Abstract
Purpose:
To evaluate long-term risk and outcomes of glaucoma in eyes with intermediate, posterior, and panuveitis managed with systemic or fluocinolone acetonide (0.59 mg, “implant”) therapy.
Design:
Prospective Follow-up of the Multicenter Uveitis Steroid Treatment (MUST) Clinical Trial Cohort
Methods:
Patients with intermediate, posterior or panuveitis randomized to implant or systemic therapy (corticosteroid plus immunosuppression in >90%) were followed prospectively for glaucoma incidence and outcome.
Results:
Among 405 uveitic at-risk eyes of 232 patients (median follow-up=6.9 years), 40% (79/196) of eyes assigned and treated with implant and 8% (17/209) of eyes assigned and treated with systemic therapy (censoring eyes receiving an implant upon implantation) developed glaucoma (Hazard Ratio (HR)=5.9 (95% CI: 3.2, 10.8); p<0.001). Adjustment for IOP elevation during follow-up only partially mitigated the association of implant treatment with glaucoma incidence: HR=3.1 (95% CI: 1.6, 6.0); p=0.001. Among 112 eyes of 83 patients developing glaucoma, the five year cumulative incidence following diagnosis of sustained (2 or more consecutive visits) worsening of mean deviation by ≥6 dB was 20% (95% CI: 12%, 33%); five year cumulative incidence of sustained worsening of cup-to-disc ratio by ≥0.2 was 26% (95% CI: 17%, 39%).
Conclusions:
Implant has substantially higher risk of glaucoma than systemic therapy, a difference not entirely explained by post-treatment IOP elevation. Management of IOP elevation was effective in preventing worsening of glaucoma for the large majority of cases, but even under expert clinical management some glaucoma worsened. Uveitis cases should be monitored carefully for IOP elevation and glaucoma indefinitely.
Intraocular pressure elevation and glaucoma frequently occur in ocular inflammatory disease, especially with high dosages of local therapies, including long-acting local therapies.1–4 Glaucoma is best defined as an optic neuropathy for which intraocular pressure (IOP) usually is a contributing factor.5 However, in the setting of ocular inflammation, often affecting younger and middle aged adults,6,7 glaucoma typically occurs following IOP elevation.1,2 A broad array of medical and surgical treatments exist to treat IOP elevation, and with sufficient effort IOP elevation typically can be controlled.8 Thus, many uveitis specialists emphasize control of immediately vision-threatening complications such as foveal scarring or macular edema on the assumption that subsequent elevations in IOP can be managed without vision loss.
We previously reported that a cohort of patients participating in a clinical trial in which patients with active or recently active intermediate, posterior or panuveitis were randomized to systemic therapy or a long-lasting intraocular corticosteroid implant had a high risk of glaucomatous optic neuropathy in during the first two years following randomization, especially in the implant group.1 We also previously have reported the incidence of glaucomatous optic neuropathy by as-randomized treatment assignment (systemic therapy vs. fluocincoloneacetonide 0.59 mg long-lasting intravitreous implant) through seven years’ follow-up.9 In the MUST Trial, elevated IOP (often over 30 mmHg) commonly preceded occurrence of glaucoma. Given that such elevation of intraocular pressure typically can be successfully treated, an important issue for patients is the extent to which glaucoma and its sequelae progress after glaucoma is detected. Here, we report the incidence of and risk factors for glaucoma through the complete follow-up of the MUST Trial cohort (up to 10 years). We also describe outcomes of uveitic eyes with glaucoma during the several years’ follow-up ultimately carried out in the study, beginning from the point of diagnosis with glaucoma.
Methods
The methods of the Multicenter Uveitis Steroid Treatment (MUST) Trial , a multicenter prospective randomized clinical trial with five year extended follow-up (www.clinicaltrials.govregistration: NCT00132691) previously have been described.10 In brief, patients gave informed consent and then were randomized to systemic therapy following Expert Panel Guidelines11 versus fluocinolone acetonide implant therapy4,12 in uveitic eyes for which the alternative treatments were indicated. Enrollment into the MUST Trial occurred from December 6, 2005–December 9, 2008, after which patients were followed for an additional two years for the primary outcome of the study.13 During the MUST Trial, subjects were followed at visits one and three months after enrollment then quarterly. After completion of the MUST Trial (after 2 to 5 years’ follow-up depending on when the subject was enrolled), willing subjectsgave informed consent and then were enrolled in the MUST Trial Follow-up Study,9,14,15 and followed at every six month follow-up visits for an additional five years, giving seven to 10 years of follow-up for the large majority of participants. Institutional Review Board approval was obtained prior to beginning the trial and updated/maintained throughout the study at the Coordinating Center (Prime IRB, Johns Hopkins Bloomberg School of Public Health Committee on Human Research), the Reading Center (University of Wisconsin Institutional Review Board) and the 19 clinical centers.
Throughout this time, IOP management was done according to best medical judgment, and data regarding IOP and glaucoma treatment and outcome were collected. Patients underwent prospective data collectionbased on protocol-specified clinical examinations and tests until study closeout.10 These included fundus photographs graded for cup-to-disc ratio by the MUST Reading Center. Humphrey Visual Fields from which Mean Deviation (representing the difference in average visual field sensitivity from that of a standard reference population) and Pattern Standard Deviation (representing whether the distribution of visual field sensitivity values across the field differed in quadrants of the field from that of a standard population) also were available. With progressive glaucomatous optic neuropathy, Mean Deviation is expected to decline (become more negative) whereas Pattern Standard Deviation is expected to rise. IOP was measured as the median of three measurements by Goldmannapplanation tonometry performed at least semi-annually. Best-corrected visual acuity also was measured at least semiannually using gold standard (ETDRS logarithmic chart) methods.16 Patient-reported quality of life measurements including the SF-36 Physical Component Score of generic health-related quality of life and the NEI-VFQ vision-related quality of life measurements summarizing the patient’s experience of peripheral vision and ocular pain17,18 were assessed.
Glaucoma was diagnosed by a process beginning with review of stereo fundus photograph images by the treatment assignment-masked study Reading Center.1 These images were obtained at baseline, 3 months, 6 months and then annually during the MUST Trial and Follow-up Study respectively, followed by a return to semiannually approximately two years into the MUST Trial Follow-up Study. Images which demonstrated a change incup-to-disc ratio of 0.1 or more for small nerves (those that were ≥2 standard deviations smaller in size from the mean of the baseline images) or a change of 0.2 or more for normal or largecup-to-disc ratio nerves were referred to the MUST Glaucoma Outcomes Committee beginning at year 2 following randomization, to determine whether incident glaucoma should be diagnosed in the eye. Reading Center cup-to-disc ratiogradings were highly reproducible.19 For eyes referred to the treatment assignment-masked MUST Glaucoma Outcomes Committee, first a treatment-masked glaucoma specialist (DSF) evaluated stereo disc color images, visual fields (obtained annually), serial IOP measurements, and other clinical data for each eye at baseline and subsequent visits to determine if he agreed with Reading Center image gradings indicating an increase in cup-to-disc ratio, and then to determine if the eye hadglaucoma or not.After this, a second treatment-masked glaucoma specialist (HA)independently reviewed the same data for the same eyes. A set of randomly selected eyes without changes also were reviewed. The rate of agreement regarding diagnosis of glaucoma between the two reviewers was 98.3%. Disagreements were settled by consensus.
As most enrolled eyes had a depressed visual field as measured by mean deviation (MD) at baseline (mean MD −5.2 dB), often related to chorioretinal damage from posterior uveitis, changes in cup-to-disc ratio were most indicative of glaucoma incidence. The Committee also reviewed stereo disc photographs from all visits corresponding to periods when clinically important worsening of the visual field was observed, even if the Reading Center had not identified a change in cup-to-disc ratio. Based on the timing of the disc imaging and visual field testing under the protocol, glaucoma could be diagnosed for the first time at the follow-up visit approximately one year after baseline. (Visits used for glaucoma outcomes occurred during a visit window, generally within +/− three months of the target date, rather than on the exact anniversary date.) Because many eyes which developed glaucoma developed it after the one year visit, eye-time both before and after occurrence of glaucoma was available for assessment.
IOP was modeled over follow-up time four ways: 1) Baseline IOP using the IOP at the baseline visit; 2) time-updated IOP using the IOP at each visit; 3) time-updated average IOP using the average of IOP measurements from baseline through each visit; and 4) time-updated maximum IOP using the highest IOP up through the visit. (Time-updated variables’ values changed and were assessed over time of follow-up in the survival analysis). Lowess curves were constructed to estimate the trajectory of IOP and cumulative average of IOP over time since initiation of treatment. The cumulative incidence of glaucoma by treatment group was calculated using Kaplan-Meier methods with 95% confidence intervals using the Huber-White variance estimation to account for correlation between eyes within the same patient. Analyses were conducted using both an intention-to-treat approach comparing treatment groups from randomization to diagnosis of glaucoma for eyes with the event or censored at last follow-up for eyes without the event; and an as-treated approach with no crossovers, namely eyes randomized to implant and receiving implant were followed from date of implant to either incident glaucoma or censoring at last follow-up visit; eyes randomized to systemic therapy were followed from date of randomization to 1) incident glaucoma or censoring at last follow-up visit if never treated with an implant; or 2) incident glaucoma if preceding implant or censoring at the last follow-up visit preceding implant if treated with an implant during follow-up. Curves of the incidence rate of glaucoma by treatment group (as treated) were estimated using weighted kernel smoothing. Risk factors for time-to-incident glaucoma were assessed using Cox regression. Goodness of fit and model selection for Cox regression models were assessed using generalized r-squared statistic, which is the proportion of explained variance for proportional hazards models,20 and Akaike Information Criteria,21 which estimate the relative amount of information lost by a given model, with less information lost indicating a better model. Multiple Cox regression analyses using time-updated covariates used a complete case approach to missing data. As sensitivity analyses, to assess whether missing data affected results substantially, last-value-carried-forward and multiple imputation approaches also were used.
The outcomes studied for eyes following diagnosis with glaucoma included cup-to-disc ratio, automated visual field mean deviation and pattern standard deviation, best-corrected visual acuity and intraocular pressure (IOP). The overall mean of each outcome and the slope across time (rounded to negative integers for yearly visits prior to glaucoma diagnosis, 0 for visit at glaucoma diagnosis, and positive integers for yearly visits after glaucoma diagnosis) for each outcome were compared before vs. at/after diagnosis of glaucoma using linear regression models with random effects for patient and eye. The comparison of the overall means was modeled using an indicator variable for before vs. at/after glaucoma diagnosis. Statistics include mean and standard error for each outcome before vs. at/after and the mean, 95% confidence interval and p-value of the difference. A separate model comparing slopes used the indicator variable for before vs at/after, slope over time and the interaction of both terms. Statistics include slopes and standard errors for each outcome separately by before vs. at/after and the mean, 95% confidence interval and p-value for the difference in slopes.
