Abstract
Degeneration of substantia nigra pars compacta dopamine neurons is a central feature in the pathology of Parkinson’s disease, which is characterized by progressive loss of motor and cognitive functions. The largest risk factors for Parkinson’s disease are age and sex; most cases occur after age 60 and males have nearly twice the incidence as females. Preclinical work has scarcely considered the influence of these two risk factors to disease risk and presentation. Here, we observed a progressive decline in dopamine neuron firing activity in male C57BL/6 mice by 18 months of age, while dopamine neurons from females remained largely unaffected. This was accompanied by increased mRNA expression of PINK1 in both males and females, and PARK2 primarily in males, both of which have been linked to Parkinson’s. Since the declining cell properties were accompanied by only slight decreases in locomotion in both sexes, it is likely that these age-related impairments in males represent a vulnerability to further insults that could predispose the neurons to neurodegenerative processes such as in Parkinson’s.
Keywords: Electrophysiology, aging, mouse, dopamine, substantia nigra, firing
Graphical Abstract
1. INTRODUCTION
Midbrain dopamine neurotransmission is involved in a wide array of functions including motor control, motivation, reward, and cognitive processes (Crocker, 1997; Wise and Rompre, 1989). The dopaminergic cell bodies of the substantia nigra (SN) pars compacta and the ventral tegmental area (VTA) form the starting points for the nigrostriatal and mesolimbic pathways, respectively (Fuxe et al., 1977). Degeneration of dopamine neurons in the SN is a hallmark feature of the pathology of Parkinson’s disease and is associated with a progressive loss of the ability to initiate movement (Hornykiewicz, 1975). Disease pathogenesis is thought to be the result of multiple complex gene/environment interactions (Duda et al., 2016) with sex and advanced age representing two prominent risk factors (Gillies et al., 2014). Parkinson’s disease incidence increases after the age of 60 (de Lau and Breteler, 2006) and several reports indicate a 1.5 to 2 times greater risk for males compared to females (Baldereschi et al., 2000; Moisan et al., 2016). Despite the awareness of these two risk factors in the clinical literature, relatively little basic research of the dopaminergic system has considered the effects of age and sex on disease development and presentation.
Previous work in our lab identified a decrease in spontaneous firing frequency and a lack of pacemaker firing fidelity in cell-attached recordings of dopamine neurons in slices from male mice over 25 months of age (Branch et al., 2014). Dopamine neurons exhibit rhythmic, spontaneous pacemaking activity in brain slices (Grace and Onn, 1989), which is the result of the coordinated activity of at least a dozen ion channels, calcium homeostasis, and energy metabolism (Duda et al., 2016). The maintenance of continuous activity renders dopamine neurons highly vulnerable to oxidative damage, which is a possible mechanism contributing to their degeneration in Parkinson’s disease (Duda et al., 2016). Dysfunction in firing may indicate deficits in the health of the cells prior to death and possibly impairment of the nigrostriatal circuit with consequences for motor function (Branch et al., 2016).
In the present study we sought to perform a full characterization of dopamine firing deficits, determine when during the mouse lifespan deficits emerge, and explore potential effects of sex. To do this, we performed current clamp recordings of SN pars compacta dopamine neurons across four age groups in male and female C57Bl/6 mice. The age groups tested were 4, 12, 18, and 24–30 months, which correspond roughly to human ages 25, 42, 56, and 69–81 (Flurkey et al., 2007). We found that dopamine neurons from females show few obvious decrements with age, while neurons from males exhibit firing deficits by 18 months. To better understand the implications of these results, we complemented these findings with an analysis of expression of relevant Parkinson’s related and ion channel genes in the SN of young and old, male and female mice, as well as a supplemental experiment testing open field locomotion.
2. MATERIALS AND METHODS
2.1. Animals
All mice were obtained from the National Institute on Aging aged rodent colony. Male and female C57BL/6 mice, ages 4, 12, 18 and ≥24 months, were housed in a humidity and temperature-controlled facility under a reverse 12:12 light-dark cycle (lights off 0900) with food and water available ad libitum. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the Oklahoma Medical Research Foundation.
2.2. Ex vivo electrophysiology
Mice were anesthetized with isoflurane before dissection of the brain, which was immediately placed in ice-cold oxygenated cutting solution containing the following (in mM): 250 sucrose, 26 NaHCO3, 2 KCl, 1.2 NaH2PO4, 11 glucose, 7 MgCl2, 0.5 CaCl2. Coronal slices (200 μm) containing the ventral midbrain were collected using a VF-200 Compresstome (Precisionary, Cambridge, MA, USA). Slices were incubated first at 34°C for 20 minutes and then at room temperature in an artificial cerebrospinal fluid (aCSF) solution containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 26 NaHCO3, 11 glucose, 1.3 MgCl2, 2 CaCl2 and with 0.01 MK-801. During recording, slices were superfused with aCSF at 34–36°C with a flow rate of 2 ml/min in the presence of 100 μM picrotoxin and 10 μM DNQX to antagonize any spontaneous GABAergic and glutamatergic synaptic activity that could influence cell firing parameters that were measured.
Dopamine neurons of the SN pars compacta were visualized by Dodt contrast with a CCD camera mounted to an upright microscope (Nikon, Melville, NY, USA) and patch clamped using glass pipettes (1.5–3 MΩ, World Precision Instruments, Sarasota, FL, USA) filled with internal solution containing (in mM): 142 K-gluconate, 2 KCl, 4 MgCl2, 10 HEPES, 0.5 EGTA, 4 ATP-Mg, 0.5 Na-GTP, 280–300 mOsm, pH adjusted to 7.2 to 7.4 with KOH. Recordings were initiated 5 minutes following breaking into the cell to allow for equilibration of internal and external solutions. Additionally, 0.05% neurobiotin (Dufour et al., 2014) was added to the pipette solution to allow for post hoc verification of the dopaminergic identity of the neurons recorded, through immunolabeling for the catecholamine marker tyrosine hydroxylase (TH). A total of 187 SN pars compacta cells were recorded from 1–2 centrally located slices per mouse (mean: 1.27±0.06), and 1–9 cells were recorded per slice (mean: 2.97±0.24). Current clamp recordings were made with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), filtered at 4 kHz. Series resistance for all recordings was maintained at <7MΩ, and both bridge balance and capacitance compensation were used. Data were collected using Axograph software (www.axograph.com). Voltages were corrected offline for a liquid junction potential of 11.1 mV between the internal and external solution. Electrophysiological data were analyzed with IgorPro (Wavemetrics, Lake Oswego, OR, USA).
