Abstract
Purpose:
Racial disparities are evident in colorectal cancer (CRC) prognosis with black patients experiencing worse outcomes than Hispanics and whites, yet mediators of these disparities are not fully known. The aim of this study is to identify variables that contribute to racial/ethnic disparities in health-related quality of life (HR-QoL) and overall survival in CRC.
Methods:
Using SF-12 questionnaires, we assessed HR-QoL in 1,132 CRC patients by calculating their physical (PCS) and mental composite summary (MCS) scores. Associations between poor PCS/MCS and sociodemographic factors were estimated and survival differences were identified by race/ethnicity.
Results:
Hispanic patients who never married were at greater risk of poor PCS (OR: 2.69; 95% CI: 1.11-6.49; P=0.028) than were currently married patients. College education was associated with a decreased risk of poor PCS in Hispanic and white, but not black, patients. Gender was significantly associated with poor MCS among white patients only. CRC patients who reported a poor PCS or MCS had poor survival, with differences in median survival times (MSTs) by race. The effect of PCS was strongest in white CRC patients with a difference in overall MST of >116 months between those with favorable versus poor physical HR-QoL. Black patients who reported poor Physical and Mental HR-QoL showed significant risk of a poor outcome.
Conclusion:
These findings suggest that racial/ethnic disparities in CRC survival may be related to differences in HR-QoL. Identified mediators of HR-QoL could supplement current CRC management strategies to improve patients’ survival.
Introduction
Racial/ethnic differences in the diagnosis and survival of colorectal cancer (CRC) are evident [1]. Incidence rates are 27% higher in black men than in white men and 22% higher in black women than in white women [2]. The trend is even more striking for mortality rates, which are 52% higher in black men than in white men and 41% higher in black women than in white women [2]. Lower rates of screening and higher rates of late-stage CRC at diagnosis among African Americans are thought to contribute to these higher mortality rates [2; 3]. In contrast, Hispanics have lower incidence rates and longer survival than do African Americans and non-Hispanic whites [4]. Racial/ethnic disparities in CRC patient survival may also reflect differences in socioeconomic status, treatments received, and comorbidities [1], yet controlling for these variables does not completely eliminate these disparities [2]. Therefore, other undefined factors may contribute to racial/ethnic disparities in CRC patient survival.
Our previous study of patients with CRC showed that health-related quality of life (HR-QoL) at baseline differs significantly by race/ethnicity and that poor HR-QoL predicts a shorter overall survival time [5]. HR-QoL, a multi-dimensional construct that encompasses the positive and negative aspects of patients’ physical, functional, emotional, and social domains [6–8], can complement other clinical measures to help provide a complete representation of health status [8–12]. An analysis of HR-QoL in clinical trials further demonstrated that HR-QoL is an important predictor of survival of many different cancers, including CRC [13; 14]. However, little is known about the differences in HR-QoL by race/ethnicity in patients with CRC and how these differences may contribute to differences in survival. To fill this gap, we retrospectively analyzed our CRC patient cohort to identify variables that contribute to racial/ethnic disparities in HR-QoL and that are associated with shortened survival in such patients.
Materials and Methods
Study population
A total of 3,374 CRC cases (age ≥18 years, diagnosed with adenocarcinoma) were identified from the MD Anderson Cancer Patient and Survivors Cohort (MDA-CPSC), a hospital-based cancer patient cohort at The University of Texas MD Anderson Cancer Center [15]. From this CRC population, we selected all non-Hispanic black (N=316) and Hispanic patients (N=366) for the current analysis.
Next, 450 non-Hispanic white patients were randomly selected for inclusion in the analyses, which gave us a total sample size of 1,132 patients. The characteristics of the white patients with CRC were representative of the larger CRC cohort [5]. All patients were recruited from MD Anderson between 1998 and 2012 during their first visit to our center, within 1 year of diagnosis. During the visits, the patients completed a baseline patient history form, which contained questions regarding age, sex, marital status, education level, and history of alcohol and tobacco use. Cancer-related and follow-up variables (cancer stage, pathology, and vital status) were obtained from the institutional tumor registry and from patients’ medical records. The study was approved by the MD Anderson Institutional Review Board.
SF-12 HR-QoL questionnaire and scoring
The patient history form includes the SF-12v1 (12-Item Short-Form Health Survey; [Medical Outcomes Study]) questionnaire to measure patient-reported HR-QoL[16]. Responses from this questionnaire were used to generate mental composite summary (MCS) and physical composite summary (PCS) scores. The pain interference question in the SF-12 was modified in the patient history form to use a 0-10 scale; therefore, these scores were recoded to the 0-5 scale of the SF-12. A norm-based scoring system[16] was used in which each item was normalized to generate a mean score of 50 based on scores from the US general population. By this normalization, a higher MCS or PCS score (≥ 50) suggested above average HR-QoL compared to the general population, while MCS or PCS score < 50 suggested a poorer HR-QoL.
Statistical analysis
Analysis of variance (ANOVA) and Wald statistics were used to compare mean PCS and MCS scores within each racial/ethnic group by patient epidemiologic and sociodemographic variables. Risk of poor PCS and MCS (< 50) by patient characteristics was assessed using logistic regression with corresponding odds ratios (ORs) and 95% confidence intervals (CIs). In separate analyses to identify risk of poor survival by poor QOL, Cox proportional hazards model adjusted for age, sex, and cancer stage was used to assess overall survival as a function of race/ethnicity and PCS/MCS score. Kaplan-Meier survival function and corresponding log-rank tests were used to plot overall survival PCS/MCS score stratified by race/ethnicity. Global tests of the Schoenfeld residuals showed that the proportional hazards assumption remained true for the PCS scores in all three racial/ethnic groups. The assumption was violated for the MCS scores in the Hispanic group and thus excluded from further interpretation. Survival time was defined as the duration from the date of diagnosis to the date of death or last follow-up visit. Follow-up information was obtained from our institutional tumor registry with a follow-up rate of 98% for our study group with the last year of follow-up in 2016. Statistical analysis was performed using Stata software (version14; StataCorp, College Station, Texas). P values less than 0.05 were considered statistically significant.