For specific eyes after diagnosis of glaucoma, the incidence of losses or gains in mean deviation (by 6 dB or more) and in cup-to-disc ratio (by 0.2 or more) were studied based on a sustained worsening or gain observed across two consecutive visits (see above for visit frequency)).
For comparisons of eye characteristics by treatment group, correlation between eyes within the same patient was accounted for using Huber-White robust variance estimation for Cox regression and logistic regression and bootstrapping clustering on patient for robust linear regression.
Results
Among the 479 uveitic eyes of 255 patients enrolled in the MUST Trial, 405 uveitic eyes (85%) of 232 patients had sufficient data available to assess the incidence of glaucoma at some point during follow-up, and hence contribute to the analysis (see CONSORT Diagram, Supplemental Figure 1). Uveitic eyes were followed for up to ten years, with those enrolled later and those lost to follow-up having less follow-up. By treatment received, patients in the Implant group compared to the Systemic group had less bilaterality of uveitis (67.5% vs. 81.7%, p=0.02), and were somewhat more likely to be male (30.8% vs. 20.0%, p=0.07). Eyes in the Implant group compared to the Systemic group had worse baseline mean deviation (−6.0 dB vs. −4.6 dB, p=0.02), and worse visual acuity (67 vs. 72standard letters, p=0.003). They also were more likely to be missing baseline cup-to-disc ratio assessments. The distributions of other baseline characteristics were similar between groups (see Table 1).
Table 1. Baseline Patient and Eye Characteristics by As-Treated Treatment Group.
Eyes with uveitis treated with [fluocinolone acetonide) Implant or Systemic Therapy in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study
| Implant | Systemic | P-value* | |
|---|---|---|---|
| Patient characteristics | |||
| No. patients - n | 117 | 115 | |
| Male – n (%) | 36 (30.8%) | 23 (20.0%) | 0.07 |
| Race / Ethnicity | 0.27 | ||
| White, non-Hispanic - n (%) | 68 (58.1%) | 65 (56.5%) | |
| Hispanic - n (%) | 16 (13.7%) | 11 (9.6%) | |
| Black, non-Hispanic - n (%) | 30 (25.6%) | 30 (26.1%) | |
| Other - n (%) | 3 (2.6%) | 9 (7.8%) | |
| Age (yrs) - mean(SD) | 45 (14) | 46 (15) | 0.53 |
| Body mass index (kg/m2) - mean(SD) | 31 (8) | 31 (9) | 0.87 |
| Uveitis characteristics | |||
| Uveitis Stratum (Intermediate vs. Posterior or Panuveitis) - n (%) | 47 (40.2%) | 45 (39.1%) | 0.89 |
| Bilateral Uveitis - n (%) | 79 (67.5%) | 94 (81.7%) | 0.02 |
| Quality of Life measures | |||
| SF-36 Physical Component Score - mean(SD) | 47 (10) | 48 (10) | 0.25 |
| SF-36 Mental Component Score - mean (SD) | 48 (13) | 48 (11) | 0.77 |
| VFQ-25 Composite - mean(SD) | 59 (22) | 63 (20) | 0.17 |
| EQ-5D - mean(SD) | 0.81 (0.18) | 0.83 (0.15) | 0.45 |
| Eye characteristics | |||
| No. eyes - n | 196 | 209 | |
| Treatment | |||
| OP medication use | 33 (16.8%) | 26 (12.4%) | 0.34 |
| Glaucoma risk factor characteristics | |||
| Mean deviation (dB) - median[IQR] | −6.0 [−11.0, −3.5] | −4.6 [−8.1, −2.7] | 0.02 |
| Cup-to-disc ratio - median(IQR] | 0.29 [0.20, 0.37] | 0.30 [0.21, 0.38] | 0.53 |
| Missing data for cup-to disc ratio | 61 (31.1%) | 29 (15.8%) | 0.002 |
| Intraocular pressure (mmHg) - median[IQR] | 14 [12, 17] | 14 [12, 17] | 0.81 |
| Other eye characteristics | |||
| Visual acuity (standardized letters) -median[IQR] | 67 [46, 78] | 72 [57, 81] | 0.003 |
| Anterior chamber cells | |||
| Grade 1+ or higher (≥ 6 cells) - n (%) | 101 (51.5%) | 95 (45.4%) | 0.34 |
| Anterior chamber flare | |||
| Grade 1+ or higher (≥ faint) - n (%) | 102 (52.0%) | 94 (45.0%) | 0.27 |
| Anterior vitreous cells | |||
| Grade 1+ or higher (≥ 11 cells) - n (%) | 149 (81.0%) | 168 (83.6%) | 0.59 |
| Vitreous haze | |||
| Grade 1+ or higher- n (%) | 119 (66.5%) | 138 (69.0) | 0.67 |
For patient characteristics, p-values are derived from unequal variance t-tests for continuous variables and Fisher’s Exact Test for categorical variables. For eye characteristics, p-values are derived from robust linear regression with bootstrapped standard errors accounting for clustering for continuous variables and logistic regression with robust estimation of standard errors to account for clustering for binary variables.
As in our previous reports based on less follow-up time,1,9,13,15 the mean IOP over time (Figure 1, left and right) and the incidence of glaucoma (Figure 2) were higher in the implant group than the systemic group.9,13–15 The Kaplan-Meier estimates of the overall proportion developing IOP elevation≥30 mmHg by 2.5, 5, 7.5 and 10 years respectively were: 22% (95% Confidence Interval (CI): 18%, 26%); 28% (95% CI: 24%, 32%); 29% (95% CI: 25%, 33%);and 30% (95% CI: 25%, 35%). The Kaplan-Meier estimates of the overall proportion developing glaucoma by 2.5, 5, 7.5 and 10 years respectively were: 10% (95% CI: 8%, 14%); 22% (95% CI: 18%, 26%); 28% (95% CI: 24%, 33%); and 32% (95% CI: 26%, 38%). In an intention-to-treat analysis, 36% (80/220) of eyes in the implant group vs. 15% (32/209) of eyes in the systemic group developed glaucoma (crude hazard ratio (HR)=2.8, 95% confidence interval (CI)=1.8, 4.6, P<0.001). In an as-treated analysis, with median follow-up=6.0 years after randomization, 40% (79/196) of eyes assigned to and receiving the implant vs. 8% (17/209) of eyes assigned to and receiving systemic treatment (censored at time of implant if an implant was placed) developed glaucoma (crude HR=5.8, 95% CI=3.2, 10.5; p<0.001). In addition, 38% (15/39) of eyes assigned to systemic group but receiving the implant during follow-up developed glaucoma a median of 2.8 years following implant surgery. Although the incidence rate for glaucoma decreased over time after randomization, the proportional hazards assumption was not violated (p=0.13) (Supplemental Figure 2)
Figure 1. Intraocular Pressure (IOP) Over Time by As-Treated Treatment Group.
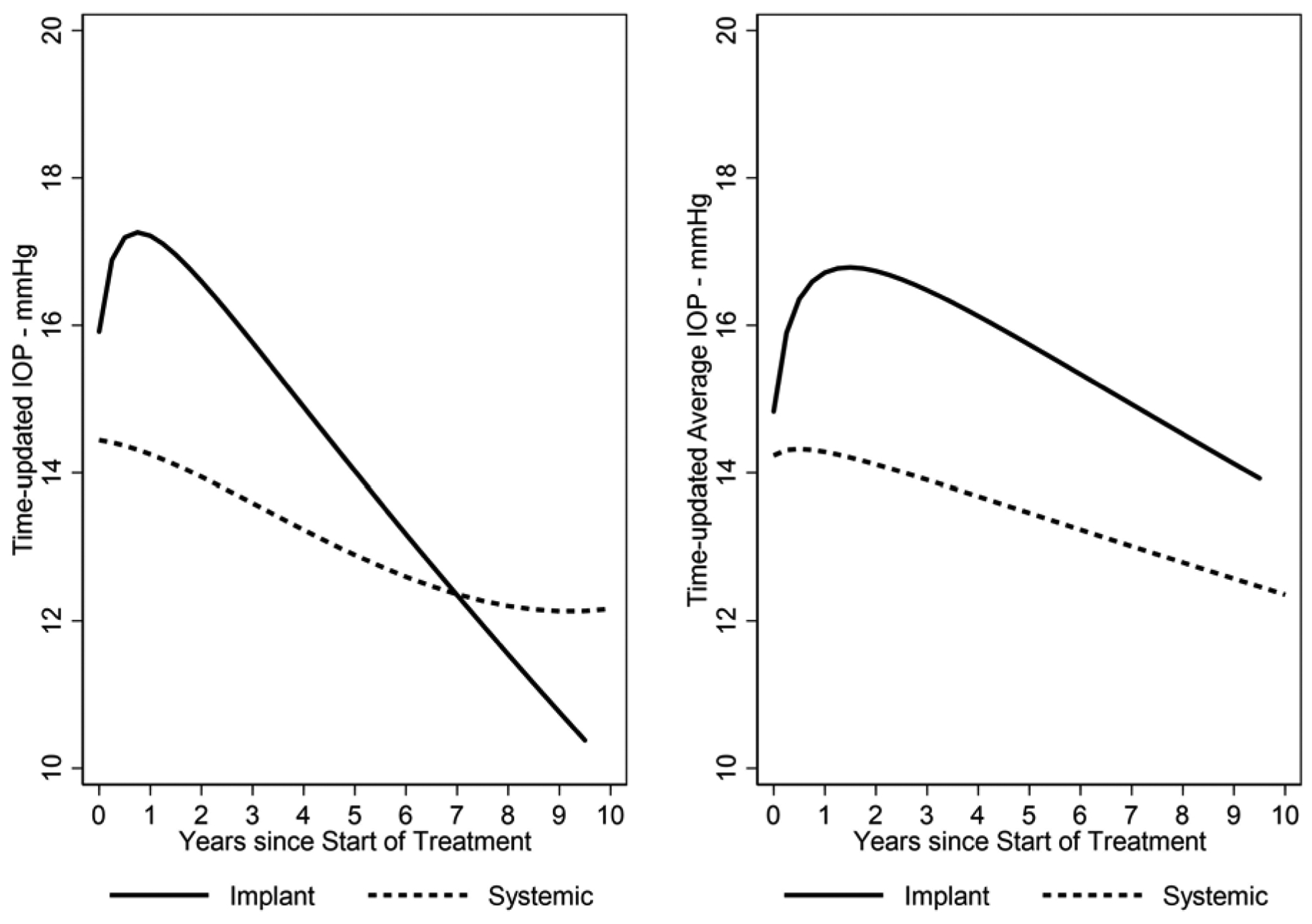
Right panel: Lowess curve summarizing IOP over time by treatment group. Left panel: Lowess curve summarizing the average of cumulative IOP over time by treatment group. Eyes with uveitis treated with [fluocinolone acetonide) Implant or Systemic Therapy in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
Figure 2. Cumulative Incidence of Glaucoma by As-Treated Treatment Group.