2.3. Immunohistochemistry
For identification of neurobiotin-filled cells, brain slices from electrophysiology experiments were fixed overnight in 4% PFA. Polyclonal rabbit anti-TH (Millipore-Sigma, Burlington, catalog no. AB152) was used as a primary antibody and goat anti-rabbit Alexa Fluor 594 (Abcam, Cambridge, catalog no. ab150080) was used as a secondary antibody. Normal goat serum (Jackson ImmunoResearch, West Grove) was used in all blocking steps. Brain sections were equilibrated to room temperature (RT) for 30 minutes, rinsed in phosphate-buffered saline (PBS) and incubated in a blocking solution (BS; 5% normal goat serum in 0.2% PBST, phosphate buffer saline tris) for 4 hours at RT on a platform shaker. Sections were then incubated overnight with primary TH antibody (1:300 dilution in 1% normal goat serum in 0.2% PBST) at 4°C. After rinsing with 0.2% PBST, sections were incubated with Alexa Fluor 594 (1:200 dilution in PBS) for 2 hours at RT and protected from light with foil. Natural streptavidin protein Dylight 488 (1:500 dilution in PBS, Abcam, Cambridge, catalog no. ab134349) was added to label neurobiotin. 100 ng/ml DAPI in 0.2% PBST (1.5 hrs. incubation) was added in the last rinse after secondary antibody incubation. This was followed by three rinse steps (1-hour, 30 min, 30 min) with PBST to remove excess staining. After rinsing with PBST and PBS, sections were mounted onto glass slides using Shur/Mount Aqueous (EMS, Hatfield). Coverslips were applied and sealed with nail polish. Images were obtained using a Zeiss LSM-710 confocal microscope.
2.4. qPCR
The SN was dissected from mice that were 4 months (5 males, 5 females) and 27 months old (5 males and 5 females). Samples were immediately snap-frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from frozen tissue using RNeasy plus mini kit (Qiagen, CA, USA). The amount of total RNA was quantified using Nanodrop 2000 spectrophotometer and RNA quality and purity was evaluated using an Agilent 2200 TapeStation system (Agilent Technologies, CA, USA). Gene expression levels were assayed by qPCR, as previously described (Mangold et al., 2017; Masser et al., 2014). Briefly, cDNA was synthesized from 80 ng RNA using Quantabio qScript cDNA Sythesis Kit. Pre-designed probe and primer fluorogenic exonuclease assays (TaqMan, Life Technologies, Watham, MA, Table S1) were used to perform qPCR on the QuantStudio™ 12K Flex Real-Time PCR System (Applied Biosystems). Relative gene expression (RQ) was normalized to endogenous control GAPDH using the 2−ΔΔCt method in the Expression Suite v1.0.3 software.
2.5. Statistical analysis
Data are represented in box and whisker plots, to show distributions of data, or line graphs with means ± standard error of the mean. Data were analyzed by two-way ANOVA (factors: sex, age) and two-way repeated measures (RM) ANOVA, followed by Bonferroni-corrected t-test post hoc (Sigma Stat, San Jose, CA, USA). Alpha was set a priori to 0.05.
3. RESULTS
3.1. Dopamine neuron pacemaking becomes disrupted in males, but not females, by 18 months of age
Dopamine neurons were identified in the ventral half of the SN of coronal midbrain slices based on their large cell bodies, characteristic slow firing rate (<7Hz) (Grace & Onn, 1989), and the presence of a large voltage sag as a result of the hyperpolarization-activated cation current, IH (Dufour et al., 2014; Neuhoff et al., 2002). Recorded cells were filled with neurobiotin to confirm their dopaminergic identity through post hoc immunostaining for TH, the rate limiting enzyme of dopamine synthesis (Dufour et al., 2014; Fig 1A). Dopamine neurons were recorded from male and female mice across four age groups: 4 months (n=21 cells from 6 male mice; n=23 from 3 females), 12 months (n=20 from 8 males; n=17 from 6 females), 18 months (n=21 from 4 males; n=19 from 4 females) and 24–30 months (n=31 from 10 males; n=26 from 7 females). A range of spontaneous firing frequencies were recorded in current clamp for at least 2 minutes each, across all ages in dopamine neurons from males (from 0.6 to 4.4 Hz) and females (0.76 to 5.3 Hz). An age-sex interaction was detected in firing frequency (F3,170=2.71, p=0.047; Fig 1B,C), with males showing lower firing frequencies than females at ages 18 months and older. This decrease in firing rate in males was numerically more pronounced at 18 months (33.96% decrease) than at 24–30 months (16.53% decrease).
Figure 1.
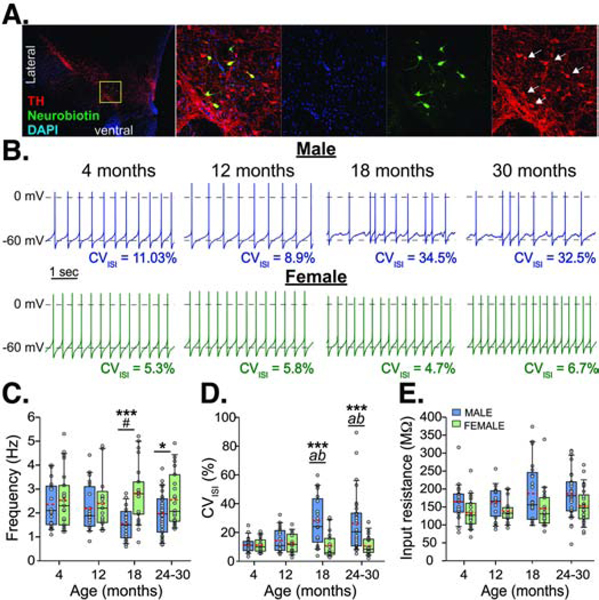
Evolution of spontaneous firing activity across ages. A. Images from a recorded slice immunolabeled for tyrosine hydroxylase (TH, a marker for DA neurons) and neurobiotin (a tracer added to the patch pipette). Far left, image of the midbrain dopamine region from the whole slice (a bisected coronal section). Images to the right show magnified images (zoom in of the yellow box), of DAPI counterstain (blue), and labeling for neurobiotin (green) and TH (red). White arrows (far right) indicate co-labeled cells. B. Representative recordings of spontaneous firing in current clamp from cells at 4, 12, 18, and 30 months from males (top, blue) and females (bottom, green). Box and whisker plots show firing rate (C), coefficient of variation for the inter-spike interval (CVISI) (D), and input resistance (E). Red dashed lines indicate means. Males differ from females, *p<0.05, ***p<0.001; a - 18 or 24–30 month males differ from 4 month males, p<0.001; b - 18 or 24–30 month males differ from 12 month males p≤0.005; # – 18 month males versus 4 month males, p=0.054.
