Abstract
Introduction:
Vascular access for central venous catheter (CVC) placement is technically challenging in children. Ultrasound (US) guidance is recommended for pediatric CVC placement, yet many practitioners rely on imprecise anatomic landmark techniques risking procedure failure due to difficulty mastering US guidance. A novel navigation system provides a visual overlay on real-time US images to depict needle trajectory and tip location during cannulation. We report the first pediatric study assessing feasibility and preliminary safety of using a computer-assisted needle navigation system to aid in central venous access.
Methods:
A prospective, IRB-approved feasibility study was performed. All participants provided written informed consent. Ten patients (mean age 11.4 years, 5 males) underwent CVC placement with US and navigation system guidance. All procedures were performed by interventional radiologists expert in vascular access. Feasibility was measured through binary (yes/no) responses from participating users assessing device usability and feasibility. Number of needle passes and procedure time measures were also recorded.
Results:
Internal jugular veins (7 right, 3 left) were cannulated in all patients with no complications. Users confirmed navigation system feasibility in all 10 participants. Mean vein diameter and depth was 13.3×9.8±3.4×2.1 and 7.0±1.7 mm, respectively. Successful cannulation occurred in all patients and required only a single needle pass in 9/10 patients. Mean device set-up and vascular access times were 5:31±2:28 and 1:48±2:35 minutes, respectively.
Conclusion:
This pilot study suggests that it is feasible to use a novel computer-assisted needle navigation system to safely obtain central venous access under US guidance in pediatric patients.
Trial Registry:
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT04031495)
Registration Number:
Keywords: Ultrasound, Central Venous Catheter, Pediatrics, Interventional Radiology, New Devices
Introduction:
Central venous catheter (CVC) placement in children is often more technically challenging than in adults because the target veins are smaller, more mobile, and more anatomically variable1. Ultrasound (US) guidance to assist with venous cannulation during CVC placement has long shown benefit in adults. More recently, pediatric trials have also demonstrated that US visualization improves needle accuracy while reducing procedure time and complication rates2–5. Several organizations now recommend US guidance during CVC placement in children6–8.
Despite these recommendations, many clinicians, including those placing catheters in intensive care units or emergency departments, rely on anatomic landmark techniques rather than image guidance9. Many pediatric medical centers have purchased point-of-care US units to encourage use for CVC placement, but adoption has been impeded by lack of experience and the steep learning curve necessary to master the real-time US-guided technique. Therefore, a clinical need exists to facilitate US-guided central vein cannulation for less experienced operators, so that CVCs can be successfully placed in even the smallest target veins. Described here is the first report assessing the feasibility and safety of using a computer-assisted needle navigation system to obtain central venous access in pediatric patients.
The SCENERGY navigation system (Clear Guide Medical, Baltimore, MD, USA) consists of an optical camera head that attaches to a standard US transducer to track an access needle. A second screen, in addition to a standard US display, provides guidance overlay on real-time US images to assist in needle placement. By depicting the angle of needle trajectory and confirming needle orientation relative to the US beam, the device may decrease vascular catheterization times in adults11. Prior to this study, this system’s feasibility, safety, and efficacy has yet to be studied in children.
We hypothesized that it is feasible to use the SCENERGY navigation system to obtain vascular access for CVC placement in pediatric patients. In the study reported here, vascular access experts determined the feasibility of using the guidance system for venous cannulation in children, with plans to extend the user profile to less experienced practitioners in subsequent studies. Secondary/exploratory objectives were to evaluate needle placement efficiency and central venous cannulation time.
Methods:
Patient Population:
The prospective pilot study included patients age 5–17 years who underwent US-guided central venous catheterization required for clinical care at our institution. This Health Insurance Portability and Accountability Act (HIPAA)-compliant study was institutional review board (IRB) approved (PR00011099) and registered with ClinicalTrials.gov (NCT04031495). Written informed consent was obtained for each participant. Patients were enrolled from April to July 2019.