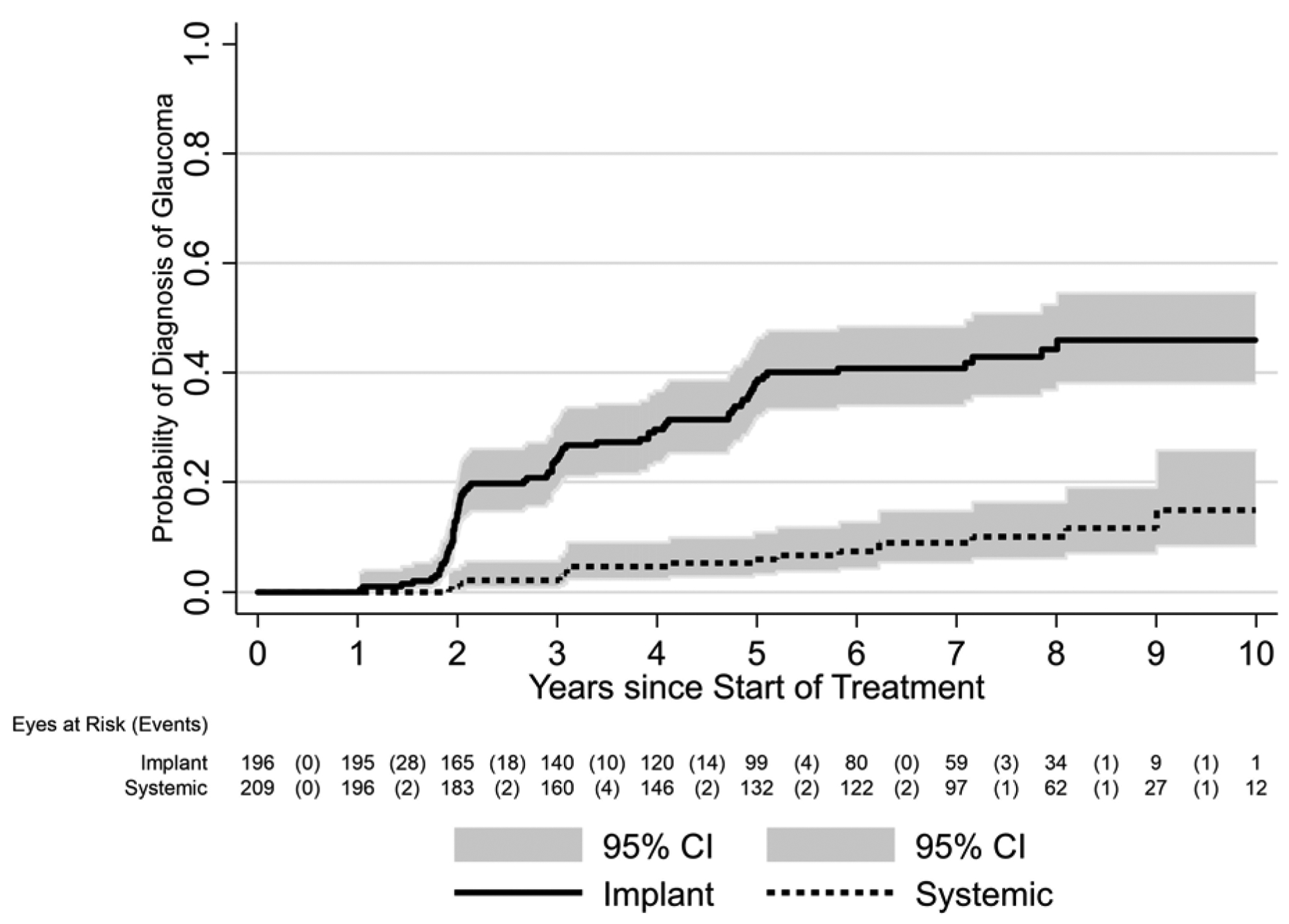
Eyes with uveitis treated with [fluocinolone acetonide) Implant or Systemic Therapy in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
Risk Factors for Glaucoma
Among potential baseline risk factors assessed (Table 2), Implant treatment (adjusted hazard ratio (aHR)=6.3, 95% CI: 3.4–11.8, compared to Systemic treatment) was the strongest predictor. Other risk factors included Black race (aHR=1.73, 95% CI: 1.01, 2.94 vs other race/ethnicity), use of IOP-lowering medication at baseline (aHR=2.24, 95% CI: 1.24, 4.05), and higher cup-to-disc ratio (HR=1.36 for each 0.1 higher baseline cup-to-disc ratio, 95% CI: 1.07–1.62). Higher baseline IOP was associated with higher crude incidence of glaucoma, but this association was no longer significant after adjusting for other factors. The MUST Trial protocol did not permit enrollment of eyes with baseline IOP≥24 mmHg or cases already with advanced glaucoma.13
Table 2.
Baseline Risk Factors for Incident Glaucoma, eyes with uveitis treated with [fluocinolone acetonide 0.59 mg) Implant or Systemic Therapy in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study
| Crude | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| Risk factor | Comparison | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Design | |||||||
| Treatment group | Implant vs. Systemic | 5.8 | 3.2, 10.5 | <0.001 | 6.3 | 3.4, 11.8 | <0.001 |
| Stratum | Posterior or Panuveitis vs. Intermediate Uveitis | 1.15 | 0.70, 1.89 | 0.58 | |||
| Demographics | |||||||
| Age | 50+ vs <50 yrs | 0.76 | 0.47, 1.24 | 0.27 | |||
| Race | Black vs other | 1.28 | 0.74, 2.23 | 0.37 | 1.73 | 1.01, 2.94 | 0.04 |
| Sex | Male vs. Female | 1.01 | 0.60, 1.73 | 0.96 | |||
| Body mass index | / kg/m2 | 0.98 | 0.95, 1.01 | 0.16 | |||
| Baseline use of IOP-lowering medication | Yes vs no | 2.19 | 1.23, 3.88 | 0.008 | 2.24 | 1.24, 4.05 | 0.008 |
| Eye BL characteristics | |||||||
| Higher intraocular pressure | / mmHg | 1.07 | 1.01, 1.14 | 0.02 | |||
| Larger cup-to-disc ratio | /0.1 | 1.26 | 1.05, 1.51 | 0.02 | 1.32 | 1.07, 1.62 | 0.01 |
| Mean deviation | /dB | 0.98 | 0.95, 1.01 | 0.26 | |||
| Bilateral disease | Yes vs. no | 0.81 | 0.40, 1.62 | 0.54 | |||
Used multiple imputation for CDR (due to 24% missing data) and forward selection model with probability of entry = 0.05 using candidate set of all baseline risk factors. Baseline intraocular pressure was not selected by forward selection, see Methods). BL=baseline; CI=confidence interval.
In addition, elevation of IOP during follow-up after uveitis treatment initiation was a strong predictor for glaucoma. The relationship between IOP over time and the incidence of glaucoma is summarized in Table 3. Higher IOP during follow-up was associated with increased risk of glaucoma in an approximate dose-response fashion, whether IOP was summarized using a time-updated approach (the IOP level measured at the time of assessment for glaucoma), the average IOP measurement at follow-up visits prior to and at the time of assessment for glaucoma diagnosis, or the highest observed IOP at or prior to the time of assessment for glaucoma. In the Systemic uveitis as-treated group, current IOP of 16–20 mmHg was associated with 5.3-fold higher incidence of glaucoma than IOP<16 mmHg and average IOP of 16–20 mmHg was associated with a 3.5-fold higher incidence of glaucoma than average IOP<16, both with progressively higher glaucoma incidence as IOP progressed upward from there. For maximum observed IOP (IOPmax), the incidence of glaucoma was 3.0-fold higher for IOPmax of 21–24 than 16–20, and progressed as IOPmax became higher. The risk of glaucoma increased in a similar manner across increasing IOP categories in both the implant and systemic groups using categories defined as cumulative average IOP (interaction p=0.52) and IOPmax (interaction p=0.71) but for time-updated IOP-glaucoma risk association was significantly lower (interaction p=0.002) forthe implant group (RR=1.3/IOP time-updated category) than the systemic group (RR=2.3/time-updated IOP category).
Table 3.
Intraocular Pressure (IOP) as Predictor of Incident Glaucoma Stratified by Treatment, eyes with uveitis treated with [fluocinolone acetonide) Implant or Systemic Therapy in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study
| Implant (events=77; n=196 eyes; 3,201 complete case 3-mo eye-visits) | Systemic (events=16; n=209 eyes; 4,024 complete case 3-mo eye-visits) | ||||||
|---|---|---|---|---|---|---|---|
| IOP Definition‡ | IOP category -mmHg | Rate /100 Eye-Years | Events/3-mo eye-visit | P-value* vs. Reference (Ref) group | Rate /100 Eye-Years | Events/ 3-mo eye-visit | P-value* vs. Ref group |
| Baseline | <16 | 8.4 | 40/1901 | Ref | 1.2 | 8/2642 | Ref |
| 16–20 | 11.2 | 34/1214 | 0.18 | 2.8 | 8/1351 | 0.09 | |
| 21–24 | 14.0 | 3/86 | 0.33 | 0.0 | 0/242 | -- | |
| 25–29 | -- | 0/0 | -- | 0.0 | 0/9 | -- | |
| 30–39 | -- | 0/0 | -- | -- | 0/0 | -- | |
| 40–49 | -- | 0/0 | -- | -- | 0/0 | -- | |
| 50+ | -- | 0/0 | -- | -- | 0/0 | -- | |
| HR (95% CI) /cat† | 1.3 (0.9, 1.9) | 0.12 | 1.3 (0.7, 2.4) | 0.42 | |||
| Time-updated | <16 | 8.8 | 37/1654 | Ref | 0.6 | 4/2748 | Ref |
| 16–20 | 8.2 | 19/925 | 0.96 | 3.2 | 8/1008 | 0.004 | |
| 21–24 | 13.5 | 9/266 | 0.05 | 4.8 | 2/163 | 0.003 | |
| 25–29 | 4.6 | 2/173 | 0.71 | 6.4 | 1/62 | 0.001 | |
| 30–39 | 11.4 | 4/140 | 0.18 | 12.5 | 1/32 | <0.001 | |
| 40–49 | 51.3 | 5/39 | 0.001 | 0.0 | 0/8 | -- | |
| 50+ | 44.4 | 1/9 | <0.001 | 0.0 | 0/1 | -- | |
| HR (95% CI) /cat† | 1.3 (1.1, 1.5) | 0.001 | 2.3 (1.7, 3.1) | <0.001 | |||
| Time-updated Cumulative Average | <16 | 3.6 | 10/1153 | Ref | 0.8 | 5/2551 | Ref |
| 16–20 | 10.0 | 37/1455 | 0.02 | 2.8 | 9/1344 | 0.05 | |
| 21–24 | 20.0 | 23/460 | <0.001 | 7.2 | 2/107 | 0.001 | |
| 25–29 | 16.4 | 5/122 | <0.001 | 0.0 | 0/18 | -- | |
| 30–39 | 53.2 | 2/15 | <0.001 | 0.0 | 0/4 | -- | |
| 40–49 | 0.0 | 0/7 | -- | -- | -- | -- | |
| 50+ | -- | -- | -- | -- | -- | ||
| HR (95% CI) / cat† | 2.0 (1.6, 2.5) | <0.001 | 2.8 (1.5, 5.1) | 0.001 | |||
| Time-updated Maximum | <16 | 0.0 | 0/508 | NC | 0.0 | 0/987 | NC |
| 16–20 | 3.0 | 5/667 | Ref | 0.8 | 4/1824 | Ref | |
| 21–24 | 3.6 | 5/538 | 0.98 | 2.4 | 4/704 | 0.32 | |
| 25–29 | 7.2 | 9/499 | 0.36 | 6.0 | 4/270 | 0.03 | |
| 30–39 | 24.8 | 40/687 | <0.001 | 8.8 | 4/182 | 0.007 | |
| 40–49 | 20.3 | 13256 | 0.005 | 0.0 | 0/52 | NC | |
| 50+ | 35.1 | 5/57 | 0.002 | 0.0 | 0/5 | NC | |
| HR (95% CI) /cat† | 1.6 (1.4, 1.9) | <0.001 | 1.7 (1.3, 2.3) | <0.001 | |||
Note: Eyes with IOP>24 mmHg at baseline were not eligible for enrollment into the study. Mo=month. HR=hazard ratio; CI=confidence interval. Cat=category. Ref=reference group. Event=diagnosis with glaucoma.