To assess pacemaker fidelity, the coefficient of variation of the inter-spike interval (CVISI) in spontaneous firing was measured during the most stable 30 seconds of firing of the minutes-long recordings from each cell. An age-sex interaction was detected (F3,170=6.25, p<0.001; Fig 1D), with males showing an increase in variability of firing by 18 months that remained through 24–30 months. Although the mean CVISI was much higher in males ages 18 months and older, the range of data (3.83% to 89.48%) suggests that there was a population of dopamine cells present that maintained regular pacemaking ability. Around a third of the total cells recorded from males ages 18 to 30 months had a CVISI less than 15% (23.8% of cells from 18 months and 38.7% of cells from 24 to 30 months; Fig S1). Those cells came from just half of the animals in each of the 18 and 24–30 month age groups. Of those animals, 30.95±1.94% of the cells had low CVISI in the 18-month-old group and 57.67±10.16% of the cells from the 24–30 month old group. This is consistent with substantial animal-to-animal variability in the oldest age group, as we previously observed using cell-attached recordings from aged male mice (Branch et al., 2014). Females did not show any change across age and still maintained low variability in pacemaking at ages 18 months and older (1.95% to 28.94%), with 73.33% of cells having a CVISI below 15% (Fig S1). No change in input resistance with age was observed, as previously reported (Branch et al., 2014), however, dopamine neurons from females overall showed lower input resistance compared with males (F1,170=14.45, p<0.001; Fig 1E; males=174.73±5.87 MΩ; females=142.51±6.12 MΩ). A main effect of sex was detected for membrane capacitance (F1,170=5.19, p=0.024) with females overall having slightly lower values than males (males=42.27±0.94 pF; female=39.17±0.98 pF).
3.2. Action potential waveforms show minor divergence across sex and age
To further assess the underlying properties of the dopamine firing activity in males and females across age, the waveforms of the action potentials during spontaneous firing activity were averaged and analyzed for the following parameters: spike height (measured from peak to trough), half width (the duration measured at the midpoint of the spike), afterhyperpolarization potential (AHP; raw value taken at the trough) and peak overshoot value (raw potential at peak depolarization). The action potential threshold and maximum rise and decay velocities were also measured by using phase plane plots averaged for each cell. Age-sex interactions were detected for spike height (F3,170=3.207, p=0.025; Fig 2C), half width (F3,170=3.351, p=0.02; Fig 2D), AHP (F3,170=3.467, p=0.018; Fig 2E), peak overshoot value (F3,170=3.139, p=0.027; Fig 2F), and maximum rise velocity (F3,170=5.344, p=0.002; Fig 2G). For maximum decay velocity, only main effects of age and sex were detected (age: F3,170=6.778, p<0.001; sex: F1,170=12.346, p<0.001, Fig 2H), where females showed overall higher velocities than males and decreased velocities were observed by 18 months. When analyzed alone, the only difference detected in males was decreased velocity at 24 months compared with 12 months (F3,89=5.159, p=0.002). No differences were detected in action potential thresholds across ages and between sexes (in mV, 4 months: males=−42.37±0.54, females=−43.65±0.41; 12 months: males=−44.57±0.75, females=−42.46±0.42; 18 months: males=−44.13±0.54, females=−41.92±0.82; 24–30 months: males=−43.56±0.48, females=−43.24±0.66; not shown).
Figure 2.
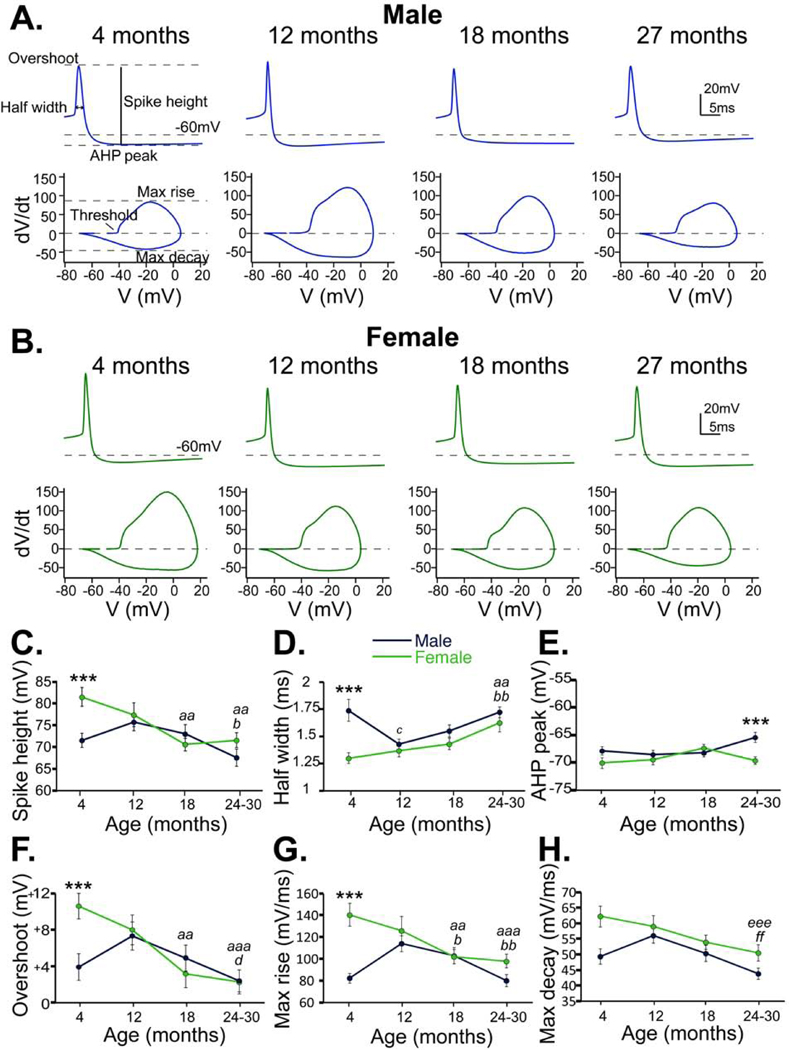
Action potential waveforms in males and females across age. A. Top, representative averages of action potentials from cells in each of the 4 age groups of males (blue). Bottom, phase plane plots of the representative action potential traces. Individual parameters are indicated in the 4-month-old action potential and phase plane plot on the left. B. Representative action potential averages (top) and phase plane plots (bottom) from cells in each of the 4 age groups of females (green). Males (blue) and females (green) are compared in spike height (C), half width (D), AHP (E), peak overshoot (F), maximum rise velocity (G) and maximum decay velocity (H). Bonferroni corrected t-test: *** males and females differ, p<0.001; Females 18 months or 24–30 months compared with 4 months, aa – p<0.005, aaa – p<0.001; Males 24–30 months compared with 12 months, b – p<0.05, bb – p<0.01; Males 12 months compared with 4 months, c – p<0.02; Females 24–30 months compared with 12 months, d – p<0.05; Age group 24–30 months compared with 12 months, eee – p<0.001; Age group 24–30 months compared with 4 months, ff – p=0.003
From these data, it is evident that there exists a sexual disparity in action potential waveform from SN dopamine neurons in young adulthood. At 4 months, cells from females showed larger and narrower spikes with faster kinetics than in males. Females showed a larger peak overshoot value than males at this age, which accounts for the difference in spike height, since there was no difference in AHP. Despite no changes in pacemaker firing across ages in females, there are indeed indications of either decreased ion channel number or function, as evidenced by the linear decrease in spike height and broadening of action potentials across ages. The observed decrease in spike height was likely due to a decrease in the peak overshoot value since there was no change in AHP across ages. The decrease in both rise and decay kinetics, correlating with the widening half width, suggests a reduced activation of both sodium and potassium channels.
