Abstract
Purpose/ Background:
Venlafaxine is a commonly used antidepressant with both serotonergic and noradrenergic activity. There are concerns it may prolong the corrected QT interval (QTc), and older adults may be at higher risk for this adverse effect, especially at higher dosages of the medication.
Methods/Procedures:
In this secondary analysis of a prospective clinical trial, we measured changes in QTc and other ECG parameters in 169 adults aged ≥ 60 with a major depressive disorder treated acutely with venlafaxine extended release (XR) up to 300 mg daily. We examined the relationship of venlafaxine dosage and ECG parameters, as well as the relationship between serum levels of venlafaxine and ECG parameters.
Findings/Results:
Venlafaxine exposure was not associated with an increase in QTc. Heart rate increased with venlafaxine treatment, while the PR interval shortened, and QRS width did not change significantly. QTc change from baseline was not associated with venlafaxine dosages or serum concentrations. Age, sex, cardiovascular comorbidities, and depression remission status did not predict changes in QTc with venlafaxine.
Implications/Conclusions:
Venlafaxine treatment did not prolong QTc or other ECG parameters, even in high dosages in older depressed adults. These findings indicate that venlafaxine does not significantly affect cardiac conduction in most older patients.
Keywords: Venlafaxine, QTc, ECG, Serum levels, Late life depression
Introduction
Venlafaxine is a serotonin-norepinephrine reuptake inhibitor (SNRI) recommended for1 treating late-life depression because of its effectiveness2, low cost, ease of use with once-daily dosing in the extended release (XR) form, and minimal drug-drug interactions3. The effectiveness of venlafaxine has been attributed partly to noradrenergic reuptake inhibition4, particularly at dosages higher than 150 mg/day4,5. Venlafaxine’s noradrenergic activity, however, has led to concerns regarding potential cardiovascular adverse effects, such as causing hypertension and orthostatic hypotension6,7. Adverse effects of antidepressants such as venlafaxine may be especially troubling for older adults who have age-related physiological changes, multiple comorbid medical conditions, and a high risk of complications from polypharmacy8.
Concerns have been raised regarding the effect of SNRIs and selective serotonin reuptake inhibitors (SSRIs) on electrocardiogram (ECG) changes, particularly the corrected QT interval (QTc). Several antidepressants have been postulated to prolong the QTc, which is a risk factor for sudden cardiac death9 by increasing the risk for Torsades de Pointes (TdP), an arrhythmia that can lead to ventricular fibrillation and cardiac arrest. Increased QTc interval has become a proxy marker for drug-induced risk of sudden cardiac death10 and several studies have demonstrated that some specific antidepressants prolong the QTc11,12. While some studies have cause concern regarding SSRIs such as citalopram and QTc prolongation13, there is question regarding the true risk for resulting ventricular arrhythmias or death14. The risk of QTc prolongation and its clinical significance from therapeutic doses of different antidepressants remains a controversial topic.
Preclinical evaluations of venlafaxine have not indicated that venlafaxine likely causes drug-induced QTc prolongation. Blockade of the hERG channel, a potassium channel that when mutated causes long QT syndrome and arrhythmias15, is now used in drug development as an indicator for potential QTc-prolonging risk. Venlafaxine is not a potent inhibitor of hERG, and only demonstrates blockade at levels significantly higher than in therapeutic range, with an IC50 of 28μM (effective concentration of 659 nM)16. While the hERG IC50 of desvenlafaxine has not been published to our knowledge, the product monograph for Pristiq, desvenlafaxine’s brand name, indicates that desvenlafaxine did not inhibit hERG up to desvenlafaxine concentrations of 195 μM.
Clinical evaluations of venlafaxine, however, have been less conclusive. Some case reports and studies have suggested that venlafaxine may prolong the QTc6,17–19, while others have not found this effect20–25. Variations in venlafaxine metabolism due to age, genetic variants in CYP2D626,27, and co-prescribed medications may contribute to these discrepant findings. For example, genetic variations leading to high serum venlafaxine and O-desmethylvenlafaxine concentrations have been postulated to contribute to possible cardiac toxicity in overdose28. A study from post-mortem samples suggested that peripheral serum concentrations of venlafaxine correlate with cardiac serum or tissue concentrations of venlafaxine and O-desmethylvenlafaxine29. Therefore, it is important to examine not only the dosage but also the serum concentration of venlafaxine and O-desmethylvenlafaxine when assessing its effect on ECG.