Derived from Cox regression accounting for between-eye correlation
P-value for interaction of treatment group and IOP category = 0.86 for Baseline IOP; 0.002 for Time-updated IOP; 0.52 for Time-updated Average IOP; and 0.71 for Time-updated Max IOP
IOP was defined 4 ways: 1) Baseline IOP using the IOP at the baseline visit; 2) time-updated IOP using the IOP at that visit; 3) time-updated average IOP using the average IOP up through that visit; and 4) time-updated max IOP using the highest IOP up through that visit.
In order to assess further whether IOP elevation occurring after treatment initiation explained the strong association between treatment with implant and glaucoma incidence, we adjusted for IOP elevation during follow-up in various ways, which lessened the association between Implant treatment and glaucoma (Table 4). However, Implant treatment remained a strong predictor of glaucoma after such adjustment (lowest aHR=3.1, 95% CI: 1.6, 6.0). Inclusion of both Implant treatment and intraocular pressure elevation during follow-up improved model fit (the proportion of explained variance was 0.34 for treatment alone, and 0.54 after adjusting for the maximum observed IOP). Associations were similar after adjustment for potential effects of intraocular corticosteroid injections.
Table 4.
A. Relative risk* of Glaucoma by Implant vs. Systemic Treatment (Tx) adjusted for Alternative Time-Updated¶ Definitions of Intraocular Pressure (IOP) Categorization, eyes with uveitis treated with [fluocinolone acetonide) Implant or Systemic Therapy in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study
B. Relative risk* of Glaucoma by Alternative Intraocular Pressure Categorizations in the Implant and Systemic Treatment Groups and Both Combined; eyes with uveitis treated with [fluocinolone acetonide) Implant or Systemic Therapy in the MUST Trial and Follow-up Study
| Model | Relative Risk Treatment (Implant vs Systemic) | 95% CI | P-value | Generalized R-squared† | AIC‡ |
|---|---|---|---|---|---|
| Tx | 5.9 | 3.2, 10.8 | <0.001 | 0.34 | 1024.2 |
| Tx + Baseline IOP | 5.8 | 3.2, 10.6 | <0.001 | 0.37 | 1020.5 |
| Tx + Time-updated IOP | 5.1 | 2.7, 9.5 | <0.001 | 0.44 | 1006.6 |
| Tx + Time-updated Average IOP | 3.6 | 1.9, 6.9 | <0.001 | 0.53 | 984.1 |
| Tx + Time-updated Max IOP | 3.1 | 1.6, 6.0 | 0.001 | 0.54 | 983.8 |
| Adjusted for time-dependent intra-ocular injections∥ | |||||
| Tx | 5.7 | 3.1, 10.3 | <0.001 | 0.35 | 1024.3 |
| Tx + Baseline IOP | 5.6 | 3.1, 10.2 | <0.001 | 0.38 | 1020.5 |
| Tx + Time-updated IOP | 4.9 | 2.6, 9.1 | <0.001 | 0.44 | 1007.1 |
| Tx + Time-updated Average IOP | 3.5 | 1.9, 6.7 | <0.001 | 0.54 | 984.9 |
| Tx + Time-updated Max IOP | 2.9 | 1.5, 5.7 | 0.001 | 0.54 | 983.9 |
| Relative Risk IOP (per mmHg) | |||||
| Both Groups | |||||
| Baseline IOP | 1.07 | 1.01, 1.13 | 0.02 | 0.04 | 1074.3 |
| Time-updated IOP | 1.07 | 1.05, 1.09 | <0.001 | 0.20 | 1048.1 |
| Time-updated Average IOP | 1.23 | 1.18, 1.28 | <0.001 | 0.43 | 1006.8 |
| Time-updated Max IOP | 1.09 | 1.07, 1.11 | <0.001 | 0.46 | 998.8 |
| Systemic Group | |||||
| Baseline IOP | 1.11 | 1.01, 1.21 | 0.03 | 0.09 | 147.2 |
| Time-updated IOP | 1.22 | 1.14, 1.30 | <0.001 | 0.63 | 129.3 |
| Time-updated Average IOP | 1.27 | 1.12, 1.44 | <0.001 | 0.40 | 138.2 |
| Time-updated Max IOP | 1.12 | 1.06, 1.18 | <0.001 | 0.45 | 136.8 |
| Implant Group | |||||
| Baseline IOP | 1.07 | 1.00, 1.14 | 0.04 | 0.04 | 811.8 |
| Time-updated IOP | 1.05 | 1.03, 1.07 | <0.001 | 0.10 | 803.9 |
| Time-updated Average IOP | 1.18 | 1.13, 1.23 | <0.001 | 0.28 | 778.6 |
| Time-updated Max IOP | 1.07 | 1.05, 1.09 | <0.001 | 0.29 | 776.4 |
Derived from Cox regression accounting for between-eye correlation; 6,832 complete case observations
Propor:on of explained variance for proportional hazards models.20
Akaike Information Criterion (best model is one with lowest AIC)21
IOP was defined 4 ways: 1) Baseline IOP using the IOP at the baseline visit; 2) time-updated IOP using the IOP at that visit; 3) time-updated average IOP using the average IOP up through that visit; and 4) time-updated max IOP using the highest IOP up through that visit.
Relative risk (95% CI) for glaucoma in patients receiving vs not receiving intra-ocular injections was 0.56 (0.23, 1.33); p=0.19 in model including Tx; 0.55 (0.23, 1.30); p=0.17 in model including Tx + Baseline IOP; 0.60 (0.25, 1.43); p=0.25 in model including Tx + time-updated IOP; 0.62 (0.25, 1.47); p=0.28 in model including Tx + time-updated average IOP; and 0.55 (0.23, 1.32); p=0.18 in model including Tx + time-updated max IOP.
Sensitivity analyses using last-value-carried forward and multiple imputation yielded similar results (see Supplemental Table 2).
Average Outcomes of Uveitic Eyes with Glaucoma Over Time
One hundred twelve eyes (of 83 patients) which developed glaucoma had a median follow-up time after glaucoma diagnosis of 4.5 (interquartile range 3.0 to 6.5) years; these (17 .7% with systemic treatment) were included in the outcome of glaucoma analyses. Figure 3 displays the distribution of outcome measurements of interest over the 6 years before and after diagnosis of glaucoma for uveitic eyes that developed glaucoma at some point during the study. With the limited power available for comparison given the few cases with systemic treatment, there were no clear differences in outcome between treatment groups (see Supplement Figure 3). The meancup-to-disc ratio (Figure 3a), which was the basis for referral for consideration of diagnosis of glaucoma, increased (worsened) to 0.58 after diagnosis from 0.40 before diagnosis [difference= 0.18, 95% CI=0.17, 0.19; p<0.001]. The slope decreased (improved) to 0.003/yr after diagnosis from 0.026/yr before diagnosis [difference=−0.023, 95% CI=−0.030, −0.017; p<0.001]. Regarding visual field sensitivity, the mean Mean Deviation (Figure 3b) decreased (worsened) to −13.3 dB after diagnosis from −8.5 dB before diagnosis [difference=−4.8, 95% CI=−5.4, −4.1; p<0.001]. The slope decreased (worsened) to −0.6 dB/yr after diagnosis from 0.0 dB/yr before diagnosis [difference=−0.6, 95% CI=−0.9, −0.2; p=0.001]. The mean Pattern Standard Deviation (variability across quadrants in visual field) (Figure 3c) increased (worsened) to 6.4 dB after diagnosis from 4.0 dB before diagnosis [difference = 2.4, 95% CI=2.1, 2.7; p<0.001]. The slope did not significantly change: 0.21 dB/yrof worsening after diagnosis vs. 0.10 dB/yr before diagnosis [difference=0.11, 95% CI=−0.05, 0.26; p=0.18]. The mean best-corrected visual acuity (Figure 3d) decreased (worsened) by approximately one line to 59.9 standardized letters after diagnosis from 64.6 standardized letters before diagnosis [difference = −4.7, 95% CI=−6.0, −3.5; p<0.001]. The slope also decreased to −2.7 letters/yr (approximately half a line) after diagnosis from 0.1 letters/yr before diagnosis [difference = −2.8, 95% CI=−3.5, −2.1; p<0.001]. The mean IOP (Figure 3e) decreased (improved) to 14.4 mmHg after diagnosis (where IOP-lowering treatment typically was stepped up) from 18.8 mmHg before diagnosis [difference = −3.9, 95% CI=−4.5, −3.3; p<0.001]. The slope decreased to −0.9 mmHg/yr after diagnosis from 0.3 mmHg/yr before diagnosis [difference = −1.1, 95% CI=−1.5, −0.8; p<0.001], indicating ongoing lowering of IOP over time after glaucoma diagnosis.