Action potential waveforms from cells in males showed a biphasic evolution in spike height, half width, and kinetics across the lifespan. Between 4 and 12 months, there was an increase in spike height and kinetics, after which they linearly declined. Interestingly, no differences were detected between the waveforms at 4 months and 18–30 months, yet after 18 months is when the disruption in pacemaker firing occurs. There were also no differences in waveform detected between females and males after 18 months, except for a decrease in AHP at 24–30 months compared with females. These data suggest that action potentials of SN dopamine neurons evolve through different mechanisms across the lifespan in males and females.
3.3. Dopamine neurons from males, but not females, are more prone to depolarization block by 18 months of age
The membrane excitability of neurons was measured through frequency-current (F-I) curves in response to current injections increasing in 20pA steps up to 400pA, starting from a holding potential of −60mV to preclude pacemaker firing. Dopamine neurons were recorded across four age groups including 4 months (n=24 from 9 males; n=21 from 3 females), 12 months (n=17 from 7 males; n=18 from 7 females); 18 months (n=22 from 7 males; n=18 from 5 females), and 24–30 months (n=29 from 9 males; n=25 from 10 females). The initial frequency was determined from the first three action potentials. In males and females, no differences were detected for the frequency between ages (Fig 3C and 3D) nor were any differences in frequency curve slope between males and females across ages (Fig 3G).
Figure 3.
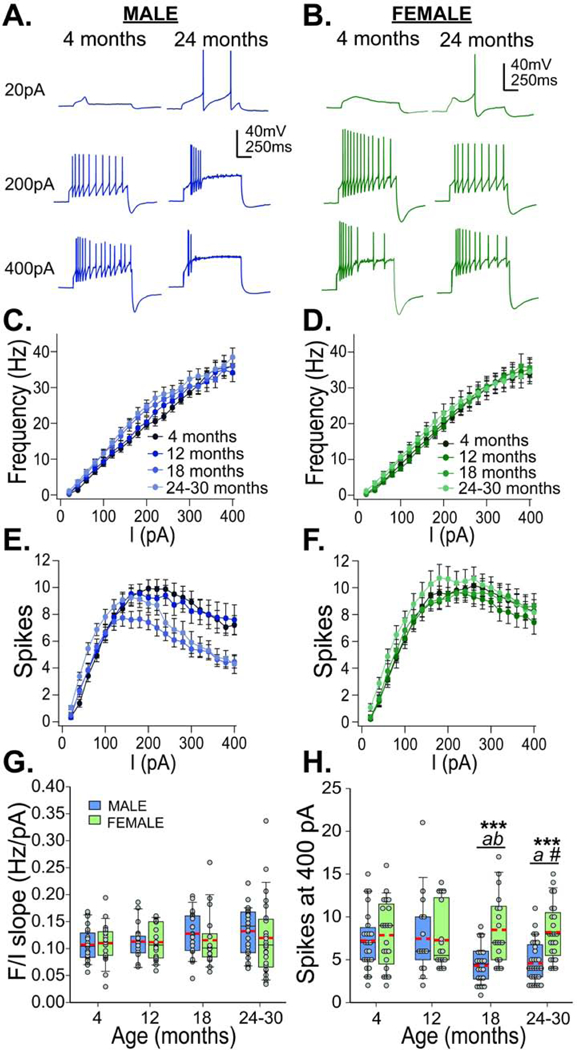
Membrane excitability across ages. Representative traces of current step injections at 20, 200, and 400 pA from males (A; blue; 4 months left, 24 months right) and females (B; green; 4 months left, 24 months right). F-I curves (frequency x injected current) and number of spikes x injected current are plotted for each age group of males (C. & E., blue, left) and females (D. & F., green, right). Box and whisker plots show slopes of F/I curve (G) and number of spikes at 400 pA current injection (H) across ages in males (blue) and females (green). Red dashed lines show means. Bonferroni corrected t-test: *** males and females differ, p<0.001; a – 18 and 24 month males differ from 12 month males, p<0.05; b – 18 month males differ from 4 month males, p<0.05; # − 24 month males versus 4 month males, p=0.057
Males showed a robust age-current interaction for number of spikes (F57,1672=21.142, p<0.001, Fig 3E). 18-month old males showed decreases in spikes evoked by a 200pA current injection and 24–30 months old males showed decreases by a 300pA injection. Females did not show any change in the number of spikes across ages (Fig 3F). Overall, females showed higher numbers of spikes at all ages compared with males (F1,166=7.378, p=0.007) and a higher maximum number of spikes (F1,166=6.666, p=0.011). Males 18 months and older showed fewer spikes for the highest current injection (400pA) compared with females (F3,166=3.717, p=0.013; Fig 3H). An analysis of kinetics showed that action potentials from 4 month old females showed larger spike heights and faster kinetics with current injections than males at 4 months and both males and females at 24–30 months (Table S2). No bimodal distribution was observed for the number of spikes from dopamine neurons recorded from males; almost 90% of cells from males 18 months and older showed spike numbers below the average for young males at a 400pA current injection. This decrease in spikes at higher current injections in older males seems to indicate a greater tendency of dopamine cells to enter depolarization block than in younger males or females at any age.