Description of Technology:
The SCENERGY navigation system (Clear Guide Medical, Baltimore, MD, USA) was designed to attach to an existing US machine and probe (Figure 1A). A Philips iu22 ultrasound and L15–7io “hockey-stick” probe routinely used for vascular access were utilized for this study. As illustrated in Figure 1B, the navigation system consisted of a small dual-head optical camera that attached to the probe using a mounting bracket. This camera tracked the vascular access needle using the two sterile TipTAG markers attached to the needle shaft near the hub as shown in Figure 1D. The system determined needle orientation and tip location relative to the TipTAG markers through a simple image calibration process. Additionally, a proprietary display was connected to the iu22 using a video output connection and frame grabber card. The optical camera tracked the needle using image processing techniques and overlaid the projected path of the needle and the needle tip location on live US, as indicated in Figure 1C. Both a standard US orientation and top-down view of the probe face was shown on the user interface. A green dashed line denoted the projected needle trajectory and became highlighted when the trajectory was in-plane with the US beam. The green line turned purple to highlight the actual needle depth and tip location in tissue.
Figure 1.
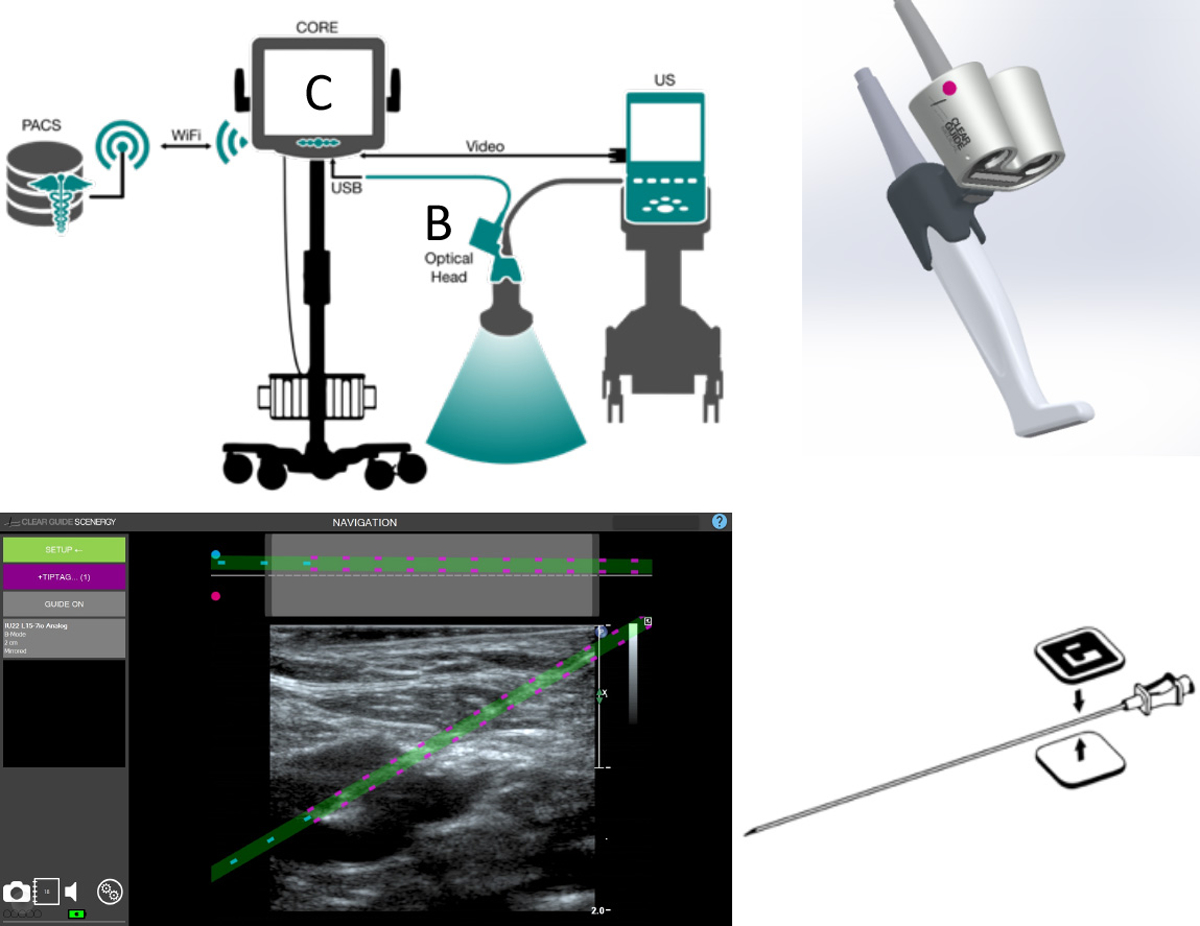
(a) The SCENERGY navigation system consisted of a (b) small dual-head optical camera attached to the probe using a mounting bracket and (c) a proprietary display was connected to the iu22 US machine to track the cannulation needle and overlay the projected path of the needle on live US. (d) The camera tracked the needle using TipTAGs, two sterile markers that enabled the system to determine the needle orientation and tip location.