Figure 3. Distribution of parameters related to glaucoma outcome among uveitic eyes from six years before to six years after their diagnosis with glaucoma, eyes from the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
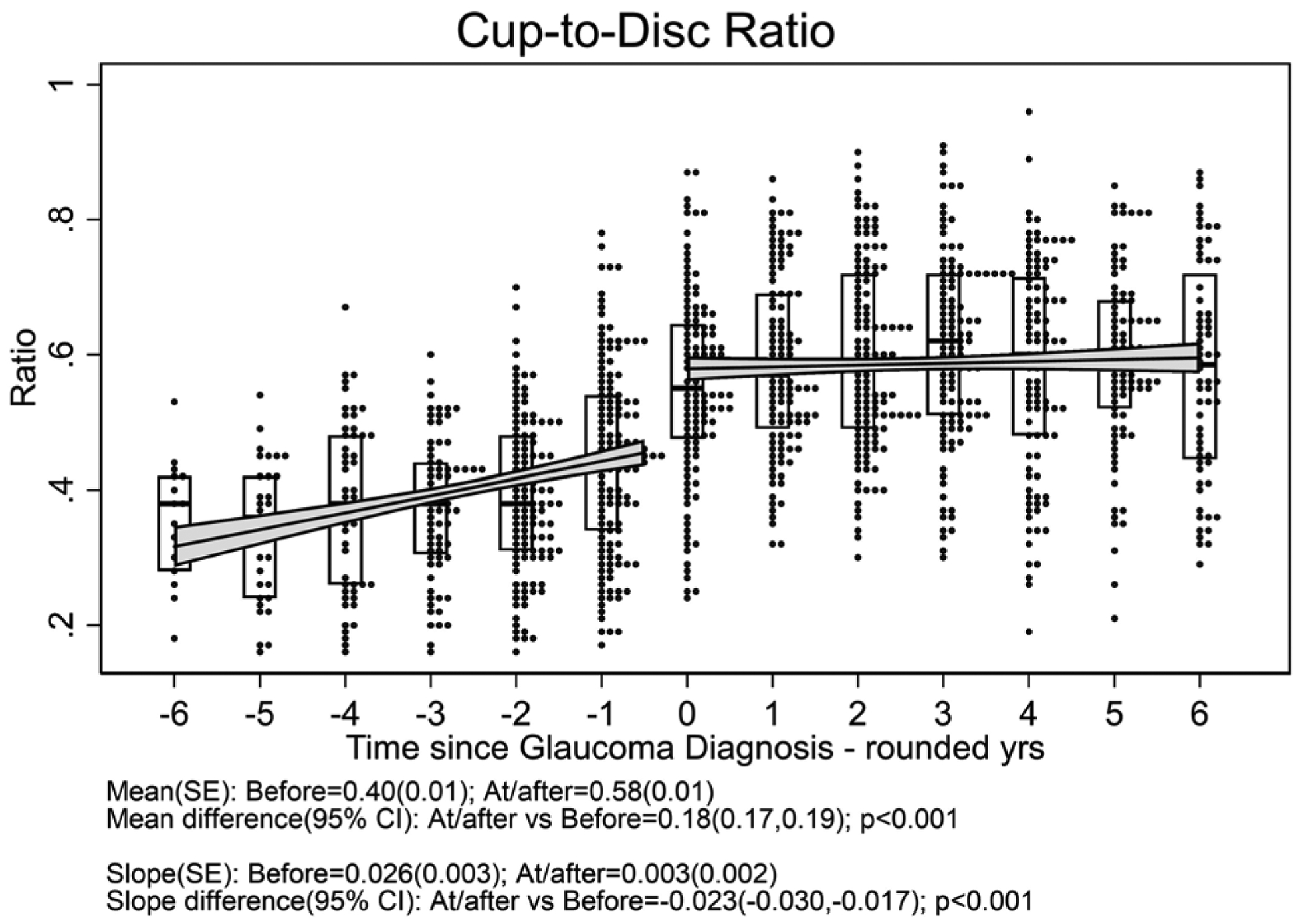
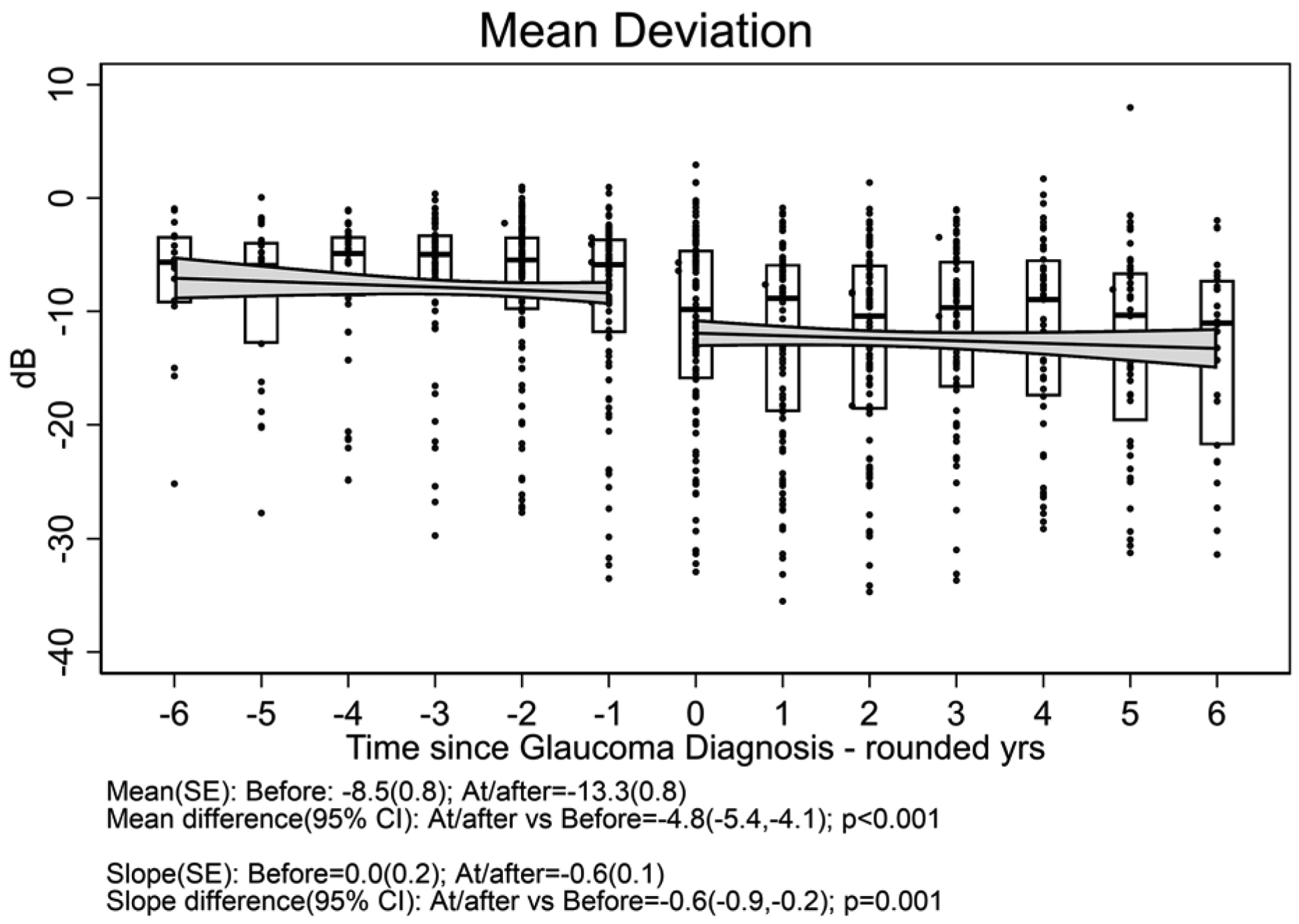
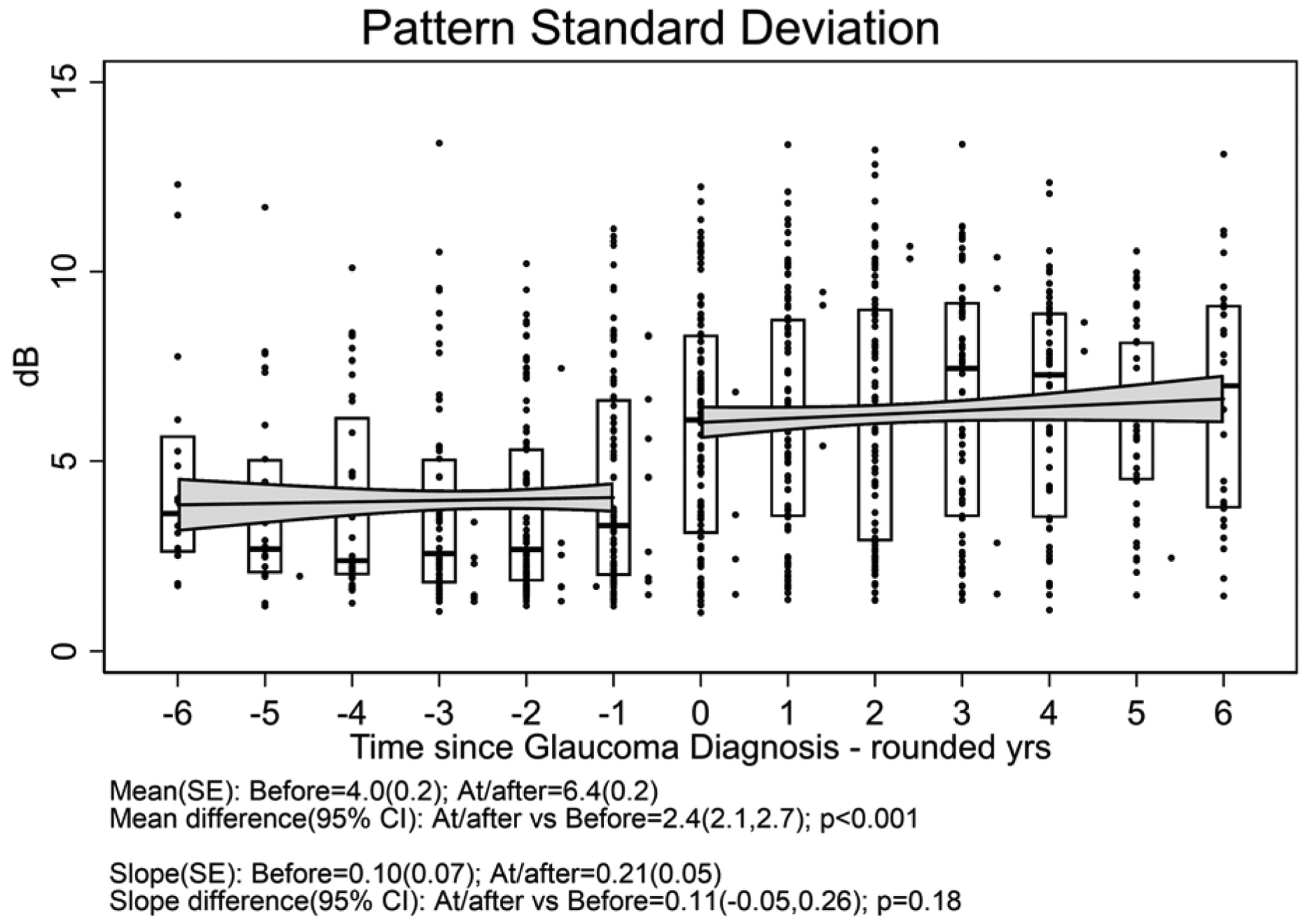
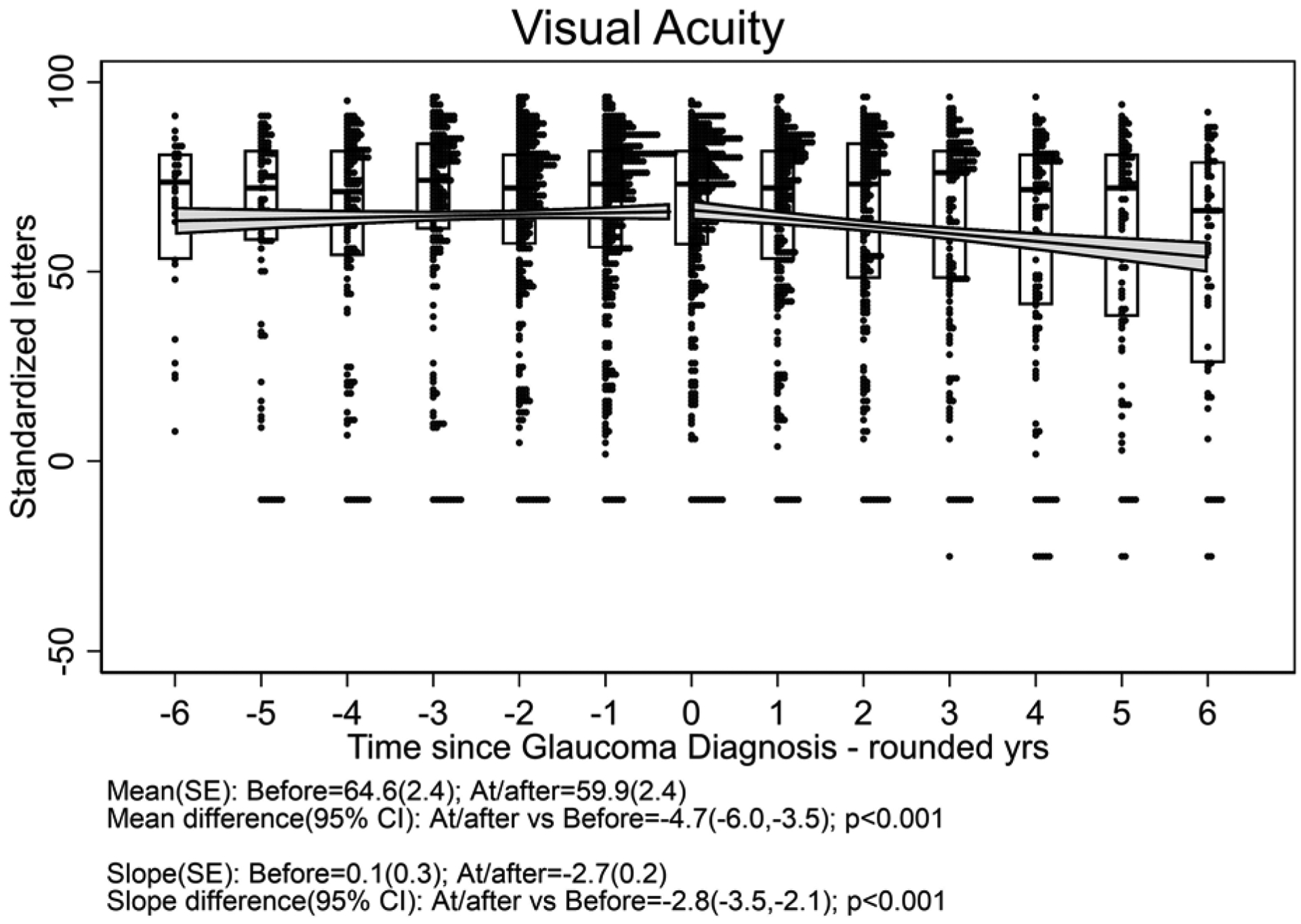
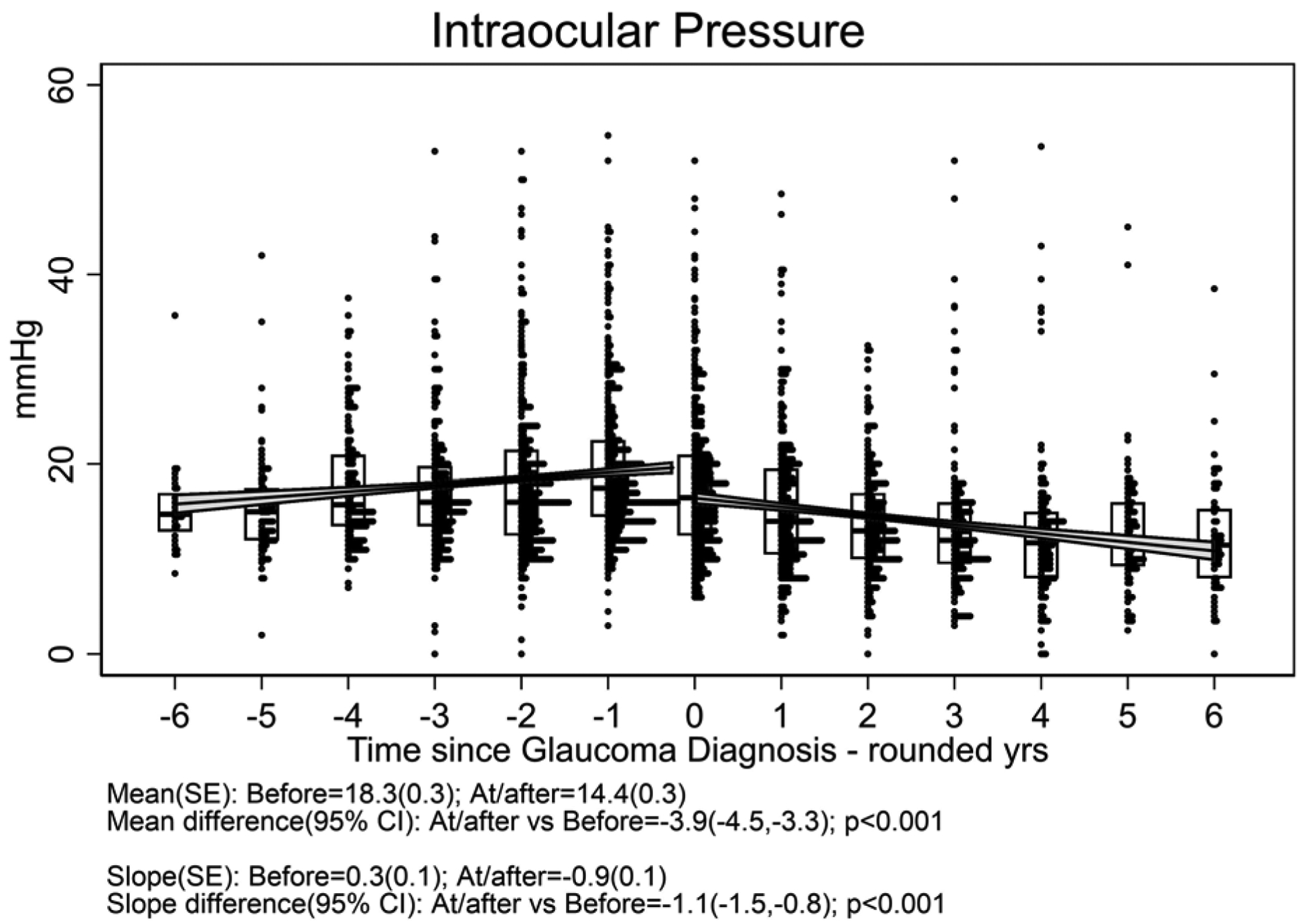
a: Cup-to-disc ratio; b:Mean deviation; c) Pattern standard deviation; d) Best-corrected visual acuity; e) Intraocular pressure. Overall, mean Visual Acuity was worse by approximately five letters (one line) at/after diagnosis with glaucoma compared with before (p<0.001), and the trajectory of change in visual acuity after glaucoma diagnosis was toward worsening by approximately 2.5 letters (one-half line) per year. Overall, mean IOP was worse approximately 4 mmHg lower at/after diagnosis with glaucoma than before (p<0.001), and the trajectory of change in mean IOP was upward before vs. downward after glaucoma diagnosis (p<0.001).
Longitudinal Eye-Level Outcomes for Uveitic Eyes with Glaucoma