Results
Characteristics of the study population
The study population consisted of 1,132 patients with CRC from three racial/ethnic groups: non-Hispanic white (N=450), Hispanic (N=366), and non-Hispanic black (N=316) (Table 1). The average follow-up times for the white, Hispanic, and black patients were 45.9 months, 47.9 months, and 42.5 months, respectively. A majority of the patients were diagnosed with colon tumors (70.9%). There were slightly more men (56.5%) than women (43.8%). Most patients were married (76.9% of whites, 73.5% of Hispanics, and 57.0% of blacks). Most patients, regardless of race/ethnicity, graduated from high school (48.7% of whites, 44.8% of Hispanics, and 51.9% of blacks), and 42.9% of white patients, 22.7% of Hispanic patients, and 26.6% of black patients had at least some college-level education. We also found a higher percentage of current users of alcohol among white patients (41.1%) compared than among Hispanic (28.4%) and black (24.4%) patients. Black patients had a slightly higher percentage of past users of alcohol (26.6%) than did Hispanic (22.4%) and white (16.9%) patients.
Table 1.
CRC patient population by race/ethnicity (N=1,132)
| White N (%) | Hispanic N (%) | Black N (%) | |
|---|---|---|---|
| Total | 450 (100) | 366 (100) | 316 (100) |
| Mean length of follow-up, months (standard deviation) | 45.9 (38.9) | 47.9(39.4) | 42.5 (37.4) |
| Sex | |||
| Male | 261 (58.0) | 213 (58.2) | 166 (52.5) |
| Female | 189 (42.0) | 153(41.8) | 150 (47.5) |
| Age, years | |||
| <50 | 156 (34.7) | 127 (34.7) | 92 (29.1) |
| 50-59 | 133 (29.6) | 123 (33.6) | 104 (32.9) |
| 60-69 | 98 (21.8) | 76 (20.8) | 79 (25.0) |
| ≥70 | 63 (14.0) | 40 (10.9) | 41 (13.0) |
| Marital status | |||
| Married | 346 (76.9) | 269 (73.5) | 180 (57.0) |
| Widowed | 24 (5.3) | 20 (5.50) | 39(12.3) |
| Separated | 1 (0.2) | 2 (0.50) | 3 (0.9) |
| Divorced | 32(7.1) | 25 (6.80) | 29 (9.2) |
| Never married | 47 (10.4) | 20 (5.50) | 65 (20.6) |
| Education level | |||
| Less than high school | 25 (5.6) | 91 (24.9) | 48 (15.2) |
| High school/AA/VOC | 219(48.7) | 164 (44.8) | 164 (51.9) |
| At least some college | 193 (42.9) | 83 (22.7) | 84 (26.6) |
| Missing | 13 (2.9) | 28 (7.7) | 20 (6.3) |
| Alcohol use | |||
| Never | 185 (41.1) | 176 (48.1) | 152 (48.1) |
| Yes, but quit | 75 (16.7) | 82 (22.4) | 84 (26.6) |
| Yes, currently | 185 (41.1) | 104 (28.4) | 77 (24.4) |
| Missing | 5(1.1) | 4(1.1) | 3 (0.9) |
| Tobacco use | |||
| Never | 227 (50.4) | 195 (53.3) | 177 (56.0) |
| Yes, but quit | 174 (38.7) | 132 (36.1) | 106 (33.5) |
| Yes, currently | 42 (9.5) | 31 (8.5) | 31 (9.8) |
| Missing | 7(1.6) | 8 (2.2) | 2 (0.6) |
| Cancer site | |||
| Rectum | 139 (30.9) | 115 (31.4) | 79 (25.0) |
| Colon | 311 (69.1) | 251 (68.6) | 237 (75.0) |
| stage | |||
| I | 43 (9.6) | 23 (6.3) | 32 (10.1) |
| II | 73 (16.2) | 74 (20.2) | 54(17.1) |
| III | 143 (31.8) | 134 (36.6) | 100 (31.6) |
| IV | 108 (24.0) | 83 (22.7) | 86 (27.2) |
| Missing | 83 (18.4) | 52 (14.2) | 44 (13.9) |
AA, associate of arts; VOC, vocational.
Patterns and risk of poor HR-QoL by race/ethnicity
Overall, the CRC patient population reported a high burden of poor baseline HR-QoL, with mean PCS and MCS scores below “average” for the general US population after normalization (Tables 2 and 3). This burden was most striking for physical HR-QoL, with over 65% of CRC patients reporting a poor PCS regardless of race/ethnicity (Supplementary Figure S1). We also observed that the proportion of CRC patients with favorable physical HR-QoL differed by racial/ethnic group. Only 35% of white patients reported above average PCS, which dropped to 30% for Hispanic and 27% for black patients (Figure S1). In contrast, the burden of poor mental HR-QoL was less in this cohort, and differences in it according to race/ethnicity were less evident.