Central Venous Cannulation Procedure:
All central venous cannulations were performed under real-time US guidance by experienced (>10 years) pediatric interventional radiologists. The iu22 ultrasound machine was coupled to the SCENERGY navigation system. US was used to evaluate the vein of interest, ensuring the vessel was patent and compressible. Skin overlying the vein was prepped and draped in a sterile fashion, and lidocaine was used for local anesthesia. A custom, sterilized US probe cover named the SteriMASK was placed over the US probe and attached optical head (Figure 2A). Sterile TipTAGs were applied to a 21-gauge needle and held up to the optical camera so the system could locate the needle and calibrate its length. Using short axis US view and an in-plane needle approach with the SCENERGY system, the 21 -gauge needle was directed from the skin to the target vein using the system’s live display with needle overlay to guide cannulation. The operator had the option to refer to adjacent US images without the overlay if necessary. A short axis US view of the vessel and in-plane needle orientation was used to allow for visualization and separation of the internal jugular vein and common carotid artery while also allowing visualization of real-time needle advancement from the skin entry to the vein wall. Given the current device design, an out-of-plane needle approach is limited to 15 degrees from normal because of the need for the camera head to visualize the TipTags used to determine needle tip and trajectory.
Figure 2.
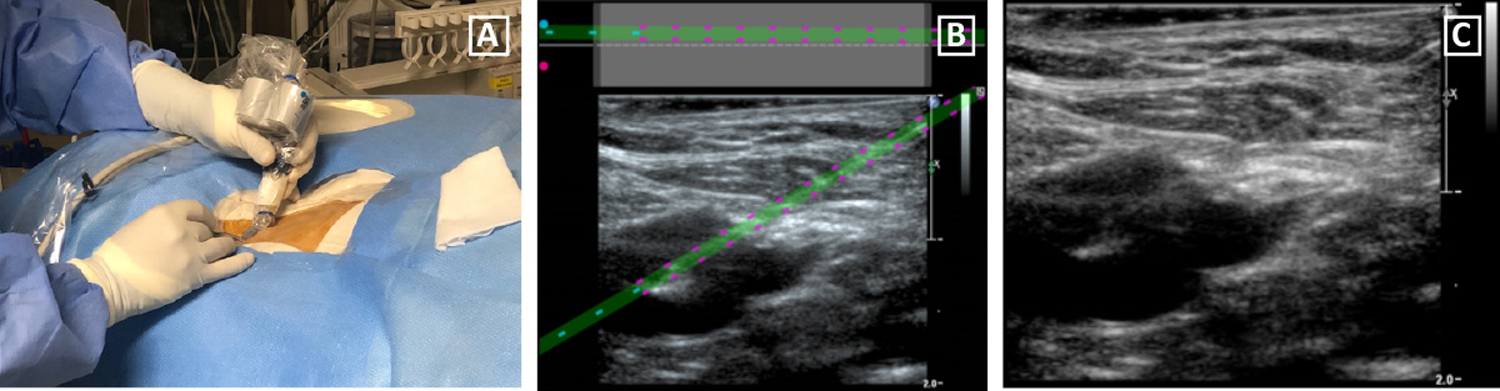
(a) After covering the L15–7io US probe and optical head with a sterilized SteriMASK probe cover, a 21-gauge needle was directed toward the vein of interest using US guidance and the navigation system. (b) The camera accurately portrayed needle trajectory and position compared to (c) the user-planned approach.
Qualitative Analysis:
The primary objective of this study was to determine feasibility of using the SCENERGY system for venous cannulation in pediatric patients undergoing central venous catheterization. For each patient, feasibility was measured through binary (yes/no) responses from the participating interventional radiologist to three specific statements. The statements were 1) attachment of the optical camera on the US probe compromises the ability to position the probe on the skin as needed for vascular access, 2) attachment of the optical camera on the probe compromises US image quality, and 3) the needle trajectory planned by the navigation system follows the actual user-planned trajectory with freehand guidance. Only the answers 1) no, 2) no, and 3) yes signified that it was feasible to use the guidance system in this population. Subjective feedback regarding system performance were also gathered from the users following each case.
Quantitative Analysis:
The secondary/exploratory objectives were to determine needle placement efficiency and central venous cannulation time. Needle placement efficiency to obtain central venous cannulation was measured by the number of needle passes, number of needle adjustments, and number of vascular punctures. The number of needle passes was defined as the amount of times the skin was entered with a needle. If the needle was retracted and then redirected to change trajectory, this was defined as needle adjustment. The number of vascular punctures was the number of times the vein itself was punctured.
Total time for central venous cannulation was measured by the equipment set-up time and needle alignment and cannulation time. Equipment set-up time was defined as the time elapsed from the beginning of probe cover placement to the end of TipTAG calibration. Needle alignment and cannulation time was the time required to optimize needle trajectory till the final image capture displaying the intravascular needle.
Results:
Ten patients (5 males, 5 females) underwent CVC placement with ultrasound guidance coupled to the navigation system. Patient demographics are shown in Table 1. Mean patient age was 11.4 years (Range: 5 – 17 years). Mean patient weight and standard deviation was 41 ± 24.2 kg (Range: 11.9 – 78.9 kg). There were no complications.
Table 1:
Patient demographics
| Subject Number: | Age (yrs): | Sex: | Race: | Weight (kg): | Height (cm): | Body Mass Index (kg/m2): |
|---|---|---|---|---|---|---|
| 1 | 10 | M | Caucasian | 25.6 | 140.0 | 12.7 |
| 2 | 6 | M | Caucasian | 11.9 | 90.0 | 14.7 |
| 3 | 11 | F | Black/African American | 28.3 | 138.5 | 14.8 |
| 4 | 14 | M | Hispanic/Latino | 77.0 | 170.0 | 26.6 |
| 5 | 14 | F | Hispanic/Latino | 66.4 | 151.5 | 28.9 |
| 6 | 11 | F | Black/African American | 25.3 | 139.0 | 13.1 |
| 7 | 5 | M | Caucasian | 21.3 | 114.5 | 16.3 |
| 8 | 13 | F | Caucasian | 36.4 | 154.0 | 15.2 |
| 9 | 17 | M | Hispanic/Latino | 78.9 | 174.0 | 26.1 |
| 10 | 13 | F | Hispanic/Latino | 39.0 | 139.0 | 20.2 |
M = male, F = female
Qualitative Analysis:
All ten patients underwent successful CVC placement. Table 2 describes the procedure, accessed veins, catheter type and length, as well as feasibility results. The internal jugular vein was cannulated in all patients (7 right-sided, 3 left-sided). The mean catheter length and standard deviation was 17.3 ± 2.3 cm. The mean vein diameter and standard deviation was 13.3 × 9.8 ± 3.4 × 2.1 mm and mean vein depth was 7.0 ± 1.7 mm. For all 10 patients, the operator confirmed that it was feasible to use the needle-guidance system. The camera did not compromise probe or needle positioning or ultrasound image quality while accurately portraying needle trajectory compared to user-planned trajectory (Figure 2B and 2C).