3.4. Dopamine neurons from males 18 months and older, but not females, show a longer rebound spike delay
SN dopamine neurons are characterized by IH, which can be measured in current clamp as a large sag during hyperpolarizing current injections (Dufour et al., 2014). We next assessed IH and rebound firing properties in dopamine cells by delivering a hyperpolarizing current injection from resting membrane potential for 1 second, bringing the potential at the end of the sag to - 83.73±1.007 mV (average across all cells). Sag and rebound spiking were recorded from animals at 4 months (n=13 from 4 males; n=15 from 3 females), 12 months (n=13 from 5 males; n=12 from 5 females), 18 months (n=17 from 4 males; n=17 from 3 females) and 24–30 months (n=16 from 8 males; n=21 from 7 females). No change was detected in sag amplitude across ages and between sexes (Fig 4B), suggesting no change in IH. This agrees with our previous findings of no change in IH with age in males, measured in voltage clamp (Branch et al., 2014). However, we did detect a robust age-sex interaction for rebound spike delay (F3,116=5.177, p=0.002; Fig 4C). At ages 18 months and older, neuron from males exhibited an average of 483% greater rebound spike delay than those from females of the same age. As with the CVISI, a significant proportion of dopamine cells from males ages 18 months and older showed rebound spike delays in the range of the young males, encompassing 35.3% of the cells from the 18-month-old group and 50% of the cells from the 24–30 month age group (Fig S2). Further analysis showed that long rebound spike delays in cells from old males correlated with increasing CVISI and lower firing frequencies (Fig S3). At 18 months, at least one “healthy” dopamine cell was recorded from each of the mice in that group, while at 24–30 months those cells came from just 62.5% of the mice.
Figure 4.
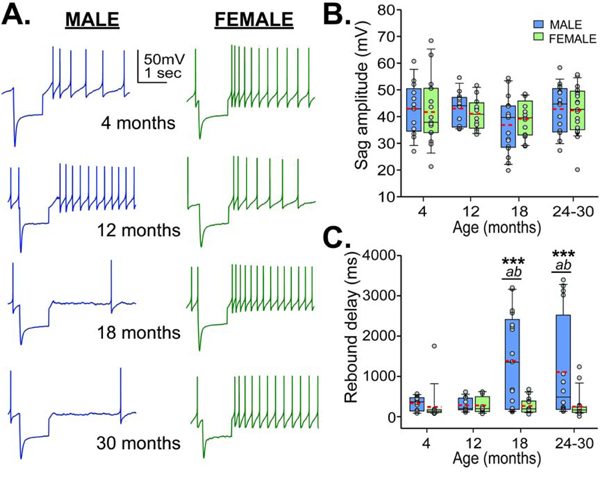
Sag and post inhibitory rebound spiking across ages. A. Representative traces of hyperpolarizing current injections at 4, 12, 18, and 24–30 months from males (left, blue) and females (right, green). Box and whisker plots show sag amplitude (B) across ages (males, blue; females, green) and time delay until rebound spike (C). Red dashed lines show means. Bonferroni corrected t-test: *** males and females differ, p<0.001; a – 18 and 24 month males differ from 12 month males, p<0.01; b – 18 and 24 month males differ from 4 month males, p<0.02
3.5. PINK1 and PARK2 expression increases with age in the SN, primarily in males
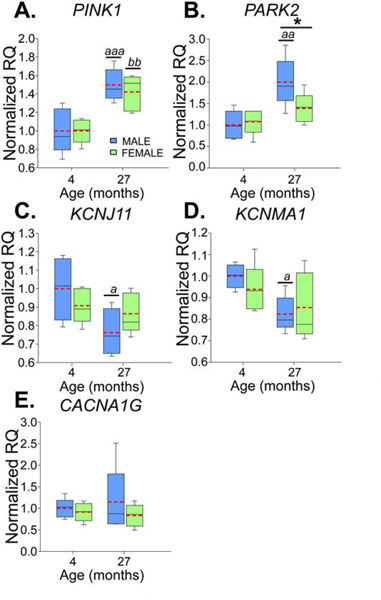
To test if alterations in mitochondrial function and calcium signaling play a role in the deficits in firing activity observed in males with age and the differences with females, qPCR was performed to evaluate the expression of mRNA for selected ion channel and Parkinson’s-related genes: PARK2, PINK1, KCNJ11, CACNA1G and KCNMA1. SN tissue was harvested from 4-month-old (n=5 males; n=5 females) and 27-month-old mice (n=5 males; n=4 females). In old mice, PINK1 expression increased by 46% (age: F1,16=29.636, p<0.001; Fig 5A) and PARK2 expression increased by 63% (age: F1,16=13.268, p=0.002, Fig 5B). Additionally, for PARK2 there was a trend toward an age-sex interaction (F1,16=3.667, p=0.074), with males having an increase in expression of 85% (Bonferroni t-test, p=0.001) and females having an increase of 39% (Bonferroni t-test, p=0.24). Decreases were observed in expression of mRNA for the K-ATP channel subunit (KCNJ11) between young and old animals (F1,15=5.717, p=0.03, Fig 5C). Overall there was a 14.8% decrease in K-ATP mRNA expression between young and old mice, with males showing a 23.8% decrease (Bonferroni t-test, p=0.014) and females showing no change (Bonferroni t-test, p=0.6). A decrease in mRNA for BK channels (KCNMA1, “big” conductance calcium-activated potassium channels) was observed with age (F1,16=7.286, p=0.016, Fig 5D). Comparing young and old animals alone, there was a 13.4% decrease with age; 17.6% in males (Bonferroni t-test, p=0.02) and 8.9% in females (Bonferroni t-test, p=0.23). No significant differences were detected in expression of T-type calcium channel subunit mRNA (CACNA1G, Fig 5E).