Table 2:
Patient characteristics assessed by PCS score and stratified by race/ethnicity
| White | Hispanic | Black | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | P value | Mean (SD) | P value | Mean (SD) | P value | |
| Total | 42.6 (11.0) | 41.9 (11.1) | 40.0 (11.9) | |||
| Sex | ||||||
| Male | 44.4 (10.9) | 42.8 (11.1) | 41.0 (12.4) | |||
| Female | 40.3 (10.8) | 1.00x10−4 | 40.6 (11.0) | 0.07 | 39.0 (11.3) | 0.14 |
| Age, years | ||||||
| <50 | 42.6 (11.1) | 41.0 (11.9) | 39.8 (11.5) | |||
| 50-59 | 42.8 (10.8) | 42.5 (10.6) | 40.6 (12.4) | |||
| 60-69 | 43.2 (11.4) | 42.2 (10.8) | 39.7 (12.2) | |||
| ≥70 | 41.4 (11.0) | 0.77 | 41.9 (10.7) | 0.75 | 40.0 (11.5) | 0.96 |
| Marital status | ||||||
| Married | 43.2 (10.9) | 43.0 (10.7) | 40.4 (12.1) | |||
| Widowed | 40.9 (10.6) | 41.4 (11.3) | 40.4 (11.9) | |||
| Separated | 45.6 (0) | 52.4 (5.4) | 50.5 (5.1) | |||
| Divorced | 41.4 (11.7) | 39.5 (12.0) | 39.3 (11.4) | |||
| Never married | 40.1 (11.9) | 0.35 | 37.0 (11.5) | 0.004 | 38.7 (11.8) | 0.48 |
| Education level | ||||||
| Less than high school | 39.1 (10.8) | 38.1 (10.8) | 37.4 (12.9) | |||
| High school/AA/VOC | 41.2 (11.0) | 41.6 (10.7) | 39.7 (11.9) | |||
| At least some college | 44.5 (10.8) | 0.0030 | 46.9 (9.9) | 3.78x10−7 | 42.7 (11.1) | 0.067 |
| Alcohol use | ||||||
| Never | 40.5 (11.3) | 41.5 (10.9) | 40.9 (11.5) | |||
| Yes, currently | 38.6 (11.7) | 45.4 (10.3) | 42.6 (11.5) | |||
| Yes, but quit | 46.2 (9.5) | 1.12 × 10−6 | 37.6 (10.8) | 6.86 × 10−6 | 36.4 (12.3) | 0.0021 |
| Tobacco use | ||||||
| Never | 43.6 (10.5) | 41.7 (11.2) | 42.1 (11.2) | |||
| Yes, currently | 42.1 (11.5) | 39.9 (11.8) | 36.1 (11.5) | |||
| Yes, but quit | 39.1 (11.8) | 0.041 | 42.2 (10.7) | 0.60 | 37.8 (12.5) | 0.0018 |
| Cancer site | ||||||
| Rectum | 45.7 (11.6) | 41.6 (11.7) | 40.5 (12.4) | |||
| Colon | 41.3 (10.5) | 1.00×10−4 | 42.0 (10.8) | 0.72 | 39.9 (11.8) | 0.71 |
| Stage | ||||||
| I | 46.7 (11.2) | 47.1 (9.7) | 44.9 (11.3) | |||
| II | 45.5 (10.1) | 43.7 (11.1) | 42.1 (11.6) | |||
| III | 44.6 (10.1) | 43.6 (10.2) | 39.6 (12.0) | |||
| IV | 40.3 (11.0) | 5.00×10−4 | 37.2 (11.4) | 1.08×10−5 | 37.3 (11.7) | 0.0085 |
AA, associate of arts; VOC, vocational school. P values <0.05 (bold) were considered statistically significant.
Table 3:
Patient characteristics assessed by MCS score and stratified by race/ethnicity
| White | Hispanic | Black | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | P value | Mean (SD) | P value | Mean (SD) | P value | |
| Total | 48.2 (10.4) | 47.6 (10.8) | 47.9 (11.3) | |||
| Sex | ||||||
| Male | 49.8 (9.6) | 47.6 (11.4) | 48.6 (10.8) | |||
| Female | 46.1 (11.0) | 2.00x10−4 | 47.7 (9.9) | 0.96 | 47.0 (11.3) | 0.21 |
| Age, years | ||||||
| <50 | 48.3 (9.9) | 46.3 (10.5) | 46.9 (10.9) | |||
| 50-59 | 47.3 (10.9) | 47.8 (10.9) | 47.9 (11.3) | |||
| 60-69 | 48.5 (10.0) | 48.6 (10.7) | 48.5 (10.9) | |||
| ≥70 | 49.7 (11.2) | 0.47 | 49.8 (11.0) | 0.24 | 49.0 (13.1) | 0.71 |
| Marital status | ||||||
| Married | 48.5 (10.2) | 48.4 (10.2) | 48.3 (11.3) | |||
| Widowed | 48.5 (12.2) | 46.6 (12.3) | 46.7 (11.3) | |||
| Separated | 39.6 (0.0) | 57.3 (3.5) | 46.6 (14.6) | |||
| Divorced | 46.2 (11.4) | 46.9 (12.3) | 47.6 (12.8) | |||
| Never married | 47.6 (10.3) | 0.68 | 44.1 (11.7) | 0.066 | 47.5 (10.8) | 0.93 |
| Education level | ||||||
| Less than high school | 47.1 (9.5) | 45.7 (11.6) | 45.9 (12.1) | |||
| High school/AA/VOC | 47.3 (10.8) | 47.6 (10.2) | 47.9 (11.7) | |||
| At least some college | 49.5 (9.8) | 0.076 | 50.2 (9.8) | 0.021 | 49.3 (9.9) | 0.41 |
| Alcohol use | ||||||
| Never | 49.8 (9.3) | 48.5 (10.0) | 48.3 (11.0) | |||
| Yes, currently | 47.6(11.1) | 47.5 (11.1) | 49.7 (10.8) | |||
| Yes, but quit | 45.9 (10.8) | 0.065 | 45.8 (11.8) | 0.16 | 45.4 (12.0) | 0.047 |
| Tobacco use | ||||||
| Never | 46.1 (10.6) | 47.6 (10.4) | 48.3 (11.2) | |||
| Yes, currently | 49.3 (10.1) | 43.4 (12.6) | 44.8 (12.7) | |||
| Yes, but quit | 47.4 (10.6) | 0.069 | 48.4 (10.8) | 0.063 | 47.8 (11.1) | 0.27 |
| Cancer site | ||||||
| Rectum | 48.8 (9.9) | 46.3 (10.9) | 49.3 (11.0) | |||
| Colon | 47.9 (10.6) | 0.44 | 48.3 (10.7) | 0.10 | 47.4 (11.4) | 0.19 |
| Stage | ||||||
| I | 49.9 (10.9) | 50.2 (9.3) | 48.0 (12.4) | |||
| II | 49.6 (10.2) | 47.9 (10.9) | 48.7 (10.6) | |||
| III | 48.9 (9.8) | 47.4 (11.1) | 49.3 (10.3) | |||
| IV | 47.5 (10.7) | 0.45 | 48.0 (11.0) | 0.73 | 46.3 (12.2) | 0.33 |
AA, associate of arts; VOC, vocational school. P values <0.05 (bold) were considered statistically significant.