Exploratory data analysis demonstrated that at visits after diagnosis with glaucoma, some eyes had transient changes in overall Mean Deviation or in cup-to-disc ratio which later improved. Therefore, to assess how often individual eye-level worsening was observed following glaucoma diagnosis, we assessed the incidence of worsening of MD or cup-to-disc ratio sustained for at least two consecutive visits in eyes at risk of further worsening. Eyes at risk of worsening after diagnosis of glaucoma for MD were those with MD better than −25 dB at glaucoma diagnosis and for cup-to-disc ratio were those with cup-to-disc ratio better than 0.9 at glaucoma diagnosis; each with non-missing data at 2 or more visits thereafter. Regarding MD worsening, 19% (15/81) of eyes developed a sustained decline of MD by ≥6 dB compared to the value the eyes had at the time of glaucoma diagnosis. Using a Kaplan-Meier approach, this translated to a cumulative probability of 20% (95% CI: 12%, 33%) of developing sustained worsening by five years after glaucoma diagnosis (Figure 4, upper left). However, 6% (5/81) developed sustained improvement by this amount with 5-year cumulative probability of 6% (95% CI: 3%, 15%) (Figure 4, upper right). Sustained catastrophic visual field loss to MD ≤ −25 dB occurred in 7% (6/81) of eyes at risk with a 5-year cumulative probability of 10% (95% CI: 4%, 22%); five of these six were in the implant group.