Figure 5.
mRNA expression differences between males (blue) and females (green) with age for PTEN induced putative kinase (PINK1, A), parkin (PARK2, B), K-ATP channels (KCNJ11, C), BK channels (KCNMA1, D), and T-type calcium channels (CACNA1G, E). Box and whisker plots show relative quantification (RQ) for male (blue) and female (green) mice aged 4 and 27 months. Red dashed lines indicate means. Bonferroni t-test: * males differ from females, p<0.05; old males differ from young males, a - p<0.05, aa - p<0.005, aaa - p<0.001; old females differ from young females, bb - p<0.005
4. DISCUSSION
Here we report the first in-depth characterization of dopamine neuron firing parameters across the lifespan in both male and female mice. We observed that dopamine neurons from male mice begin showing signs of deterioration in firing activity by 18 months of age, while dopamine neurons from females were more resilient throughout the lifespan. Impairments observed in dopamine neurons from males included decreases in firing rate, disrupted pacemaking, increased propensity to enter depolarization block, and prolonged delays in rebound spiking. Impairments in rebound spiking were correlated with decreased firing rate and pacemaking deficits, suggesting disruption in a similar underlying process (Fig S3). Interestingly, enhanced depolarization block was observed in the majority of cells from males 18 months and older. Accompanying those changes were increases in PINK1 and PARK2 mRNA expression with age, suggesting altered response to mitochondrial stress. Overall, we interpret the age-dependent alterations in dopamine neuronal properties in the males as possibly contributing a vulnerability to further insults rather than a disease process per se, while dopamine neurons from females show resilience to these effects of normal aging.
4.1. Sex divergence in dopamine neuron action potential generation with age
Firing properties of SN dopamine neurons have only been lightly studied in females and not at all studied in aged female mice. We identified robust phenotypical differences in SN dopamine neuron firing properties between aged male and female mice. At the youngest age tested (4 months), SN dopamine neurons from females exhibited a larger spike height and faster action potential kinetics compared to males. Indeed, the only age-related change in neuronal firing properties that occurred in females was a linear decrease in spike height and kinetics. In contrast, by 24 months it appeared that individual cells from males segregated into two populations that could be designated as “aged-healthy” and “aged-impaired.” A similar phenomenon has been widely documented with other phenotypes in rodents over 24 months of age (Menard et al., 2015). To obtain a sense of how dopaminergic decline may affect motor behavior, we also conducted an open field locomotor experiment during the dark cycle for mice of in all age groups (Fig S4). We observed a modest decrease in spontaneous locomotor activity across ages with the only sex effect being higher basal activity in young females, which has been previously observed (Van Swearingen et al., 2013). Fischer et al. (2016) previously reported similar decreases in male and female animals tested in the dark phase, however in the light phase they only observed a decrease in locomotor activity in males at 28 months of age. Therefore, while declining firing properties of dopamine neurons from males were not accompanied by a sexually divergent effect in spontaneous locomotion, they could instead reflect an intrinsic vulnerability to additional insults.
In the context of Parkinson’s disease, evidence from lesion-based models suggest biological differences in SN dopamine neurons for males compared with females. Administration of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) or 6-OHDA (6-hydroxydopamine), toxins which target mitochondrial respiration (Glinka et al., 1997; Przedborski et al., 2004), produce a greater loss of SN dopamine neurons in males compared with females (for review see Gillies et al., 2014). This is also true in aged rats, where following toxin exposure females experience far less of a loss of dopamine neurons compared with males (Tamas et al., 2005). Genes escaping Xlinked inactivation have failed to account for the decreased susceptibility of females to Parkinson’s (Sharma et al., 2017), but ovarian steroid studies have accounted for the resilience of females to oxidative damage. In whole brain samples, young females show lower levels of oxidative stress than males, which was absent in animals that were either ovariectomized or aged (Gaignard et al., 2015). Indeed, human clinical studies have shown an increased risk of developing Parkinson’s after hysterectomy with removal of both ovaries (Benedetti et al., 2001). While mice do not formally enter menopause like humans, by 18 months of age they are reproductively senescent and have very low plasma levels of estrogen and progesterone (Lu et al., 1979). As the degeneration of dopamine neurons is a slow process, it is possible the months of cycling hormones can afford females with the prolonged protection needed to delay the decline of dopamine neuron function.
4.2. Vulnerability of SN dopamine neurons and risk of Parkinson’s disease
Previous work from our lab using a progressive mouse model of Parkinson’s disease through impairment of mitochondrial function found that deficits in multiple electrical parameters, including pacemaking, precede morphological decline and cell death (Branch et al., 2016; Lynch et al., 2018). Aging is thought to create a pre-Parkinsonian environment in dopamine cells through oxidative damage, mitochondrial dysfunction, and inflammation, thereby promoting neurodegeneration following exposure to additional insults such as toxins or infections (Collier et al., 2017). Dopamine neurons of the SN are noteworthy for their vulnerability to oxidative stress (Duda et al., 2016) as opposed to dopamine neurons of the VTA, which are more resistant to neurodegeneration (Damier et al., 1999). As autonomous pacemakers, SN dopamine cells depend on large, well-controlled calcium oscillations for their continuous firing activity (Puopolo et al., 2007), which creates great energy demands for the cell (Duda et al., 2016). Mitochondrial damage produced, for example, through age-related accumulation in oxidative stress can increase intracellular calcium concentrations and yield adverse consequences such as excitotoxicity, apoptosis, and activation of detrimental enzymatic cascades (Mattson, 2007).
The increase we observed in PINK1 and PARK2 expression, particularly in males, could represent an adaptive response to age-related mitochondrial stress and possibly elevated intracellular calcium. PINK1 encodes PTEN induced putative kinase 1, which accumulates on damaged mitochondria and works synergistically with parkin (encoded by PARK2), the E3 ubiquitin ligase, to facilitate mitochondrial degradation (Srivastava, 2017). Notably, mutations in PINK1 and PARK2 have been identified in autosomal recessive forms of early onset Parkinson’s disease, causing accumulation of damaged mitochondria and leading to dopamine neuron degeneration. Moreover, high intracellular calcium levels can induce an increase in PINK1 expression (Gomez-Sanchez et al., 2014). Interestingly, PINK1 mRNA is upregulated in SN dopamine neurons in brains of old male human subjects, but not in females (Cantuti-Castelvetri et al., 2007).