In each of the racial/ethnic groups, we observed differences in patient characteristics associated with HR-QoL. For instance, the mean PCS score for white female patients (40.3) was significantly lower than that for white male patients (44.4; P=1.00x10−4) (Table 2). Also, the mean MCS score was significantly lower for white women than for white men (46.1 vs. 48.9; P=2.00x10−4) (Table 3). This difference corresponded to a more than two-fold greater risk of poor physical HR-QoL (OR: 2.06; 95% CI: 1.32-3.21; P=1.50x10−3; Figure 1a) and poor mental HR-QoL (OR: 2.03; 95% CI: 1.33-3.10; P=1.04x10−3; Figure 1b) for white female patients than for white male patients. We observed similar associations for physical HR-QoL and sex among black and Hispanic patients (Figure 1a), although the effect sizes were slightly higher in black patients (OR: 2.28; 95% CI: 1.29-4.02; P=4.50x10−3), and were not statistically significant in Hispanic patients (P=0.09). Among women, the effect of a poor MCS score was not as remarkable in Hispanic or black patients as it was in white patients (Table 3, Figure 1b).
Figure 1. Sociodemographic and clinical mediators of poor HR-QoL by race/ethnicity.
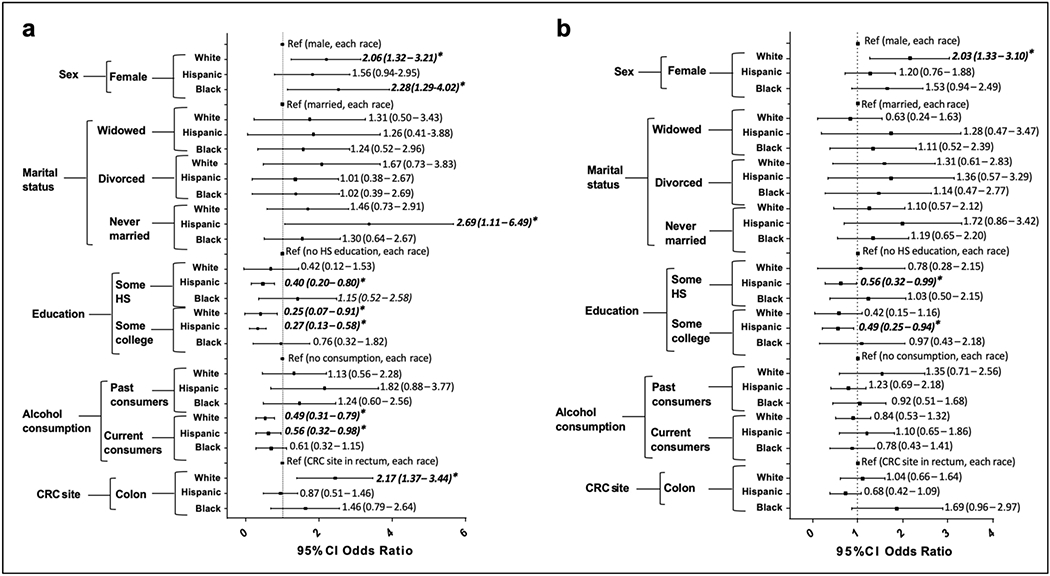
Forest plots show odds ratios of poor PCS score (< 50) (a) and MCS score (< 50) (b) by sex, marital status, education level, alcohol consumption, and CRC site, for the white, Hispanic, and black patient cohorts. Odds ratios (bold and italics) with asterisks * are considered statistically significant (P value < 0.05).
Among Hispanic patients, we observed significant associations between marital status and education level and physical HR-QoL. Individuals who never married had a more than 2.5-fold greater risk (OR: 2.69; 95% CI: 1.11-6.49; P=0.028) of poor physical HR-QoL than did their married counterparts and had a lower mean PCS score (37.0) than did patients with other marital statuses. We did not observe significant associations between marriage and HR-QoL in the white or black populations. The association between having favorable physical HR-QoL and at least some college education was strong for Hispanic patients (P=3.78x10−7), significant for white patients (P=0.0030), and borderline significant for black patients (P=0.067) (Table 2). We also identified a reduced risk of both poor PCS (OR: 0.27; 95% CI: 0.13-0.58; P<8.2x10−4; Figure 1a) and MCS (OR: 0.49; 95% CI: 0.25-0.94; P=0.031; Figure 1b) in Hispanic patients with at least some college-level education than in those with less than a high school education. Likewise, we observed a reduced risk of poor physical HR-QoL (OR: 0.25; 95% CI: 0.07-0.91; P=0.036; Figure 1a) in white patients with at least some college education than in those with less than a high school education.
In addition, current alcohol consumption had a protective effect in white and Hispanic patients by 48% (P=3.1x10−3) and 44% (P=0.043; Figure 1a), respectively. However, for black patients, the association between current alcohol use and poor PCS (P=0.13) and MCS (P=0.41) was insignificant.
Tumors occurring in the colon, as opposed to those in the rectum, were significantly associated with a poor PCS score (OR: 2.17; 95% CI: 1.37-3.44; P=1.03x10−3), but not a poor MSC score for white patients (Figure 1a). Risk of poor PCS and MCS scores did not differ by cancer location for Hispanic and black patients. As expected, cancer stage IV was a major determinant of poor physical HR-QoL score in our cases.
Low PCS and MCS scores and poor survival of CRC patients
Racial/ethnic differences in overall survival were observed for the CRC patient population (log-rank P=0.023; Figure 2a). The median survival times (MSTs) for the Hispanic and white patients were nearly double that of the black patients (107.8 and 90.5 months vs. 57.6 months, respectively).
Figure 2. Overall survival of CRC patients by race/ethnicity.
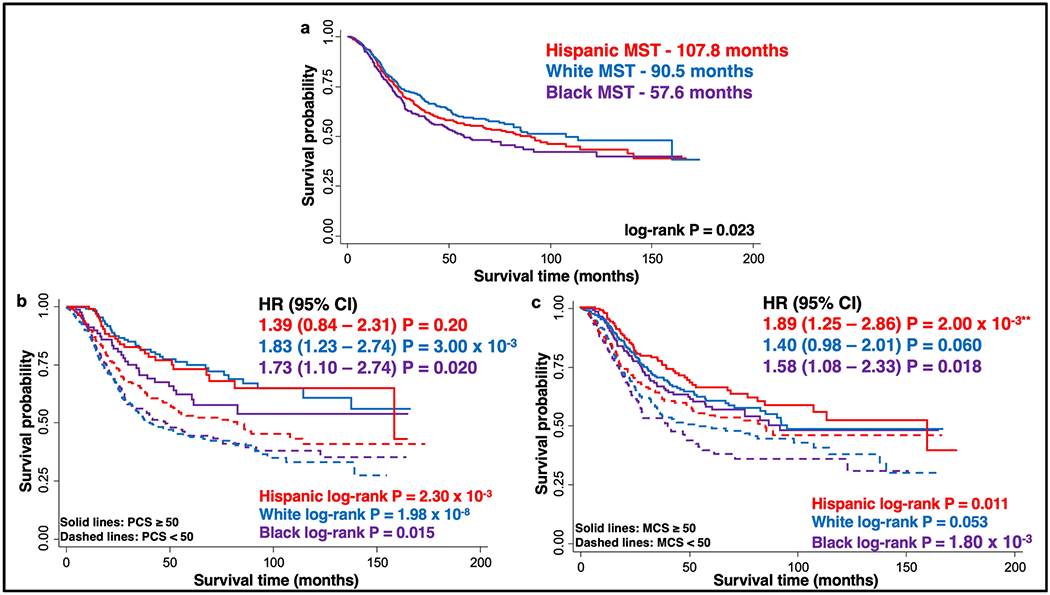
Kaplan Meier curves show overall survival of the entire CRC patient cohort stratified by race/ethnicity (white, Hispanic, black) (a) and further stratified by PCS (b) and MCS (c), with P values calculated using the log-rank test. Also included is Hazard Ratio (HR) with 95%CI of poor PCS (b) and MCS (c) on overall survival, for each stratified race/ethnicity. HR is adjusted for age, gender, and stage and PCS/MCS ≥ 50 is used as reference. Overall survival was reduced for PCS/MCS < 50 (dotted lines) compared with PCS/MCS ≥ 50 (solid lines). **Proportional hazards assumption not valid for this subgroup. MST: median survival time.
In our previous analysis, we showed a reduction in overall survival for patients when stratified by PCS or MCS scores [5]. When stratified by race/ethnicity, similar significant separations by PCS and MCS scores were present for each racial/ethnic group (Figures 2b and 2c). The largest gap between PCS strata was evident for the white CRC population. Individuals with favorable PCS at diagnosis had an MST of over 160 months, which dropped to only 43.3 months for those with poor PCS (log-rank P=1.98x10−8; Figure 2b). This corresponds to a 1.83-fold increase in risk (95% CI: 1.23-2.74; P=3.00x10−3; Figure 2b). Strikingly, the survival curve for black patients with a favorable PCS was similar to that for Hispanic cases who reported a poor PCS (Figure 2b). For black patients, poor PCS scores were associated with a significant 1.73-fold increased risk of reduced overall survival (95% CI: 1.10-2.74; P=0.020). Similarly, Hispanic patients with poor physical HR-QoL were at a 1.39-fold increased risk of poor survival. However, this association was not statistically significant (P=0.20). In agreement with the findings in our previous report, the differences in overall survival duration between CRC patients with poor versus favorable MCS scores were not as dramatic as those observed for patients with poor versus favorable PCS scores, although they were significant for the Hispanic and black patients and was borderline significant for the white study group (Figure 2c). Black patients with favorable MCS scores had survival durations similar to those of white patients with favorable MCS scores (MSTs, 91.8 and 95.0 months, respectively). Yet, the effect of poor MCS was most pronounced in black patients, dropping the MST to only 40.8 months compared to 54.1 and 81.9 months for white and Hispanic patients, respectively. This corresponds to a significant 58% increased risk of poor survival in black cases (HR: 1.58; 95% CI: 1.08-2.33; P=0.018; Figure 2c). Poor Mental HR-QoL was associated with a 1.40-fold increased risk of poor survival in white patients, although this effect was not statistically significant (HR: 1.40; 95% CI: 0.98-2.01; P=0.06). The proportional hazard assumption was violated for the Hispanic MCS subgroup analysis and was excluded from further interpretation.