While studies of the impact of antidepressants on ECG changes typically focus on the QTc due to its association with sudden cardiac death, other clinically-meaningful ECG parameters can also be altered by antidepressants, such as PR interval prolongation and QRS widening with tricyclic antidepressants30. Therefore, we performed a secondary analysis from a previously published clinical trial31 to examine the relationship between measured venlafaxine and O-desmethylvenlafaxine serum concentration and pre- and post-treatment ECGs in older adults with a major depressive disorder (MDD) treated with venlafaxine 150–300 mg/day over the course of up to 14 weeks. We hypothesized that if venlafaxine is associated with changes in the QTc, QRS, or PR intervals, these changes would correlate with both dosages and serum concentrations.
Materials and Methods
Data analyzed in this study was obtained during the open-label phase of the multicenter (Pittsburgh, St. Louis, and Toronto) clinical trial “Incomplete Response in Late Life Depression: Getting to Remission” (IRL-GRey). The protocol for this NIMH-funded clinical trial has been described previously (ClinicalTrials.gov identifier: NCT00892047)31. Briefly, participants were aged 60 years or older, had a diagnosis of a current major depressive episode via the Structured Clinical Interview for Axis I DSM-IV Disorders32, and had a Montgomery-Asberg Depression Scale (MADRS)33 score ≥ 15. We included only participants who had both baseline and follow-up ECGs and those with evidence of venlafaxine intake as determined by serum measurements. We excluded those already using venlafaxine at the start of study. Open treatment with venlafaxine XR was started in participants following a protocolized titration schedule. The initial dosage was 37.5 mg/day, titrated over 2 weeks to a target dosage of 150 mg/day in increments of 37.5–75 mg during intervals of at least 3 days. After 6 weeks, venlafaxine XR was further titrated to a maximum of 300 mg/day in participants who did not demonstrate remission of their depressive symptoms (defined as a MADRS score ≤ 10). Overall, participants received 12–14 weeks of venlafaxine open-label treatment.
Measures
Clinical characteristics including medical comorbidities were assessed at a baseline visit via medical record diagnoses and quantified with the Cumulative Illness Rating Scale for Geriatrics (CIRS-G)34. Serious adverse events were recorded, including death and any admissions to a hospital.
ECGs were obtained at a pretreatment visit and at the final visit of the open-label treatment phase. The St Louis and Pittsburgh sites used the MAC 5500 HD (General Electric, Chicago, IL) ECG equipment, which calculates the QTc using Bazett’s formula, QTcB = QT / √RR. At the Toronto site, ECGs were obtained with either a Philips Pagewriter Touch Cardiograph (Philips, Amsterdam, Netherlands), which uses the same QTcB formula, or a Hewlett Packard Pagewriter Xli (Hewlett Packard, Palo Alto, CA), which uses Fridericia’s formula; QTcF = QT/ (RR)1/3. For this analysis, we recalculated all the QTc intervals using Fridericia’s formula based on the QT and RR output. We considered a QTc to be “prolonged” if it was ≥ 450 ms for males and ≥ 460 ms for females35.
ECG parameters as generated by the ECG machines were entered into a database throughout the study. Due to the secondary nature of our analysis, the ECGs themselves were not available for review. Therefore, qualitative descriptions such as abnormal rhythms or bundle branch blocks were not available for all ECGs and were not included in analyses or exclusion criteria. Included participants were, however, required to have both pre- and post-treatment ECGs in order to account for baseline differences in ECG characteristics that could impact ECG interval calculations, allowing for isolation of venlafaxine-specific effects.
A blood sample was collected at the final visit. Due to logistical reasons and the secondary nature of this analysis, the samples were not required to be drawn at a specific dosage interval, but were obtained at the same visit as the ECG for the majority of participants. Serum specimens were obtained and kept at – 80 °C until the serum venlafaxine and O- desmethylvenlafaxine concentrations were determined. Standards were from Cerilliant (Round Rock, TX), and reagents (acids, bases, solvents) were Fluka brand (Honeywell, Morristown, NJ). Venlafaxine and its active metabolite O-desmethylvenlafaxine were quantified by LC-MS/MS after solvent extraction at alkaline pH; 100 μL of sample or standard was combined with 50 μL of deuterated internal standard mix (venlafaxine-D6, 100 ng/mL in 30% methanol) and 50 μL of 1N ammonium hydroxide (pH 9.5–10), then extracted with 3 mL of ethyl acetate. Extract was acidified with 50 μL of HCl-methanol, dried under nitrogen and reconstituted in 200 μL of mobile phase (55 % of 10 mM ammonium acetate pH 5, 10 % of 0.1 % formic acid, and 35 % acetonitrile). A 20 μL injection was made onto a Kromasil C18 column (50 mm × 4.6 mm, 3.5 μm) on an Acquity UPLC system (Waters, Milford, MA) connected to a TSQ Quantum Ultra (AM) triple quadrupole mass spectrometer (ThermoFisher Scientific, Waltham, MA). