Abstract
Objective:
Treatment with BCR-ABL tyrosine kinase inhibitors (TKIs) is the standard of care for patients with chronic myeloid leukemia, however evidence indicates these compounds may have cardiovascular side-effects. This study sought to determine if ex vivo exposure of human adipose arterioles to the BCR-ABL TKIs imatinib and nilotinib causes endothelial dysfunction.
Methods:
Human adipose arterioles were incubated overnight in cell culture media containing vehicle (PBS), imatinib (10 μmol/L) or nilotinib (100 μmol/L). Arterioles were cannulated onto glass pipettes and flow mediated dilation (FMD) was assessed via video microscopy. To determine the mechanism of vasodilation, FMD was re-assessed in the presence of either the nitric oxide synthase inhibitor L-NAME (100 μmol/L) or the H2O2 scavenger PEG-Catalase (500 U/mL).
Results:
Neither imatinib nor nilotinib affected the magnitude of FMD (max dilation = 78±17% vehicle, 80 ± 24% nilotinib, 73 ± 13% imatinib). FMD was decreased by L-NAME in vehicle-treated arterioles (max dilation = 47±29%). Conversely, L-NAME had no effect on FMD in imatinib- or nilotinib-treated vessels (max dilation = 79±14% and 80 ± 24%, respectively), rather FMD was inhibited by PEG-Catalase (max dilation = 29±11% and 29 ± 14%, respectively).
Conclusion:
Incubating human arterioles with imatinib or nilotinib switches the mediator of FMD from vasoprotective nitric oxide to pro-inflammatory H2O2.
Keywords: chronic myelogenous leukemia, microcirculation, nitric Oxide, tyrosine kinase inhibitor, vasodilation
1 |. INTRODUCTION
BCR-ABL TKIs have become the frontline treatment for patients with CML and have resulted in significantly prolonged life expectancy (58% survival over 7 years to 85% over 8 years1,2). Currently, the standard of care is to continue TKIs indefinitely; thus, attention must be paid to potential long-term side effects. As reports have shown, some of these side effects are cardiovascular in nature, and the mechanisms are still poorly defined.3 Of the five TKIs that are currently first-line therapy for patients with CML (imatinib, dasatinib, nilotinib, bosutinib, and ponatinib), nilotinib and ponatinib have been associated with a significant incidence of cardiovascular disease (16%2 and 27%,4 respectively). While much of the research in this area has been focused on cardiac function, the direct effects of these pharmacological agents on the systemic vasculature remain largely unknown.
Studies have shown NO–mediated dilation is impaired in large vessels after several anti-cancer therapies5–7; however, little direct evidence exists on the effect of anti-cancer therapy on the human microcirculation. The contribution and predictive value of endothelial microvascular dysfunction to the development of cardiovascular disease is well established and may be a superior predictor of cardiac events than large vessel disease.8,9 Thus, understanding the effects of anti-cancer therapy (particularly therapies with prolonged use) on the vascular endothelium may open novel approaches for treatment and/or screening of adverse side effects for cancer patients generally, and those with CML specifically. In this study, we sought to determine whether ex vivo treatment of human adipose arterioles with two different BCR-ABL TKIs (nilotinib and imatinib) caused endothelial dysfunction in the human microvasculature by assessing the magnitude and vasoactive mediator of FMD.
2 |. METHODS
All study procedures were approved by the Institutional Review Board at the Medical College of Wisconsin (study ID number: PRO00000114).
2.1 |. Microvascular function studies
A total of 30 human arterioles 100–200 μmol/L in diameter were dissected from 21 unique adipose samples and were used for video microscopy as previously described.10,11 The samples were obtained from 4 men and 15 women. Two samples were from individuals of unknown sex. When possible, multiple arterioles were studied from the same individual, but not within the same treatment or inhibitor group.
Because the tissue was discarded during the normal surgical procedure and samples were de-identified, informed consent was not required from the patients. Only tissue from patients without a history of cardiovascular disease was used for this study.
Arterioles were incubated in full cell culture media (EGM™−2 Endothelial Cell Growth Medium-2 BulletKit™ with 5% fetal bovine serum; Lonza Group Ltd) containing either vehicle (phosphate-buffered saline; PBS) or the clinically used TKIs imatinib (10 μmol/L; Novartis Pharma AG) or nilotinib (100 nmol/L; Novartis Pharma AG) and incubated at 37°C overnight (15–20 hours). The concentrations of imatinib and nilotinib were chosen based on studies that have shown imatinib effectively inhibits ex vivo cell growth at concentrations between 5 and 15 μmol/L while nilotinib has similar inhibitory effects at concentrations ranging from 100 nmol/L to 1 μmol/L.12–15 Additionally, nilotinib has also been shown to inhibit BCR-ABL1 with about 25-fold greater potency than imatinib.16 Clinically, the target plasma trough concentrations for imatinib and nilotinib are >1000 ng/mL (2.0 μmol/L) and >500 ng/mL (943 nmol/L), respectively.16
Vasodilation to changes in intraluminal flow (pressure gradient 0–100 cm H2O) was evaluated using video microscopy. Briefly, the microvessels were cannulated onto glass micropipettes of equal internal diameter in an organ bath filled with Krebs solution containing (in mmol/L) 123 NaCl, 4.4 KCl, 2.5 CaCl2, 1.2 MgSO4, 20 NaHCO3, 1.2 KH2PO4, and 11 glucose. The microvessels were then pressurized to 60 mm Hg, heated to 37°C, and bubbled with an air mixture of 21% O2-5% CO2-74% N2 to maintain pH constant at 7.4 for 1 hour. After the equilibration period, FMD was assessed in the absence of any pharmaceutical reagents. The mechanism of FMD was then determined in the same cannulated arteriole by adding either the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (L-NAME; 100 μmol/L; MilliporeSigma) or the H2O2 scavenger PEG-Catalase (500 U/mL; MilliporeSigma) for 30 minutes to the organ bath. When two vessels were studied from the same individual to assess the effects of L-NAME or PEG-Catalase on FMD within a given treatment group (control, imatinib, nilotinib), the two control curves were averaged and reported as a single n. At the conclusion of each experiment, maximal dilation to the phosphodiesterase inhibitor papaverine (100 μmol/L; MilliporeSigma) was assessed.
2.2 |. Statistical methods
To determine the effects of nilotinib or imatinib on the magnitude of vasodilation compared to vehicle-treated arterioles, a 2-way ANOVA with repeated measures was used after testing the data for normal distribution with the Shapiro-Wilk test. Similar analysis was performed to determine the effects of L-NAME or PEG-Catalase on vasodilation compared to vehicle control in all three treatment groups. A Bonferroni post hoc test for multiple comparisons was performed to test for differences vs. the control group (ie, PBS Vehicle or Control Response). The ED50, defined as the effective dose of flow required to cause 50% of the maximal dilation, was calculated using a four-parameter dose-response curve model with a least squares regression fitting method in GraphPad Prism version 8.3.1 (GraphPad Software). The ED50 values were compared using the extra-sum-of-squares F test. The area under the curve for the dilator response from each arteriole ± pharmacological inhibitors was also calculated using GraphPad Prism, and the within-group means were compared using a one-way repeated measures ANOVA with a post hoc Bonferroni test for multiple comparisons. All data are reported as mean ± SD, and all n’s represent biological replicates, not technical replicates. A P-value < .05 was considered statistically significant.
3 |. RESULTS
The mean age of the patients from whom tissue samples were obtained from was 54 ± 14 years at the time of surgery. All patients were free of cardiovascular disease and had a mean body mass index of 30 ± 5 kg/m2. The average maximum diameter of all microvessels used in this study was 180 ± 75 μm, and the vessels were preconstricted with endothelin-1 to an average of 51 ± 13% of their maximum diameter prior to the assessment of FMD. There was no difference in maximum vessel diameter or percent constriction between the groups, and neither PEG-Catalase nor L-NAME had effect on the percent constriction to endothelin-1 (P > .05).
As shown in Figure 1, neither nilotinib (n = 7) nor imatinib (n = 7) had an effect on the magnitude of FMD compared to vehicle (n = 8)-treated vessels (vehicle maximum dilation = 78±17%; nilotinib maximum dilation = 80±24%; imatinib maximum dilation = 73±13%). The ED50 also did not differ between the three groups (vehicle = 23 cm H2O; nilotinib = 17 cm H2O; imatinib = 16 cm H2O). Finally, all arterioles dilated to >90% of their maximal diameter in response to papaverine, indicating that neither treatment had a negative effect on vascular smooth muscle function.
FIGURE 1.
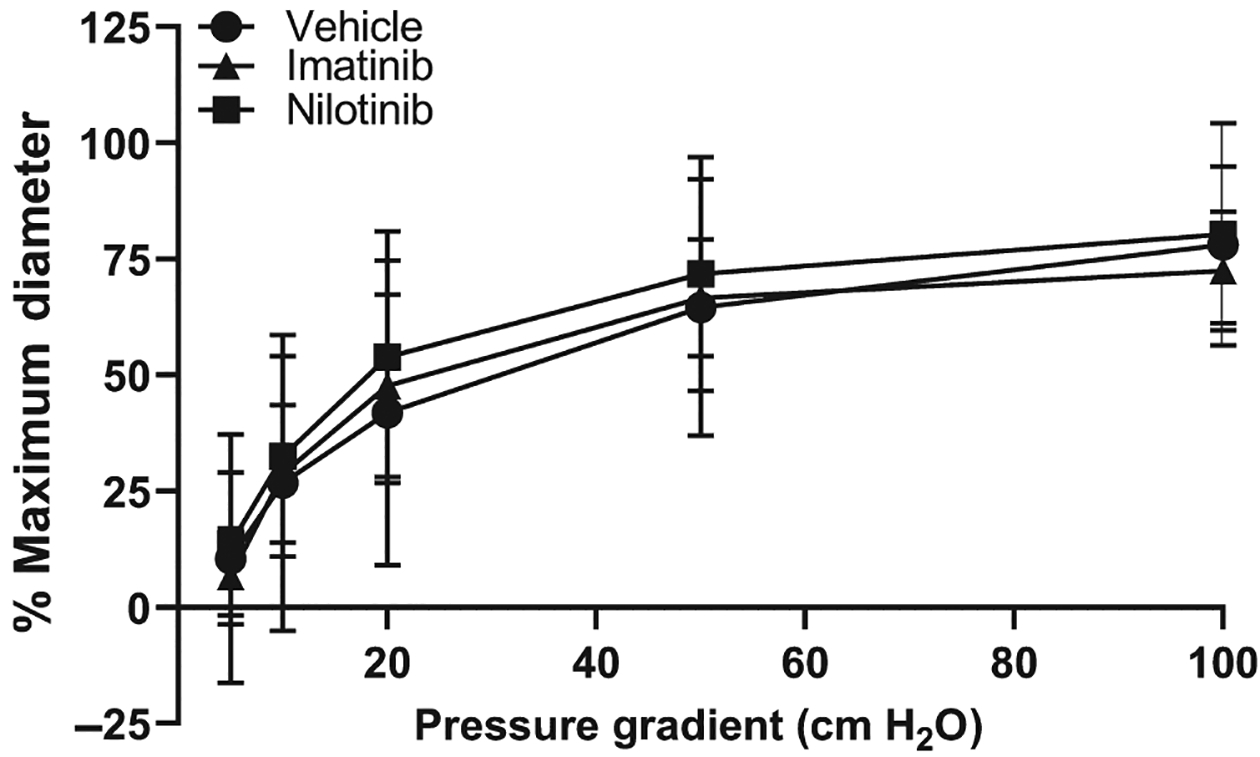
Flow-mediated dilation of human adipose arterioles incubated in either PBS vehicle, nilotinib, or imatinib overnight. Neither imatinib (n = 7) nor nilotinib (n = 7) affected the magnitude of flow-mediated dilation compared to PBS vehicle (n = 8)
Consistent with our previous work,17 FMD was significantly reduced by the nitric oxide synthase inhibitor L-NAME (n = 5) in vehicle-treated vessels compared to the untreated control response (Figure 2A; maximum dilation = 47±29% vs. 78 ± 17%, respectively; P = .045). PEG-Catalase, a scavenger of H2O2, had no effect on FMD in the vehicle-treated vessels (n = 7; maximum dilation = 72±32%). The ED50 for each of the groups was as follows: Control, 23 cm H20; L-NAME, 17 cm H2O; and PEG-Catalase, 19 cm H2O. Conversely, as shown in Figure 2B,C, L-NAME had no effect on FMD in either imatinib- or nilotinib-treated vessels (imatinib maximum dilation = 79±14% L-NAME vs 73 ± 13% untreated control, n = 7; nilotinib maximum dilation = 79±24% L-NAME vs 80 ± 24% untreated control, n = 4); rather, PEG-Catalase caused a reduction in FMD (imatinib maximum dilation = 29±11%, n = 7, P < .001; nilotinib maximum dilation = 29±14%, n = 5, P = .009). For nilotinib-treated vessels, the ED50 in all groups was as follows: Control, 17 cm H2O; L-NAME, 14 cm H2O; and PEG-Catalase, 33 cm H2O. For imatinib-treated vessels, the ED50 was as follows: Control, 16 cm H2O; L-NAME, 19 cm H2O; and PEG-Catalase, 64 cm H2O. As shown in Figure 2D, the area under the curve for vessels incubated with PEG-Catalase was significantly less in vessels incubated with imatinib (P = .003) or nilotinib (P < .001) compared to control, indicating the majority of the vasodilator response was mediated by H2O2 after BCR-ABL TKI treatment.
FIGURE 2.
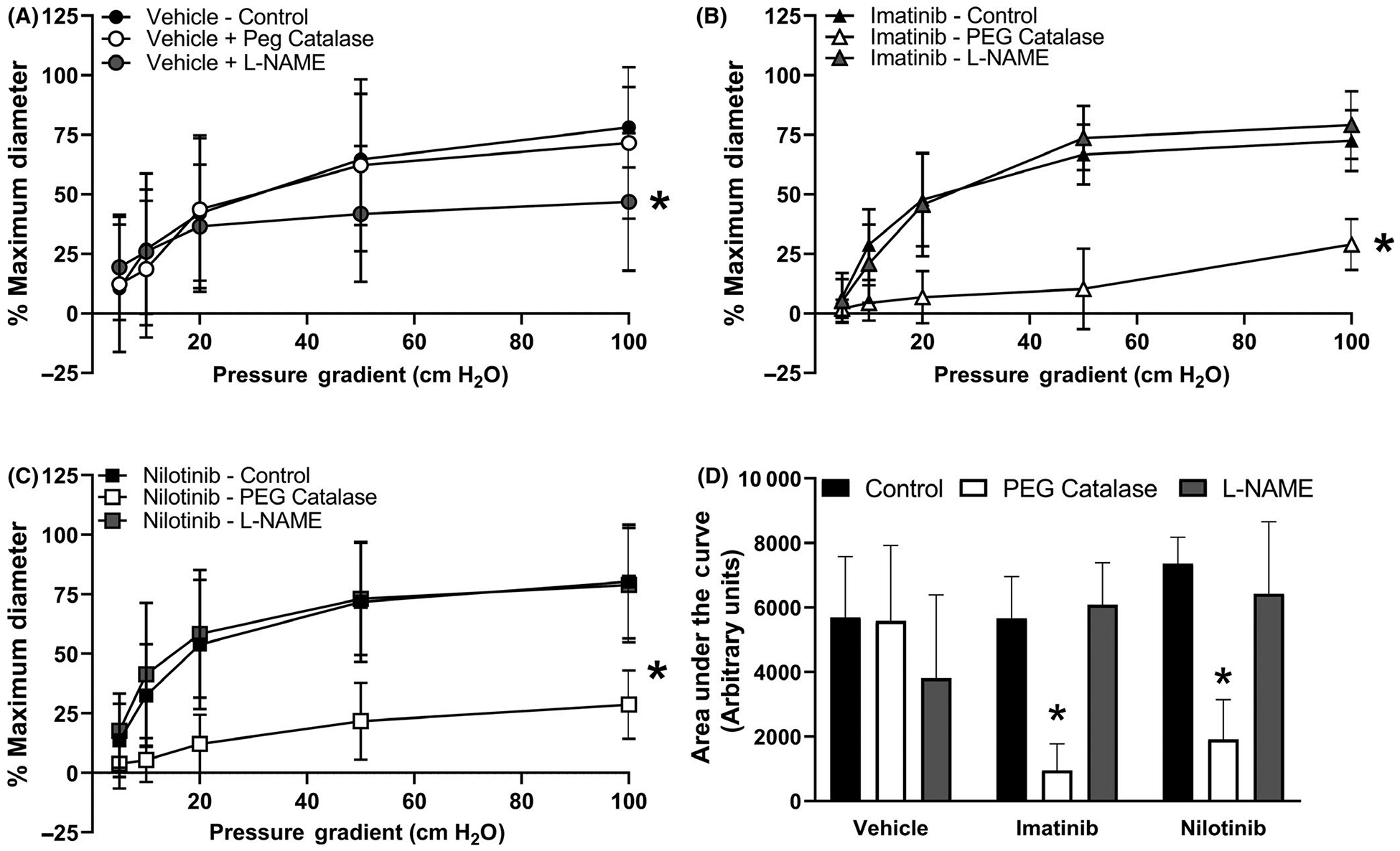
Flow-mediated dilation in the presence or absence of either L-NAME or PEG-Catalase. Panel A. Consistent with our previous work, in arterioles incubated in PBS vehicle, the nitric oxide synthase inhibitor L-NAME (n = 5) reduced FMD compared to control (n = 8) while the hydrogen peroxide scavenger PEG-Catalase had no effect on FMD (n = 7). In arterioles incubated with either imatinib (Panel B) or nilotinib (Panel C), the nitric oxide synthase inhibitor L-NAME (n = 7.4) had no effect on FMD compared to control (n = 7.7) while the hydrogen peroxide scavenger PEG-Catalase reduced FMD (n = 7.5). Area under the curve analysis is shown in Panel D. In imatinib- and nilotinib-treated vessels, the area under the curve for PEG-Catalase–inhibitable dilation was reduced compared to control. All data shown as mean ± SD. *P < .05 vs control
4 |. DISCUSSION
The primary finding of this study is that ex vivo exposure of two clinically used BCR-ABL TKIs, nilotinib and imatinib, to human arterioles causes a shift in the mediator of vasodilation from vasoprotective nitric oxide to pro-inflammatory hydrogen peroxide. These findings are consistent with the microvascular phenotype described in both the coronary and peripheral microcirculations of patients with CAD or in microvessels from healthy individuals after acute stress (reviewed by Gutterman et. al).18
While both H2O2 and NO are endothelium-dependent vasodilators, H2O2 promotes inflammation and vascular remodeling and is considered pro-atherosclerotic while NO is anti-inflammatory and is considered an important vasoactive mediator of dilation in the healthy human microcirculation. Due to the predictive value of microvascular (dys)function for future cardiovascular events, this phenotype is consistent with increasing evidence of elevated cardiovascular risk in cancer patients undergoing chemotherapy as well as the established higher prevalence of CAD in childhood cancer survivors.(reviewed by Armenian et al19). Our recent study showing that anthracycline exposure causes a loss of endothelial dilator function in the human coronary circulation further amplifies the negative impact of different cancer therapies on vascular function.20
To our knowledge, this is the first study to report direct adverse effects of BCR-ABL TKIs on human microvascular endothelial function. As standard clinical practice recommends continuous treatment of patients with CML with BCR-ABL TKIs, future studies of vascular function in this patient population are warranted. It is interesting to note that of all clinically used BCR-ABL TKIs, imatinib has the fewest reported adverse events, including those of cardiovascular nature.3,21 Our data, however, show that imatinib and nilotinib have similar negative impacts on human microvascular function, which is concerning as nilotinib has been reported to have serious off-target effects.22 Clinically, BCR-ABL therapy has only been used as a primary therapy for patients with CML for about a decade, and with this in mind, it is too early to judge the long-term impact of this class of therapy on cardiovascular physiology, including in the circulation, where pathological changes may exist for years without any clinical impact. Data from our group and others support the notion that microvascular changes may precede large vessel disease (including CAD) and are superior to predicting major adverse events when compared to large vessel dysfunction.9
4.1 |. Study limitations and future directions
Future studies are necessary to determine the mechanisms by which BCR-ABL TKIs switch the mediator of dilation from vasoprotective nitric oxide to pro-inflammatory H2O2 and whether the pathways involved can be manipulated to prevent the switch in mechanism. We also did not design our study to test whether sex differences exist within the microvascular response to BCR-ABL TKI exposure, and future studies should properly evaluate if arterioles from men and women are equally affected. Finally, we did not directly test the effects of BCR-ABL TKI treatment on vascular smooth muscle function; however, all arterioles studied dilated to >90% of their maximal diameter to the phosphodiesterase inhibitor papaverine. Therefore, it is likely the changes in vasodilator mechanism observed after BCR-ABL TKI treatment are the result of altered signaling pathways in the endothelial cell layer.