Discussion
We previously identified relationships between patient characteristics and HR-QoL in a cohort of CRC patients at diagnosis, including highly significant associations between poor PCS/MCS and decreased overall survival [5]. In the current study, we further investigated this relationship in a cohort of 1,132 CRC patients, focusing on differences according to race/ethnicity to identify patterns in baseline HR-QoL that may shed light on mediators of racial/ethnic disparities in CRC prognosis.
Among the three studied racial/ethnic groups, we found that black patients experienced a high burden of poor PCS. This is in line with a previous study of over 1,778 individuals with cancer that showed that black and Hispanic cancer patients had poorer PCS scores when compared to white patients [17]. It has been suggested the differences in HR-QoL and CRC outcomes are a reflection of socioeconomic status, which influences access to care and the quality of care received. Lathan et.al. showed that a third of CRC patients had limited financial reserves and that this financial strain was associated with higher poor HR-QoL burden [18]. In another study, 68% of African-American CRC patients reported economic hardship, compared to only 50% of Hispanic patients and 40.5% of white patients [19]. However, we observed that black patients did not exhibit significant improvement in HR-QoL associated with higher education as was the case for Hispanic and white patients, suggesting that socioeconomic factors may have a lesser effect in HR-QoL and outcome in black CRC patients. The burden of poor HR-QoL and overall survival in black patients with CRC may be attributed to their having a later presentation [20–25] and younger age at diagnosis of CRC than those in Hispanic and white patients [26]. However, May et al. recently reported that the white-black disparity in CRC incidence and stage has decreased since 2010. In our study, black patients did not present with CRC at a significantly later stage or younger age than did white or Hispanic patients, and we did not assess income in the patients. Further studies of HR-QoL and outcomes in black patients with CRC are warranted to increase understanding of the complicated effects of socioeconomic status on both HR-QoL and survival.
Similar to the black population in our study, the Hispanic population had fewer individuals with at least some college education than did the white population, suggesting lower socioeconomic status. Hispanic patients with CRC had slightly lower mean MCS scores than did white and black patients. Nevertheless, poor mental HR-QoL and sociodemographic variables did not predict a shorter median survival time; instead, Hispanic CRC patients had a 13% better MST compared to white patients. A previous study also showed lower cancer mortality rates in Hispanic patients compared to other populations, in spite of the burdens of socioeconomic deprivation. This improved outcome for Hispanic patients is often termed the “Hispanic paradox” [27; 28]. Although the cause of lower mortality rate despite lower socioeconomic status, as well as poorer HR-QoL scores in Hispanics is unclear, we observed a more pronounced positive effect of education and marriage on HR-QoL in this group. College education was significantly protective of poor HR-QoL in Hispanic patients. Perhaps the potential financial safety, access to health care, and socioeconomic growth involved with higher education has a greater impact on HR-QoL in Hispanic patients [18; 26; 29], as we did not observe a similar protective effect in college-educated black patients. Another interesting finding is the significant effect of spousal support in improving HR-QoL in Hispanics, as those who never married had a more than two-fold greater risk of poor PCS score than did their married counterparts. We did not see this trend in black or white patients. One previous study showed a strong correlation between social/familial wellbeing and favorable HR-QoL in Hispanics [30]. This suggests that familial support during illness and feeling close to one’s partner [31] are especially important to Hispanics, perhaps because their partners and family members act as their active caregivers [32]. Further research is necessary to clarify the impact of familial and spousal support on HR-QoL and to ultimately better support CRC patients.
We previously demonstrated a strong correlation between female sex and poor HR-QoL in CRC patients [5]. In this analysis stratified by race/ethnicity, white and black women were at a significantly increased risk of poor PCS compared with their male counterparts. However, we observed that only white women showed a significant risk of poor mental HR-QoL compared to their male counterparts. This may be due to a lack of perceived social support. A previous study focusing on breast cancer demonstrated that white women were at risk of having lower social support than were Hispanic or African-American women [33].
Our previous analysis identified that current alcohol consumption had a protective effect on poor physical HR-QoL in CRC patients [5]. In this study, we observed that this trend was present in white and Hispanic CRC patients only. This is somewhat consistent with a previous study where abstainers and heavy drinkers had higher depression scores than did moderate alcohol users in response to acute and chronic stressors [34]. The same research group further identified the same effect in Mexican-Americans and non-Hispanic whites, but not in African-Americans [35]. However, to our knowledge, neither of these relationships have been thoroughly understood in the context of CRC.
In our study, stage was a major determinant of poor survival regardless of race/ethnicity. Nevertheless, differences in survival and HR-QoL by race/ethnicity were evident. For example, the survival gap between high PCS and low PCS was striking in white patients compared to other races/ethnicities. Also, the effect of poor Physical and Mental HR-QoL on survival was seen with statistical significance in black CRC patients (Figure 2b, 2c). The effect of race/ethnicity on HR-QoL could be supplemental to other clinical and pathologic features in determining prognosis for CRC patients.