Table 2:
Procedure, central veins accessed, catheter type and length, and binary results of feasibility criteria
| Subject Number: | Procedure | Vein Accessed: | Side: | Ultrasound and Needle Orientation | Catheter Type | Catheter Length (cm) | Vein Diameter (W × H in mm): | Vein Depth (mm): | Feasibility Criterium 1: | Feasibility Criterium 2: | Feasibility Criterium 3: |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mediport | IJ | R | Short Axis In Plane | 6.6 French Single Lumen |
18.0 | 11.0 × 8.5 | 6.0 | N | N | Y |
| 2 | Mediport | IJ | L | Short Axis In Plane | 5 French Single Lumen |
15.5 | 11.3 × 8.0 | 7.0 | N | N | Y |
| 3 | Mediport | IJ | R | Short Axis In Plane | 6.6 French Single Lumen |
16.5 | 10.6 × 9.2 | 5.8 | N | N | Y |
| 4 | Mediport | IJ | R | Short Axis In Plane | 6.6 French Single Lumen |
20.5 | 15.0 × 9.6 | 8.9 | N | N | Y |
| 5 | Mediport | IJ | L | Short Axis In Plane | 6.6 French Single Lumen |
22 | 16.4 × 12.6 | 9.7 | N | N | Y |
| 6 | Mediport | IJ | R | Short Axis In Plane | 6.6 French Single Lumen |
14.5 | 10.0 × 8.7 | 4.8 | N | N | Y |
| 7 | Mediport | IJ | L | Short Axis In Plane | 6 French Single Lumen |
18 | 9.7 × 6.7 | 5.9 | N | N | Y |
| 8 | Mediport | IJ | R | Short Axis In Plane | 6.6 French Single Lumen |
16 | 13.6 × 11.0 | 6.1 | N | N | Y |
| 9 | Mediport | IJ | R | Short Axis In Plane | 6.6 French Dual Lumen |
16 | 20.0 × 13.4 | 9.6 | N | N | Y |
| 10 | Tunneled Line | IJ | R | Short Axis In Plane | 5 French Dual Lumen |
16.5 | 15.5 × 10.4 | 5.9 | N | N | Y |
IJ = Internal Jugular Vein, R = Right, L = Left, W = Width, H = Height, N = No, Y = Yes
Quantitative Analysis:
Using real-time US guidance coupled with the navigation system, successful central vein cannulation occurred on the first pass without needle readjustments in 90% of the patients (Table 3). Patient 1 required two needle passes. The mean equipment set-up time and needle alignment and cannulation time was 5:31 ± 2:28 and 1:41 ± 2:17 (min), respectively.
Table 3:
Needle placement efficiency and central vein cannulation time results
| Subject Number: | # of Needle Passes: | # of Needle Adjustments: | # of Vascular Punctures: | Equipment Set-Up Time (MM:SS): | Needle Alignment and Cannulation Time (MM:SS): |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 10:38 | 03:40 |
| 2 | 1 | 0 | 1 | 05:17 | 01:19 |
| 3 | 1 | 0 | 1 | 04:05 | 01:04 |
| 4 | 1 | 0 | 1 | 09:07 | 07:29 |
| 5 | 1 | 0 | 1 | 03:04 | 00:58 |
| 6 | 1 | 0 | 1 | 03:16 | 00:27 |
| 7 | 1 | 0 | 1 | 05:42 | 01:15 |
| 8 | 1 | 0 | 1 | 04:40 | 00:19 |
| 9 | 1 | 0 | 1 | 04:46 | 00:07 |
| 10 | 1 | 0 | 1 | 04:36 | 00:12 |
Discussion:
All patients underwent successful central vein cannulation without complication using the SCENERGY navigation system coupled with real-time US-guidance. Additionally, venous cannulation was achieved on the first pass in nine of ten patients and on the second pass in the remaining patient. These results demonstrate feasibility and safety of using a computer-assisted needle navigation system to obtain real-time US-guided central venous access in pediatric patients.
CVC placement failure rates in pediatric patients range from 5% to 19% and complications are encountered between 2.5% and 22% of cases10. Several organizations recommend the use of US guidance during CVC placement in children to improve these outcomes6–8. Compared to the landmark technique, utilization of US during venous cannulation for children and infants may be of benefit due to their small vein diameter2. One study with 95 patients reported a significantly higher success rate, decreased number of attempts, and decreased cannulation time when US guidance was used3. A meta-analysis suggested the time-savings benefit may be more pronounced in inexperienced users4. Real-time US guidance allows for visualization of the target vein and surrounding anatomy as well as the needle trajectory and tip during advancement. It also allows for confirmation of puncture and possible complications. Carotid artery puncture is a potential complication during internal jugular vein cannulation, in which US was shown to reduce arterial injury (26.7% vs. 3.1%, P < 0.025)5; however, US guidance helps avoid other complications such as hematoma formation and pneumothorax as well.