4.3. Participation of ion channels in the effects of age in males
Disruption in dopamine neuron function leading to increased intracellular calcium has been long hypothesized to underlie decrements associated with aging and neurodegenerative disorders (Alzheimer’s Association Calcium Hypothesis, 2017). A continuing rise in calcium with increasing age would not only affect intracellular signaling cascades but would also alter ion channel function, thus producing disruptions in firing activity. From our cross-sectional sampling, action potential waveforms from male mice showed decreased spike height and prolonged kinetics at the oldest ages tested. However, half-width showed a biphasic effect across the lifespan in males, with decreased half-width by 12 months followed by a progressive increase with age. Our qPCR data indicated a decrease in transcript for Kcnma1, the gene that codes for the “big” conductance calcium activated potassium (BK) channel subunit in aged males. Studies in CA1 pyramidal cells found increased activity of calcium activated potassium channels, due to increased intracellular calcium with age, resulting in increased kinetics and afterhyperpolarization (Gant and Thibault, 2009). However, SN dopamine neurons are far more sensitive to calcium than many neuron types due to their low calcium buffering capacity (Duda et al., 2016). Given that signs of oxidative stress are already present in dopamine neurons from animals no older than 1 month (Guzman et al., 2018), it is possible that increases in intracellular calcium may be present at 12 months and affect action potential parameters. Antagonism of BK channels widens action potential half width but causes no change in F-I slope (Kimm et al., 2015), which is consistent with our observations in males aged 18 months and older. In addition, any lack of functioning of channels contributing to the afterhyperpolarization may also promote depolarization block, due to a lack of recovery from sodium channel inactivation (Yu et al., 2014).
We also observed decreased mRNA expression of Kcnj11, the gene that codes for Kir6.2/K-ATP channels. These channels act as energy sensors in dopamine neurons and have been proposed to play a role in the development of neurodegeneration, however their activation has paradoxically been shown to increase bursting (Schiemann et al., 2012). Dopamine cells from aged males also exhibited delayed rebound spiking, which could indicate decreased function of T-type calcium channels (Evans et al., 2017). However, we did not detect a change in mRNA transcript levels for Cacna1g, the gene coding for Cav3.1 subunits. We previously showed decreased nimodipine-sensitive (presumably L-type calcium) currents in aged male mice, but also similarly no difference in mRNA transcript levels (Branch et al., 2014). It is therefore important to remember that increased mRNA expression does not necessarily indicate parallel changes in protein expression or function. In addition to transcription, ion channel function can be regulated at the level of translation, by membrane trafficking, or functionally by (for instance) elevated levels of intracellular calcium (Simms and Zamponi, 2014). Multiple examples of altered Kcnma1 mRNA levels tracking with BK channel protein expression and/or currents have been described in the periphery (Singh et al., 2011; Kanthesh et al., 2013; Li et al., 2019) as well as centrally in the superchiasmatic nucleus, where both Kcnma1 mRNA and BK channel currents diurnally cycle and are ~2-fold higher at night (Pitts et al., 2006). Additionally, prolonged hyperglycemia is known to reduce both Kcnj11 mRNA and insulin release ~2–3 fold in a pancreatic beta cell line (Vasu et al., 2013). So while altered mRNA expression is a strong indication of system dysregulation, future studies will be needed to determine the precise effects of age and sex on protein expression, membrane channel density, and function.
5. CONCLUSION
The results presented here demonstrate the existence of functional electrophysiological differences in SN dopaminergic neurons between male and female mice in response to aging. The results provide converging evidence that SN dopamine neurons in males are more vulnerable to the effects of aging, paralleling epidemiological evidence indicating that males have nearly twice the incidence of Parkinson’s as females.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Julie Crane and Ben Fowler from the imaging core facility at the Oklahoma Medical Research Foundation for their assistance with the immunostaining and confocal imaging. We also thank Dr. Ishita Parikh for isolating the RNA and preparing cDNA from the tissue samples. This work was supported by National Institute on Aging/NIH grants R01 AG052606, P30 AG050911, and T32 AG052363, as well as funds from the Presbyterian Health Foundation and the Oklahoma Center for Adult Stem Cell Research (OCASCR). The authors declare no conflicts of interest.
This work was supported by an R01, P50, and T32 grants from NIH/NIA, as well as grants from the Presbyterian Health Foundation and the Oklahoma Center for Adult Stem Cell Research (OCASCR). This manuscript is the result of original research and has not been published previously and is not under consideration (in whole or in a part) for publication elsewhere, although a pre-print has been posted on bioRxiv. Animal procedures were approved in advance by the IACUC at the Oklahoma Medical Research Foundation. All authors have viewed and approved the contents of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VERIFICATION
The authors declare no potential conflicts of interest.