There are several limitations to this study. First, the race/ethnicity definitions used in this study were self-reported and broad, which may have limited the scope of our findings. For example, previous studies have observed clear differences in CRC incidence, survival rates, and supportive cancer care between American-born and foreign-born Hispanics [17; 23; 36–38]. Thus, one should take into account the heterogeneity of sociodemographic and cultural backgrounds within racial/ethnic groups when studying HR-QoL and CRC [39]. Furthermore, we did not have detailed information regarding comorbidities (e.g., cardiovascular disease, diabetes) in our study population, although comorbidities may have had a significant effect on HR-QoL. We selected PCS and MCS scores of 50 for our threshold to stratify our population. This normalized threshold is based on QOL distribution in the general population and may not be applicable to the cancer population. The primary focus of this manuscript was to compare the effect of PCS and MCS across racial/ethnic populations. To accomplish this, we conducted several stratified analyses instead of including an interaction term with ethnicity in our models. These stratified analyses, along with the number of logistic regression tests conducted, do present an opportunity for chance findings due to multiple testing and we acknowledge this possibility.
Given the evidence of racial/ethnic disparity of CRC prognosis, this study offers a valuable understanding of contributing factors using a routine and standard SF-12 questionnaire. Significant mediators of HR-QoL could supplement current CRC management strategies to improve patients’ survival by identifying at risk individuals of poor HR-QoL. Furthermore, future clinical research would benefit from studying the protective effect of psycho-social factors on overall survival such as seen in the Hispanic patient population.
In conclusion, our study identified patterns of epidemiologic and sociodemographic factors associated with baseline HR-QoL in CRC patients by race/ethnicity. This study also identified that the association between HR-QoL and survival vary by racial/ethnic groups. These findings underscore the importance of psycho-oncologic care in the management of CRC patients to improve outcomes. Together, the identified relationships can help us understand the underlying mediators of HR-QoL in CRC patients and to further identify those who are at higher odds for poor prognosis.
Supplementary Material
Acknowledgments
Funding: Funding support for this study was provided in part by the Center for Translational and Public Health Genomics of MD Anderson and the NIH through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare no conflicts of interest.
Statement of human rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human participant informed consent: Informed consent was obtained from all individual participants included in the study.
Availability of data and material: The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Statement on welfare of animals: This article does not contain any studies with animals performed by any of the authors.
References
- 1.White A, Vernon SW, Franzini L, & Du XL (2010). Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer, 116(19), 4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, & Jemal A (2016). Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin, 66(4), 290–308. [DOI] [PubMed] [Google Scholar]
- 3.Wallace K, DeToma A, Lewin DN, Sun S, Rockey D, Britten CD, Wu JD, Ba A, Alberg AJ, & Hill EG (2016). Racial Differences in Stage IV Colorectal Cancer Survival in Younger and Older Patients. Clin Colorectal Cancer, 16(3),178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, & Jemal A (2015). Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin, 65(6), 457–480. [DOI] [PubMed] [Google Scholar]
- 5.Reyes ME, Ye Y, Zhou Y, Liang A, Kopetz S, Rodriquez MA, Wu X, & Hildebrandt MA (2016). Predictors of health-related quality of life and association with survival may identify colorectal cancer patients at high risk of poor prognosis. Qual Life Res, 26(2),319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almutairi KM, Alhelih E, Al-Ajlan AS, & Vinluan JM (2016). A cross-sectional assessment of quality of life of colorectal cancer patients in Saudi Arabia. Clin Transl Oncol, 18(2), 144–152. [DOI] [PubMed] [Google Scholar]
- 7.Cella DF, & Tulsky DS (1993). Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest, 11(3), 327–336. [DOI] [PubMed] [Google Scholar]
- 8.Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, Osoba D, Bjordal K, & Bottomley A (2009). Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol, 10(9), 865–871. [DOI] [PubMed] [Google Scholar]
- 9.Baussard L, Stoebner-Delbarre A, Bonnabel L, Huteau ME, Gastou A, & Cousson-Gelie F (2017). Development and validation of the daily fatigue cancer scale (DFCS): Single-item questions for clinical practice. Eur J Oncol Nurs, 26, 42–48. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Mo FK, Chan SL, Hui EP, Tang NS, Koh J, Leung LK, Poon AN, Hui J, Chu CM, Lee KF, Ma BB, Lai PB, Chan AT, Yu SC, & Yeo W (2017). Prognostic values of EORTC QLQ-C30 and QLQ-HCC18 indexscores in patients with hepatocellular carcinoma - clinical application of health-related quality-of-life data. BMC Cancer, 17(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diouf M, Filleron T, Pointet AL, Dupont-Gossard AC, Malka D, Artru P, Gauthier M, Lecomte T, Aparicio T, Thirot-Bidault A, Lobry C, Fein F, Dubreuil O, Landi B, Zaanan A, Taieb J, & Bonnetain F (2016). Prognostic value of health-related quality of life in patients with metastatic pancreatic adenocarcinoma: a random forest methodology. Qual Life Res, 25(7), 1713–1723. [DOI] [PubMed] [Google Scholar]
- 12.De Aguiar SS, Bergmann A, & Mattos IE (2014). Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res, 23(2), 627–637. [DOI] [PubMed] [Google Scholar]
- 13.Gotay CC, Kawamoto CT, Bottomley A, & Efficace F (2008). The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol, 26(8), 1355–1363. [DOI] [PubMed] [Google Scholar]
