | Sensitivity at 80% specificity | AUC | Sensitivity at 80% specificity | |
| Clinical | 62.3 | 40% | 80.4 | 65% |
| EEG | 78.2 | 65% | 68.9 | 41% |
| Clinical and EEG (Logistic regression) | 80.3 | 70% | 77.8 | 57% |
| Clinical and EEG (Deep Learning) | 87.8 | 79% | 86.4 | 76% |
[1]. Clinical variables only:
The regression model had AUC=62.3 on the validation group (SE=5). At specificity of 80%, sensitivity was 40%.
[2]. EEG variables only:
The model had AUC=78.2 on the validation group (SE=4). At a specificity of 80%, sensitivity was 65%.
[3]. Combined clinical and EEG using logistic regression:
The strongest predictor from model [1], plus RACE score, was advanced into a new model that also included the four EEG variables used in model [2]. The model (see Supplement) had AUC=80.3 on the validation set (SE=6). At a specificity of 80%, sensitivity was 70%.
[4]. Combined clinical and EEG using deep learning:
The six variables used in model [3] were again evaluated but using a deep learning neural network model, which yielded AUC=87.8 in the validation group (SE=5). At a specificity of 80%, sensitivity was 80%.
All three models with EEG were significantly (p=0.016–0.004) better predictors than the clinical-only model.
EEG prediction of acute stroke with LVO or not
The same four models were evaluated but with acute stroke with LVO (or not) as the dependent measure. Findings were overall similar, with the model combining clinical and EEG using deep learning again yielding the highest AUC (Table 2, Figure 1C; Supplement).
Discussion
Earlier treatment maximizes benefits of reperfusion. Clinical scales identify treatment-eligible patients but have incomplete diagnostic precision. EEG, which immediately detects cerebral ischemia, could help but its clinical use has been limited due to lengthy times required for application of traditional gel electrodes. Advances in EEG technology, including rapidly applied dry-electrode systems3, enable rapid EEG acquisition. The current study found that, in ED patients with suspected acute stroke 12–14 hours post-onset, EEG was superior to clinical measures for diagnosing acute stroke/TIA or LVO, and that combining EEG with clinical data gives best diagnostic precision. Prehospital studies, in patients at earlier stages of stroke, are warranted.
Results indicate that EEG signals contain diagnostic information beyond what is provided by clinical assessments, and support EEG measurement to help diagnose acute stroke. For diagnosing acute stroke/TIA, clinical+EEG data had AUC=87.8, while clinical data alone had AUC=62.3. Clinical+EEG data also performed best for diagnosing LVO (AUC=86.4). AUC >0.8 is considered excellent discrimination5. Advances in EEG technology are overcoming hurdles to its implementation. The current study employed a small, portable, wireless, battery-powered, dry-electrode system that has excellent sensitivity compared to gel-lead systems3.
The main finding is that EEG and clinical data combined are better than either alone for identifying acute stroke/TIA and LVO. As a proof-of-concept study, this is a first step towards demonstrating the feasibility of EEG in the prehospital setting. Future studies should examine the diagnostic performance of EEG when administered by EMS providers. Additionally, while artifact detection was performed manually in the current study and prohibitive of prehospital applications, EEG processing and analysis must, and can be, automated. The long-term vision is to obtain prehospital EEG data that informs patient selection for reperfusion therapy, emulating prehospital EKG for diagnosing acute MI, where EMTs rapidly apply leads and obtain a computerized readout, minimally affecting on-scene time.
Supplementary Material
Sources of funding
Supported by UL1-TR001414.
Disclosures
Dr. Cramer is a consultant for Constant Therapeutics, Neurolutions, MicroTransponder, Regenera, SanBio, Fujifilm Toyama Chemical Co., and TRCare. We thank Cognionics, Inc. for providing EEG components.
Non-standard Abbreviation and Acronyms:
- LVO
Large vessel occlusion
- LKW
Last-known-well
- RACE
Rapid Arterial oCclusion Evaluation
Footnotes
Supplemental materials
Expanded Materials & Methods
Online Table I
Online Figure I
References 6–10
References
- 1.Smith EE, Kent DM, Bulsara KR, Leung LY, Lichtman JH, Reeves MJ, Towfighi A, Whiteley WN, Zahuranec DB, American Heart Association Stroke Council. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: A systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111–e122 [DOI] [PubMed] [Google Scholar]
- 2.Astrup JS L; Branston NM; Lassen NA Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57 [DOI] [PubMed] [Google Scholar]
- 3.Marini F, Lee C, Wagner J, Makeig S, Gola M. A comparative evaluation of signal quality between a research-grade and a wireless dry-electrode mobile EEG system. J Neural Eng. 2019;16:054001. [DOI] [PubMed] [Google Scholar]
- 4.Koster GT, Nguyen TTM, van Zwet EW, Garcia BL, Rowling HR, Bosch J, Schonewille WJ, Velthuis BK, van den Wijngaard IR, den Hertog HM, et al. Clinical prediction of thrombectomy eligibility: A systematic review and 4-item decision tree. Int J Stroke. 2018:1747493018801225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosmer D, Lemeshow S. Applied logistic regression. New York, NY: John Wiley and Sons; 2000:160–164. [Google Scholar]
- 6.Chi Y, Wang Y, Wang Y-T, Jung T-P, Kerth T, Cao Y. A practical mobile dry EEG system for human computer interfaces In: Schmorrow D, Fidopiastis C, eds. Foundations of augmented cognition. Berlin: Springer-Verlag; 2013:649–655. [Google Scholar]
- 7.Krebs W, Sharkey-Toppen TP, Cheek F, Cortez E, Larrimore A, Keseg D, Panchal AR. Prehospital stroke assessment for large vessel occlusions: A systematic review. Prehosp Emerg Care. 2018;22:180–188 [DOI] [PubMed] [Google Scholar]
- 8.Hastrup S, Damgaard D, Johnsen SP, Andersen G. Prehospital acute stroke severity scale to predict large artery occlusion: Design and comparison with other scales. Stroke. 2016;47:1772–1776 [DOI] [PubMed] [Google Scholar]
- 9.Dickson RL, Crowe RP, Patrick C, Crocker K, Aiken M, Adams A, Gleisberg GR, Nichols T, Mason C, Panchal AR. Performance of the RACE score for the prehospital identification of large vessel occlusion stroke in a suburban/rural EMS service. Prehosp Emerg Care. 2019;23:612–618 [DOI] [PubMed] [Google Scholar]
- 10.Tibshirani R The Lasso method for variable selection in the Cox model. Statistics in Medicine. 1997;16:385–395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