REFERENCES
- Alzheimer’s Association Calcium Hypothesis, W., 2017. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 13(2), 178–182 e117. [DOI] [PubMed] [Google Scholar]
- Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D, 2000. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 55(9), 1358–1363. [DOI] [PubMed] [Google Scholar]
- Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA, 2001. Hysterectomy, menopause, and estrogen use preceding Parkinson’s disease: an exploratory case-control study. Mov Disord 16(5), 830–837. [DOI] [PubMed] [Google Scholar]
- Branch SY, Chen C, Sharma R, Lechleiter JD, Li S, Beckstead MJ, 2016. Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson’s Disease. J Neurosci 36(14), 4026–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch SY, Sharma R, Beckstead MJ, 2014. Aging decreases L-type calcium channel currents and pacemaker firing fidelity in substantia nigra dopamine neurons. J Neurosci 34(28), 9310–9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG, 2007. Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis 26(3), 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH, 2017. Aging and Parkinson’s disease: Different sides of the same coin? Mov Disord 32(7), 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker AD, 1997. The regulation of motor control: an evaluation of the role of dopamine receptors in the substantia nigra. Rev Neurosci 8(1), 55–76. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM, 1999. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122 (Pt 8), 1437–1448. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM, 2006. Epidemiology of Parkinson’s disease. Lancet Neurol 5(6), 525–535. [DOI] [PubMed] [Google Scholar]
- Duda J, Potschke C, Liss B, 2016. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. J Neurochem 139 Suppl 1, 156–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour MA, Woodhouse A, Amendola J, Goaillard JM, 2014. Non-linear developmental trajectory of electrical phenotype in rat substantia nigra pars compacta dopaminergic neurons. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RC, Zhu M, Khaliq ZM, 2017. Dopamine Inhibition Differentially Controls Excitability of Substantia Nigra Dopamine Neuron Subpopulations through T-Type Calcium Channels. J Neurosci 37(13), 3704–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KE, Hoffman JM, Sloane LB, Gelfond JA, Soto VY, Richardson AG, Austad SN, 2016. A cross-sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated. Aging (Albany NY) 8(10), 2370–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE, 2007. Mouse Models in Aging Research, in: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL (Eds.), The Mouse in Biomedical Research (Second Edition). pp. 637–672. [Google Scholar]
- Fuxe K, Hokfelt T, Olson L, Ungerstedt U, 1977. Central monoaminergic pathways with emphasis on their relation to the so called ‘extrapyramidal motor system’. Pharmacol Ther B 3(2), 169–210. [DOI] [PubMed] [Google Scholar]
- Gaignard P, Savouroux S, Liere P, Pianos A, Therond P, Schumacher M, Slama A, Guennoun R, 2015. Effect of Sex Differences on Brain Mitochondrial Function and Its Suppression by Ovariectomy and in Aged Mice. Endocrinology 156(8), 2893–2904. [DOI] [PubMed] [Google Scholar]
- Gant JC, Thibault O, 2009. Action potential throughput in aged rat hippocampal neurons: regulation by selective forms of hyperpolarization. Neurobiol Aging 30(12), 2053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z, 2014. Sex differences in Parkinson’s disease. Front Neuroendocrinol 35(3), 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y, Gassen M, Youdim MB, 1997. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl 50, 55–66. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez R, Gegg ME, Bravo-San Pedro JM, Niso-Santano M, Alvarez-Erviti L, Pizarro-Estrella E, Gutierrez-Martin Y, Alvarez-Barrientos A, Fuentes JM, Gonzalez-Polo RA, Schapira AH, 2014. Mitochondrial impairment increases FL-PINK1 levels by calciumdependent gene expression. Neurobiol Dis 62, 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Onn SP, 1989. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9(10), 3463–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, Kondapalli J, Schumacker PT, Surmeier DJ, 2018. Systemic isradipine treatment diminishes calciumdependent mitochondrial oxidant stress. J Clin Invest 128(6), 2266–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O, 1975. Parkinson’s disease and its chemotherapy. Biochem Pharmacol 24(10), 1061–1065. [DOI] [PubMed] [Google Scholar]
- Kanthesh BM, Sandle GI, Rajendran VM, 2013. Enhanced K(+) secretion in dextran sulfate-induced colitis reflects upregulation of large conductance apical K(+) channels (BK; Kcnma1). Am J Physiol Cell Physiol 305(9), C972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm T, Khaliq ZM, Bean BP, 2015. Differential Regulation of Action Potential Shape and Burst-Frequency Firing by BK and Kv2 Channels in Substantia Nigra Dopaminergic Neurons. J Neurosci 35(50), 16404–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ding H, Zhang P, Li Z, Liu Y, Wang P, 2019. Attenuated BK channel function promotes overactive bladder in a rat model of obesity. Aging (Albany NY) 11(16), 6199–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS, 1979. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod 21(1), 193–203. [DOI] [PubMed] [Google Scholar]
- Lynch WB, Tschumi CW, Sharpe AL, Branch SY, Chen C, Ge G, Li S, Beckstead MJ, 2018. Progressively disrupted somatodendritic morphology in dopamine neurons in a mouse Parkinson’s model. Mov Disord 33, 1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold CA, Wronowski B, Du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, Freeman WM, 2017. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation 14(1), 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masser DR, Bixler GV, Brucklacher RM, Yan H, Giles CB, Wren JD, Sonntag WE, Freeman WM, 2014. Hippocampal subregions exhibit both distinct and shared transcriptomic responses to aging and nonneurodegenerative cognitive decline. J Gerontol A Biol Sci Med Sci 69(11), 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 2007. Calcium and neurodegeneration. Aging Cell 6(3), 337–350. [DOI] [PubMed] [Google Scholar]
- Menard C, Quirion R, Vigneault E, Bouchard S, Ferland G, El Mestikawy S, Gaudreau P, 2015. Glutamate presynaptic vesicular transporter and postsynaptic receptor levels correlate with spatial memory status in aging rat models. Neurobiol Aging 36(3), 1471–1482. [DOI] [PubMed] [Google Scholar]
- Moisan F, Kab S, Mohamed F, Canonico M, Le Guern M, Quintin C, Carcaillon L, Nicolau J, Duport N, Singh-Manoux A, Boussac-Zarebska M, Elbaz A, 2016. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J Neurol Neurosurg Psychiatry 87(9), 952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J, 2002. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci 22(4), 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG, 2006. Daily rhythmicity of large-conductance Ca2+ activated K+ currents in suprachiasmatic nucleus neurons. Brain Res 1071(1), 54–62. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C, Vila M, 2004. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr 36(4), 375–379. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Raviola E, Bean BP, 2007. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci 27(3), 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, Zaghloul KA, Schneider G, Liss B, Roeper J, 2012. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci 15(9), 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kaut O, Pavlova A, Frohlich H, Ahmad A, Schmitt I, El-Maarri O, Oldenburg J, Wullner U, 2017. Skewed X-chromosome inactivation and XIST locus methylation levels do not contribute to the lower prevalence of Parkinson’s disease in females. Neurobiol Aging 57, 248 e241–248 e245. [DOI] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW, 2014. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82(1), 24–45. [DOI] [PubMed] [Google Scholar]
- Singh SK, O’Hara B, Talukder JR, Rajendran VM, 2012. Aldosterone induces active K⁺ secretion by enhancing mucosal expression of Kcnn4c and Kcnma1 channels in rat distal colon. Am J Physiol Cell Physiol 302(9), C1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, 2017. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes (Basel) 8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas A, Lubics A, Szalontay L, Lengvari I, Reglodi D, 2005. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav Brain Res 158(2), 221–229. [DOI] [PubMed] [Google Scholar]
- Van Swearingen AE, Walker QD, Kuhn CM, 2013. Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology (Berl) 225(3), 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasu S, McClenaghan NH, McCluskey JT, Flatt PR, 2013. Cellular responses of novel human pancreatic β-cell line, 1.1B4 to hyperglycemia. Islets 5(4): 170–177. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP, 1989. Brain dopamine and reward. Annu Rev Psychol 40, 191–225. [DOI] [PubMed] [Google Scholar]
- Yu N, Tucker KR, Levitan ES, Shepard PD, Canavier CC 2014. Implications of cellular models of dopamine neurons for schizophrenia. Prog Mol Transl Sci 123, 53–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.