Despite evidence favoring US guidance for CVC placement, a recent survey of pediatric anesthesiologists found that 85% of respondents had access to US to assist with CVC placement but only 39% used it routinely9. This is common for practitioners who do not receive formal training in US and also for practitioners who do not routinely perform US-guided vascular access as part of daily clinical practice. Although low cost and good quality point-of-care US units are often widely available throughout many pediatric hospitals, including ours, they are not frequently used during CVC placement. Lack of formal training and the learning curve necessary to master the US-guided free-hand technique is believed to impede adoption. In response, needle navigation systems were created for providers performing point-of-care central venous cannulation to improve outcomes.
Clinical experience with computer-assisted needle navigation systems is limited in adult patients, and essentially non-existent in children. The majority of studies involving these devices and others, including the SonixGPS (UltraSonix, Richmond, BC, Canada) and Venue 50 (GE Healthcare, Chicago, IL, USA), have been pre-clinical in nature13–18. Two randomized studies using the SCENERGY and eZono 4000 (eZono, Jena, Germany) devices demonstrated 100% successful cannulation and no complications in use with adult patients; however, only the former showed a time-savings benefit11, 12. Prior to this report, no such device has been studied in specifically the pediatric population.
The present study has limitations. The primary limitation is that the measures of feasibility were subjective and relied on the perspectives of three experienced interventional radiologists who routinely perform realtime US-guided vascular access. Although these experts were ideal for initial determination of feasibility and provided useful subjective feedback regarding potential improvements for the system, it is currently unknown whether less experienced providers, the main intended operators of this technology, can utilize the device at bedside. This question will be addressed through our next planned clinical trial. The second limitation is that the accessed veins in our patients were larger (13.3 × 9.8 ± 3.4 × 2.1 mm mean diameter) than are frequently encountered in smaller pediatric patients including neonates, infants, and toddlers. These smaller children were excluded from this initial pilot study due to safety considerations, but our findings did provide confidence that the results are likely to be relevant in patients with smaller veins. This will be evaluated in the next planned trial which allows for inclusion of neonates and infants. Lastly, the quantitative results were intended to be exploratory and are not statistically powered.
Further system development and clinical investigation are warranted to evaluate the technology’s impact on central venous cannulation safety and efficiency. Optimizing the navigation system for smaller pediatric patients and for use by less experienced users are critical for clinical translation. Designs for a smaller optical head, more transparent needle overlay with a simpler and more intuitive user interface, as well as a more practical and form-fitting L15–7io probe cover are underway to improve the system for use in pediatrics.
Conclusion:
This initial pilot study demonstrates the feasibility and preliminary safety of using a novel computer-assisted needle navigation system to obtain central venous access under real-time US guidance in pediatric patients. These findings support further investigation to determine if the system can benefit less experienced operators placing CVC at the bedside with point-of-care US machines that are now widely available in most pediatric hospitals.
Acknowledgements:
The authors thank the interventional radiology team at Children’s National Hospital without whom this study would not have been possible. The authors also thank the Society of Interventional Radiology Medical Student Summer Internship for support of C.G.
Funding: Funding for this clinical study was provided through an SBIR grant [Grant Number R44HD096974, 2018]
Footnotes
IRB Approval: NCT04031495 (https://clinicaltrials.gov/ct2/show/NCT04031495)
Declaration of conflicting interests:
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of the article: P.F., A.D., and D.H. disclose that they are employees of Clear Guide Medical. The other authors have no competing interests.