Figure 4. Incidence of Worsening or Improvement (sustained over two consecutive visits) of Mean Deviation and Cup-to-Disc Ratio (indicators of Glaucoma status) over time following diagnosis with glaucoma among eyes with uveitis in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
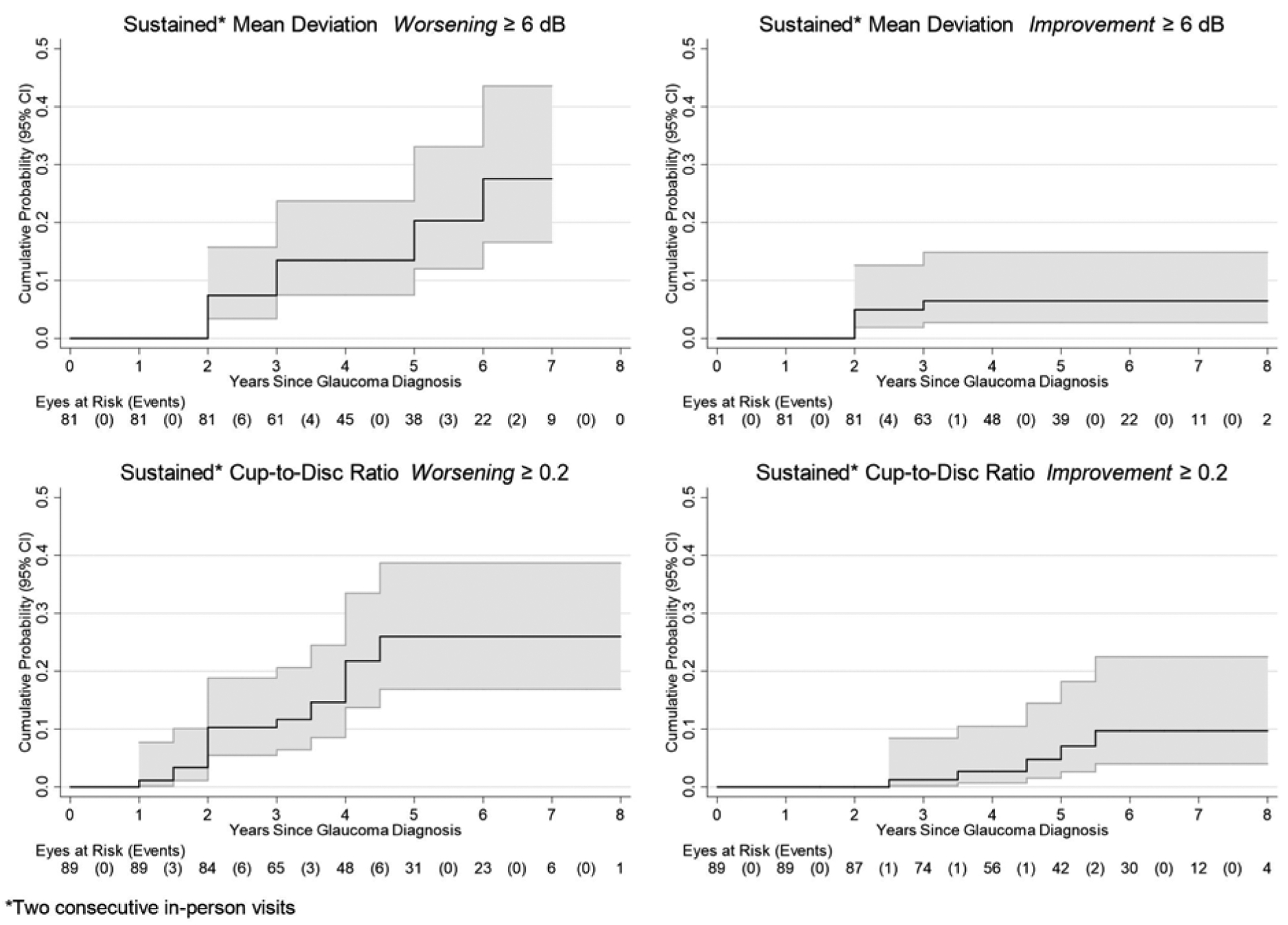
Upper left panel: Worsening of Mean Deviation by 6 dB or more; Lower left panel: Worsening of Cup-to-Disc ratio by 0.2 or more; Upper right panel: Improvement of Mean Deviation by 6 dB or more; Lower right panel: Improvement of Cup-to-Disc ratio by 0.2 or more.
Regarding cup-to-disc ratio, 20% (18/89) of eyes developed a sustained worsening of cup-to-disc ratio by ≥0.2 with 5-year cumulative probability of 26% (17%,39%) (Figure 4, lower left), whereas, 6% (5/89) developed sustained improvement in cup-to-disc ratio by this amount with 5-year cumulative probability of 7% (95% CI: 3%, 18%) (Figure 4, lower right). There were no cases of worsening to a cup-to-disc ratioof 0.9 or worse after glaucoma had been diagnosed, nor had any developed a cup-to-disc ratio of 0.9 by the time glaucoma initially was diagnosed.
Declines in MD and worsening of cup-to-disc ratio did not coincide completely; only 7% (5/72) of eyes had both a sustained decline of MD by 6 dB or more and a contemporaneous increase of cup-to-disc ratio by ≥0.2 with 5-year cumulative probability of 7% (95% CI: 3%, 18%) [9%, 95% CI: 6%, 13% if a cup-to-disc ratio increase of cup-to-disc ratio by ≥0.1 was used].
Discussion
We previously reported that IOP elevation and glaucoma affect a substantial minority of cases during the first two years of management of non-infectious intermediate, posterior and panuveitis, more frequently (but not exclusively) with long-lasting fluocinolone acetonide intraocular implant therapy than with systemic therapy.1 Our longer-term results confirm that the incidence of IOP elevation and glaucoma incidence continue to rise over time in eyes with intermediate, posterior and panuveitis with both implant and systemic treatment assignment, and are significantly higher with implant. Other reports of large populations of pediatric and adult uveitis cases suggest that the incidence of IOP elevation over time is high even in populations that had a small proportion managed with implant therapy.2,3 In our study, which had up to 10 years of follow-up, 46% (95% CI: 38%, 55%) and 15% (95% CI: 8%, 26%) were diagnosed with glaucoma by 10 years in the implant and systemic as-treated groups, respectively. Given the substantial ongoing incidence of high levels of IOP and the strong relationship between high IOP and incident glaucoma in the uveitis population, frequent monitoring and management of IOP in uveitis patients is advisable.
Eyes treated with implant therapy frequently developed intraocular pressures of 30 mmHg or higher (see Table 3), and often developed glaucoma with reduction of visual field sensitivity. Implant-treated eyes typically had excellent initial control of inflammation in our study. Because IOP elevations may not cause discomfort, such patients may be prone to think all is well when their uveitis is controlled by implant therapy, and potentially neglect follow-up, a pattern anecdotally observed in some MUST Trial cases. Therefore, frequent assessment of IOP and aggressive measures to control it are advisable following implant therapy, even when eyes seem to be stable over multiple visits. In the MUST Trial, 45.3% of cases in the implant group required incisional surgery to lower intraocular pressure within seven years.9 Given the likelihood of ultimately requiring such surgery, the large proportion of cases which developed glaucoma despite management under the protocol, and the strong association of higher maximum and average IOP with glaucoma incidence, early surgery seems advisable in cases treated with the fluocinolone acetonide 0.59 mg implant which have secondary severe elevation of IOP, to prevent (further) glaucomatous optic nerve damage from occurring. Furthermore, large elevations of IOP can occur over short intervals of time (even within the first three months after randomization IOP elevation was frequent). Frequent monitoring of IOP of non-operated cases is advisable, especially if implants are placed. While it is unclear to what extent the same approach should apply to other long-lasting implants, frequent monitoring and aggressive treatment of IOP elevation seem advisable.
Our results further extend observations1 that elevated IOP precedes the large majority of cases of glaucoma in uveitis, consistent with clinical impressions that highly elevated IOP is a primary culprit in causing glaucoma in the uveitic setting. However, in our study, epidemiological assessment of risk factors for glaucoma suggested that treatment with implant therapy was strongly associated with glaucoma incidence even after accounting for IOP elevation, with a greater than a three-fold higher IOP-adjusted glaucoma incidence. For purposes of the analysis, IOP only was measured at study visits, so there is some possibility that residual confounding between implant treatment assignment and IOP contributed to this observation. However, given that Implant treatment tended to be a stronger predictor of diagnosis of glaucoma than IOP in model fit assessments (see Table 4a and Supplemental Table 1), direct effects of implant therapy also must be considered as a possibility. One theory of glaucoma pathogenesis is that mechanical stress deforming the lamina cribrosa contributes to glaucoma by interfering with axonal transport of essential trophic factors leading to glaucomatous optic neuropathy.22–24 Corticosteroids have been reported to cause weakening of connective tissue;25 perhaps high levels of intraocular corticosteroids bathing the optic nerve over time in some cases leads to increased susceptibility of the cribriform plate to mechanical stress, contributing to glaucoma by that mechanism. However, treatment with shorter acting intraocular corticosteroid treatments in our study was not significantly predictive of incident glaucoma. Further studies are needed to assess the veracity of this hypothesis, and whether the same pattern holds with alternative long-lasting intraocular therapies using lower doses of corticosteroids.
Our results further raise concerns about IOP elevation in intermediate, posterior and panuveitis cases, in that even current or average IOP levels of 16–20 were associated with a higher and non-trivial incidence of glaucoma in systemic-treated patients (about 3%/year), whereas IOP levels in this range were associated with even higher glaucoma risk in the implant group. Only eyes never observed to have an IOP of 16 or higher completely escaped incident glaucoma. The data provide strong evidence in favor of treating all cases observed to have an IOP≥21 mmHg with IOP-lowering treatment, as such cases had a 5%/year risk of developing glaucoma while IOP is at this level.
Because many modalities exist for controlling elevated IOP once the problem is recognized,8 elevation of IOP theoretically can be managed successfully in nearly all cases. Our results suggest that the majority of incident cases of uveitic glaucoma participating in a prospective clinical trial and cohort study indeed were managed successfully, without subsequent worsening. But despite the close monitoring in this research study, a minority did progress after glaucoma was recognized, with progression of worsening visual field defects and/or worsening of cup-to-disc ratio after glaucoma diagnosis. Such worsening was over and above what had occurred by the time of glaucoma diagnosis. Possible contributors to such worsening might include initially undetected IOP spikes (especially when excellent uveitis control relieves patients of worry) and/or lack of adherence to IOP-lowering treatments; increased susceptibility of the cribriform plate to mechanical stress also may contribute (if confirmed, see above).These observations further support the urgency of carefully monitoring IOP and glaucoma status over long periods of time in intermediate, posterior and panuveitis cases.
We also observed that cases diagnosed with glaucoma tended to have reduction of best-corrected visual acuity over time following diagnosis with glaucoma. In our prior report assessing visual acuity outcome in relation to randomized treatment assignment, implant therapy assignment was associated with worsening of visual acuity compared to systemic treatment at six and seven years after treatment assignment, which appeared to be attributable to retinal scarring (likely during severe relapses that occurred when implant effect waned or subsequently).9 Given that implant-treated eyes were more likely to develop glaucoma, the pattern of visual acuity loss after glaucoma loss may have been due to confounding. Nevertheless, additional work is needed to assess whether glaucoma and its treatment over time are associated with loss of visual acuity in uveitic glaucoma cases.