5 |. PERSPECTIVES
The findings in this study warrant closer monitoring of patients with CML being treated with BCR-ABL TKIs, and there may be an opportunity to detect early changes in cardiovascular function before irreversible damage has developed. Future research in this area should be prioritized with an emphasis to understand the cardiovascular effects of BCR-ABL TKIs and other targeted anti-cancer therapies.
Funding information
R01HL133029; R01HL113612.
REFERENCES
- 1.Cortes J, Hochhaus A, Kim DW, et al. Four-year (Yr) follow-up of patients (Pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) receiving Dasatinib or Imatinib: efficacy based on early response. Blood. 2013;122:653. [Google Scholar]
- 2.Saglio G, Hochhaus A, Hughes TP, et al. ENESTnd update: Nilotinib (NIL) vs Imatinib (IM) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) and the impact of early molecular response (EMR) and sokal risk at diagnosis on long-term outcomes. Blood. 2013;122:92. [Google Scholar]
- 3.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.In brief: ponatinib (Inclusig) returns. The Medical letter on drugs and therapeutics, New Rochelle, NY: The Medical Letter, Inc; 2014;56:8 https://secure.medicalletter.org/TML-article-1434c24457561 [Google Scholar]
- 5.Jenei Z, Bardi E, Magyar MT, Horvath A, Paragh G, Kiss C. Anthracycline causes impaired vascular endothelial function and aortic stiffness in long term survivors of childhood cancer. Pathol Oncol Res. 2013;19:375–383. [DOI] [PubMed] [Google Scholar]
- 6.Lee K, Kang I, Mack WJ, et al. Effects of high-intensity interval training on vascular endothelial function and vascular wall thickness in breast cancer patients receiving anthracycline-based chemotherapy: a randomized pilot study. Breast Cancer Res Treat. 2019;177:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassilakopoulou M, Mountzios G, Papamechael C, et al. Paclitaxel chemotherapy and vascular toxicity as assessed by flow-mediated and nitrate-mediated vasodilatation. Vascul Pharmacol. 2010;53:115–121. [DOI] [PubMed] [Google Scholar]
- 8.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Hoef TP, van Lavieren MA, Damman P, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 10.Durand MJ, Phillips SA, Widlansky ME, Otterson MF, Gutterman DD. The vascular renin-angiotensin system contributes to blunted vasodilation induced by transient high pressure in human adipose microvessels. Am J Physiol Heart Circ Physiol. 2014;307:H25–H32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand MJ, Zinkevich NS, Riedel M, et al. Vascular actions of angiotensin 1–7 in the human microcirculation: novel role for telomerase. Arterioscler Thromb Vasc Biol. 2016;36:1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109:4016–4019. [DOI] [PubMed] [Google Scholar]
- 13.Konig H, Holtz M, Modi H, et al. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia. 2008;22:748–755. [DOI] [PubMed] [Google Scholar]
- 14.Merchant MS, Woo CW, Mackall CL, Thiele CJ. Potential use of imatinib in Ewing’s Sarcoma: evidence for in vitro and in vivo activity. J Natl Cancer Inst. 2002;94:1673–1679. [DOI] [PubMed] [Google Scholar]
- 15.Uziel O, Fenig E, Nordenberg J, et al. Imatinib mesylate (Gleevec) downregulates telomerase activity and inhibits proliferation in telomerase-expressing cell lines. Br J Cancer. 2005;92:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura M Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol Pharm Bull. 2015;38:645–654. [DOI] [PubMed] [Google Scholar]
- 17.Beyer AM, Zinkevich N, Miller B, et al. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol. 2017;112:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutterman DD, Chabowski DS, Kadlec AO, et al. The human microcirculation: regulation of flow and beyond. Circ Res. 2016;118:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armenian SH, Armstrong GT, Aune G, et al. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36:2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hader SN, Zinkevich N, Norwood Toro LE, et al. Detrimental effects of chemotherapy on human coronary microvascular function. Am J Physiol Heart Circ Physiol. 2019;317:H705–H710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claudiani S, Apperley JF. The argument for using imatinib in CML. Hematology Am Soc Hematol Educ Program. 2018;2018:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valent P Severe adverse events associated with the use of second-line BCR/ABL tyrosine kinase inhibitors: preferential occurrence in patients with comorbidities. Haematologica. 2011;96:1395–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]