- 14.Braun DP, Gupta D, Grutsch JF, & Staren ED (2011). Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health Qual Life Outcomes, 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Hildebrandt MA, Ye Y, Chow WH, Gu J, Cunningham S, Zhao H, Hawk ET, Wagar E, Rodriguez A, & Hamilton SR (2016). Cohort Profile: The MD Anderson Cancer Patients and Survivors Cohort (MDA-CPSC). Int J Epidemiol, 45(3), 713–713f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware J Jr., Kosinski M, & Keller SD (1996). A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care, 34(3), 220–233. [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro LC, Wheeler SB, Chen RC, Mayer DK, Lyons JC, & Reeve BB (2015). The effects of cancer and racial disparities in health-related quality of life among older Americans: a case-control, population-based study. Cancer, 121(8), 1312–1320. [DOI] [PubMed] [Google Scholar]
- 18.Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, & Schrag D (2016). Association of Financial Strain With Symptom Burden and Quality of Life for Patients With Lung or Colorectal Cancer. J Clin Oncol, 34(15), 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisu M, Kenzik KM, Oster RA, Drentea P, Ashing KT, Fouad M, & Martin MY (2015). Economic hardship of minority and non-minority cancer survivors 1 year after diagnosis: another long-term effect of cancer? Cancer, 121(8), 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phipps E, Braitman LE, Stites S, & Leighton JC (2008). Quality of life and symptom attribution in long-term colon cancer survivors. J Eval Clin Pract, 14(2), 254–258. [DOI] [PubMed] [Google Scholar]
- 21.Simpson DR, Martinez ME, Gupta S, Hattangadi-Gluth J, Mell LK, Heestand G, Fanta P, Ramamoorthy S, Le QT, & Murphy JD (2013). Racial disparity in consultation, treatment, and the impact on survival in metastatic colorectal cancer. J Natl Cancer Inst, 105(23), 1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcella S, & Miller JE (2001). Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol, 54(4), 359–366. [DOI] [PubMed] [Google Scholar]
- 23.Jackson CS, Oman M, Patel AM, & Vega KJ (2016). Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol, 7(Suppl 1), S32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Y, Wang C, Civan JM, Palazzo JP, Ye Z, Hyslop T, Lin J, Myers RE, Li B, Jiang B, Sama A, Xing J, & Yang H (2016). Effects of Cancer Stage and Treatment Differences on Racial Disparities in Survival From Colon Cancer: A United States Population-Based Study. Gastroenterology, 150(5), 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May FP, Glenn BA, Crespi C, Ponce N, Spiegel BM, & Bastani R (2016). Decreasing Black-White Disparities in Colorectal Cancer Incidence and Stage at Presentation in the United States. Cancer Epidemiol Biomarkers Prev, 26(5),762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawk R, Abner A, Ashford A, & Brown CP (2015). Differences in Colorectal Cancer Outcomes by Race and Insurance. Int J Environ Res Public Health, 13(1), ijerph13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinheiro PS, Williams M, Miller EA, Easterday S, Moonie S, & Trapido EJ (2011). Cancer survival among Latinos and the Hispanic Paradox. Cancer Causes Control, 22(4), 553–561. [DOI] [PubMed] [Google Scholar]
- 28.Philips BU Jr., Belasco E, Markides KS, & Gong G (2013). Socioeconomic deprivation as a determinant of cancer mortality and the Hispanic paradox in Texas, USA. Int J Equity Health, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du XL, Fang S, Vernon SW, El-Serag H, Shih YT, Davila J, & Rasmus ML (2007). Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer, 110(3), 660–669. [DOI] [PubMed] [Google Scholar]
- 30.Yost KJ, Hahn EA, Zaslavsky AM, Ayanian JZ, & West DW (2008). Predictors of health-related quality of life in patients with colorectal cancer. Health Qual Life Outcomes, 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, & Cella D (1999). Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res, 8(3), 181–195. [DOI] [PubMed] [Google Scholar]
- 32.Costa AL, Heitkemper MM, Alencar GP, Damiani LP, Silva RM, & Jarrett ME (2016). Social Support Is a Predictor of Lower Stress and Higher Quality of Life and Resilience in Brazilian Patients With Colorectal Cancer. Cancer Nurs, 40(5),352–360. [DOI] [PubMed] [Google Scholar]
- 33.Thompson T, Rodebaugh TL, Pérez M, Schootman M, & Jeffe DB (2013). Perceived Social Support Change in Patients with Early-stage Breast Cancer and Controls. Health psychology : official journal of the Division of Health Psychology, American Psychological Association, 32(8), 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipton RI (1994). The effect of moderate alcohol use on the relationship between stress and depression. Am J Public Health, 84(12), 1913–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton R (1997). The relationship between alcohol, stress, and depression in Mexican Americans and non-Hispanic whites. Behav Med, 23(3), 101–111. [DOI] [PubMed] [Google Scholar]
- 36.Patel MI, Schupp CW, Gomez SL, Chang ET, & Wakelee HA (2013). How Do Social Factors Explain Outcomes in Non–Small-Cell Lung Cancer Among Hispanics in California? Explaining the Hispanic Paradox. J Clin Oncol, 31(28), 3572–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, & Lee D (2009). Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev, 18(8), 2162–2169. [DOI] [PubMed] [Google Scholar]
- 38.Stern MC, Fejerman L, Das R, Setiawan VW, Cruz-Correa MR, Perez-Stable EJ, & Figueiredo JC (2016). Variability in Cancer Risk and Outcomes Within US Latinos by National Origin and Genetic Ancestry. Curr Epidemiol Rep, 3, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aragones A, Hayes SL, Chen MH, González J, & Gany FM (2014). Characterization of the Hispanic or Latino Population in Health Research: A Systematic Review. J Immigr Minor Health, 16(3), 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