The converse observation that a small number of cases of uveitic glaucoma developed improvement in cup-to-disc ratio and MD is somewhat unexpected, although some similar reports exist showing this pattern occurring in some cases with open angle glaucoma.26 Our sample size is not sufficient other than to provide preliminary data regarding the issue of potential improvements, predictive factors for improvement, and indeed whether such observations are robust over long periods of time. However, the cases reported did show sustained improvement across semi-annual or annual visits. Additional cases with transient improvements followed by reversal (data not shown) might have had measurement errors as the cause of apparent improvement.
The study was limited by non-continuous assessments of glaucoma status and measurements of outcomes of interest (e.g., IOP), and also varying follow-up intervals over time (which however were the same for all subjects), based on the logistical constraints on the frequency of assessments in a prospective study. In addition, 24% of eyes had ungradablecup-to-disc ratio at baseline (less during follow-up given cataract surgery was performed in many eyes with cataract27). However, our study had a large number of assessments, allowing us to evaluate outcomes over a clinically important period of time which was long compared to the likely delay in diagnosing glaucoma, largely overcoming this problem. We only used data from protocol visits, to avoid potential biases in adverse event reporting in this unmasked treatment study. For this reason, the first point at which glaucoma could have been diagnosed was at one year, potentially leading to some degree of glaucoma incidence underestimation by identifying glaucoma somewhat later than it occurred. Corneal thickness was not assessed (norms have not been established in uveitis cases), but probably mis-measurement of intraocular pressure based on variable corneal thickness would have had a small impact compared to the large rises in intraocular pressure observed. Misclassification of glaucoma may have occurred in a small number of cases, but is unlikely to have occurred frequently given the high level of agreement between glaucoma specialists. Ascertainment of individual-level events potentially may have been limited by measurement error in cup-to-disc ratio and visual field indices; however, the assessment of population averages or (for longitudinal assessments) the requirement that changes be sustained over a minimum of two visits likely overcame much of this problem. Also, intervals between visual fields (one year) and disc photos (six months to one year) were not always consistent; the point in requiring two consecutive visits to count events was to reduce measurement error. Changes to a degree below the thresholds selected would have been missed, but may not have been clinically important in most cases. Strengths of the study included prospective masked ascertainment of glaucoma outcomes in a large population over up to 10 years of follow-up. For the relationship between treatment assignment and the incidence of glaucoma, randomization was an additional strength.
In summary, our results demonstrate that the incidence of glaucoma is high in eyes with intermediate, posterior, and panuveitis treated with implant therapy within the first five years after implantation, and is less high in cases treated with systemic therapy (about 46% and 15% by 10 years respectively). As other kinds of long-acting implants are being developed, data regarding the long-term risk of glaucoma with such implants are needed to better understand their safety. New cases of glaucoma continued to occur through ten years of follow-up, and occurred more often with higher IOP levels (especially of 21 mmHg or higher). Further investigation of whether long-term exposure to intraocular corticosteroids increases the risk of glaucoma over and above the effect on intraocular pressure is warranted. Management of intraocular pressure elevation after glaucoma diagnosis was effective in preventing progressive loss of visual field and worsening of cup-to-disc ratio in most of these cases, but a minority worsened despite expert management. Given the high incidence of glaucoma surgery with implant treatment, and the suggested causal relationship between high levels of IOP and glaucoma, early surgery may be warranted for implanted eyes that develop clinically important IOP elevation. Ongoing follow-up and aggressive management of IOP elevation likely is necessary throughout life for intermediate, posterior and panuveitis cases regardless of the treatment approach selected.
Supplementary Material
a. Funding Support:
This study was supported by National Eye Institute (Bethesda, Maryland) Collaborative Agreements U10EY014655 (Dr. Jabs), U10EY014660 (Dr. Holbrook), and U10EY014656 (Dr. Altaweel). Additional support was provided by Research to Prevent Blindness (New York, New York). Bausch & Lomb provided support to the study in the form of donation of fluocinolone implants for patients randomized to implant therapy who were uninsured or otherwise unable to pay for implants, or were located at a site where implants could not be purchased (e.g., in the United Kingdom). Representatives of the National Eye Institute participated in the conduct of the study, including the study design and the collection, management, analysis, and interpretation of the data, as well as in the review and approval of this manuscript.None of the other sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure(s):
Michael M. Altaweel:(grant/contract to institutional employer)National Eye Institute.
Janet T. Holbrook:(consultant-DSMC member) Gilead;(grant/contract to institutional employer)National Eye Institute;National Heart, Lung, and Blood Institute; American Lung Association;Patient-Centered Outcomes Research Institute.
Douglas A. Jabs:(grant/contract to institutional employer)National Eye Institute.
John H. Kempen: (consultant-DSMC Chair) Gilead; (grant/contract to institutional employer) National Eye Institute; Sight for Souls; Christian Blind Mission International.
Lyndell L. Lim:(consultancy/lecture fees) Allergan; Bayer; Novartis;Specsavers; (grants) Bayer; National Health and Medical Council (Australia).
Elizabeth A. Sugar:(grant/contract to institutional employer)National Eye Institute.
All of the other named authors have no financial disclosures.
Conflict of interest disclosures for the remainder of the MUST Research Group are on file at the MUST Coordinating Center.
Presentations: None
ClinicalTrials.gov Identifier: NCT00132691
References
- 1.Friedman DS, Holbrook JT, Ansari H, et al. Risk of elevated intraocular pressure and glaucoma in patients with uveitis: results of the multicenter uveitis steroid treatment trial. Ophthalmology 2013; 120(8): 1571–9. doi: 10.1016/j.ophtha.2013.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniel E, Pistilli M, Kothari S, et al. Risk of Ocular Hypertension in Adults with Noninfectious Uveitis. Ophthalmology 2017; 124(8): 1196–208. doi: 10.1016/j.ophtha.2017.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kothari S, Foster CS, Pistilli M, et al. The Risk of Intraocular Pressure Elevation in Pediatric Noninfectious Uveitis. Ophthalmology 2015; 122(10): 1987–2001. doi: 10.1016/j.ophtha.2015.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. ArchOphthalmol 2008; 126(9): 1191–201. doi: 10.1001/archopht.126.9.1191 [DOI] [PubMed] [Google Scholar]
- 5.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002; 86(2): 238–42. doi: 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004; 111(3): 491–500. doi: 10.1016/j.ophtha.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 7.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol 2013; 131(11): 1405–12. doi: 10.1001/jamaophthalmol.2013.4237 [DOI] [PubMed] [Google Scholar]
- 8.Prum BE Jr., Rosenberg LF, Gedde SJ, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern((R)) Guidelines. Ophthalmology 2016; 123(1): P41–P111. doi: 10.1016/j.ophtha.2015.10.053 [DOI] [PubMed] [Google Scholar]
- 9.Writing Committee for the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study Research Group. Association Between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs Systemic Anti-inflammatory Therapy and Visual Acuity at 7 Years Among Patients With Intermediate, Posterior, or Panuveitis. JAMA 2017; 317(19): 1993–2005. doi: 10.1001/jama.2017.5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol 2010; 149(4): 550–61. doi: 10.1016/j.ajo.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000; 130(4): 492–513. doi: 10.1016/S0002-9394(00)00659-0 [DOI] [PubMed] [Google Scholar]
- 12.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006; 113(6): 1020–7. doi: 10.1016/j.ophtha.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 13.Multicenter Uveitis Steroid Treatment Trial Research Group. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 2011; 118(10): 1916–26. doi: 10.1016/j.ophtha.2011.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Multicenter Uveitis Steroid Treatment (MUST) Trial Follow-up Study Research Group. Benefits of Systemic Anti-inflammatory Therapy Versus Fluocinolone Acetonide Intraocular Implant for Intermediate, Posterior and Panuveitis: 54 month results of The Multicenter Uveitis Steroid Treatment Trial (MUST) and Follow-up Study. Ophthalmology 2015; 122(10): 1967–75. doi: 10.1016/j.ophtha.2015.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Multicenter Uveitis Steroid Treatment (MUST) Trial Follow-up Study Research Group. Quality of Life and Risks Associated with Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, or Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study. Ophthalmology 2015; 122(10): 1976–86. doi: 10.1016/j.ophtha.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempen JH, Van Natta ML, Altaweel MM, et al. Factors Predicting Visual Acuity Outcome in Intermediate, Posterior, and Panuveitis: The Multicenter Uveitis Steroid Treatment (MUST) Trial. AmJOphthalmol 2015; 160(6): 1133–41. doi: 10.1016/j.ajo.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frick KD, Drye LT, Kempen JH, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest OphthalmolVisSci 2012; 53(3): 1169–76. doi: 10.1167/iovs.11-8259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugar EA, Venugopal V, Thorne JE, et al. Longitudinal Vision-Related Quality of Life for Patients with Noninfectious Uveitis Treated with Fluocinolone Acetonide Implant or Systemic Corticosteroid Therapy. Ophthalmology 2017; 124(11): 1662–9. doi: 10.1016/j.ophtha.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gangaputra SS, Altaweel MM, Peng Q, et al. Morphologic assessment for glaucoma in the Multicenter Uveitis Steroid Treatment (MUST) trial. OculImmunolInflamm 2011; 19(4): 267–74. doi: 10.3109/09273948.2011.583376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Quigley JX R;. Goodness of Fit in Survival Analysis In: Ermitage PC T;, editor. Encyclopedia of Biostatistics. New York: John Wiley & Sons, Ltd; 2005. p. 1–14. [Google Scholar]
- 21.Akaike H. A new look at the statistical model identification. IEEE TransAutomatContr 1974; 19(6): 716–23. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 22.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 1981; 99(4): 635–49. doi: 10.1001/archopht.1981.03930010635009 [DOI] [PubMed] [Google Scholar]
- 23.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci 2000; 41(11): 3460–6. [PubMed] [Google Scholar]
- 24.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 2005; 24(1): 39–73. doi: 10.1016/j.preteyeres.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 25.Pace CS, Blanchet NP, Isaacs JE. Soft Tissue Atrophy Related to Corticosteroid Injection: Review of the Literature and Implications for Hand Surgeons. J Hand Surg Am. 2018;43(6):558–563. doi: 10.1016/j.jhsa.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Cohen SL, Rosen AI, Tan X, Kingdom FA. Improvement of the visual field index in clinical glaucoma care. Can J Ophthalmol 2016; 51(6): 445–51. doi: 10.1016/j.jcjo.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 27.Sen HN, Abreu FM, Louis TA, et al. Cataract Surgery Outcomes in Uveitis: The Multicenter Uveitis Steroid Treatment Trial. Ophthalmology 2016; 123(1): 183–90. doi: 10.1016/j.ophtha.2015.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