References:
- 1.Souza Neto E, Grousson S, Duflo F, Tahon F, Mottolese C, Dailler F. Ultrasonographic anatomic variations of the major veins in paediatric patients. British journal of anaesthesia. 2014;112(5):879–84. [DOI] [PubMed] [Google Scholar]
- 2.Costello JM, Clapper TC, Wypij D. Minimizing complications associated with percutaneous central venous catheter placement in children: recent advances. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(3):273–83. [DOI] [PubMed] [Google Scholar]
- 3.Verghese ST, McGill WA, Patel RI, Sell JE, Midgley FM, Ruttimann UE. Ultrasound-guided internal jugular venous cannulation in infants: A prospective comparison with the traditional palpation method. Anesthesiology. 1999;91(1):71–77. [DOI] [PubMed] [Google Scholar]
- 4.Sigaut S, Skhiri A, Stany I, Glomer J, Nivoche Y, Constant I, et al. Ultrasound guided internal jugular vein access in children and infant: A meta-analysis of published studies. Paediatric anaesthesia. 2009;19(12):1199–1206. [DOI] [PubMed] [Google Scholar]
- 5.Chuan WX, Wei W, Yu L. A randomized-controlled study of ultrasound prelocation vs anatomical landmark-guided cannulation of the internal jugular vein in infants and children. Paediatric anaesthesia. 2005;15(9):733–738. [DOI] [PubMed] [Google Scholar]
- 6.Rothschild JM. Ultrasound guidance of central vein catheterization On Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Rockville, MD: AHRQ Publications; 2001. p.245–55. [Google Scholar]
- 7.Guidance on the use of ultrasound locating devices for placing central venous catheters London UK: National Institute for Clinical Excellence (NICE); 2002. Technology appraisal guidance no. 49. [Google Scholar]
- 8.Revised statement on recommendations for use of real-time ultrasound guidance for placement of central venous catheters. Bulletin of the American College of Surgeons. 2011;96(2):36–7. [PubMed] [Google Scholar]
- 9.Bosman M, Kavanagh RJ. Two dimensional ultrasound guidance in central venous catheter placement: A postal survey of the practice and opinions of consultant pediatric anesthetists in the United Kingdom. Paediatric anaesthesia. 2006;16(5):530–537, [DOI] [PubMed] [Google Scholar]
- 10.Froehlich CD, Rigby MR, Rosenberg ES, Li R, Roerig PL, Easley KA, et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Critical care medicine. 2009;37(3):1090–6. [DOI] [PubMed] [Google Scholar]
- 11.DeAngelis M New guidance technology results in reducing mid-line catheterization times. International anesthesia research society; May; Washington, D.C. 2017. [Google Scholar]
- 12.Chew SC, Beh ZY, Hakumat Rai VR, Jamaluddin MF, Ng CC, Chinna K, et al. Ultrasound-guided central venous vascular access-novel needle navigation technology compared with conventional method: A randomized study. The journal of vascular access. 2019: In press. [DOI] [PubMed] [Google Scholar]
- 13.Thomas A, Ewald J, Kelly I, Pierce M, Thomas J, Mattison B, et al. Conventional vs computer-assisted stereoscopic ultrasound needle guidance for renal access: A randomized crossover bench-top trial. Journal of endourology. 2018;32(5):424–30. [DOI] [PubMed] [Google Scholar]
- 14.Tielens LK, Damen RB, Lerou JG, Scheffer GJ, Bruhn J. Ultrasound-guided needle handling using a guidance positioning system in a phantom. Anaesthesia. 2014;69(1):24–31. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Min JY, Song J, Song K, Song JH, Byon HJ. The effect of electromagnetic guidance system on early learning curve of ultrasound for novices. Korean journal of anesthesiology. 2016;69(1): 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopac DS, Chen J, Tang R, Sawka A, Vaghadia H. Comparison of a novel real-time SonixGPS needle-tracking ultrasound technique with traditional ultrasound for vascular access in a phantom gel model. Journal of vascular surgery. 2013;58(3):735–741. [DOI] [PubMed] [Google Scholar]
- 17.Sander D, Schick V, Ecker H, Lindacher F, Felsch M, Spelten O, et al. Novel navigated ultrasound compare with conventional ultrasound for vascular access: a prospective study in a gel phantom model. Journal of cardiothoracic and vascular anesthesia. 2015;29(5): 1261–1265. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AN, Peiffer JS, Halmann N, Delaney L, Owen CA, Hersh J. Ultrasound guided needle technique accuracy: prospective comparison of passive magnetic tracking versus unassisted echogenic needle localization. Regional anesthesia pain medicine. 2017;42(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]


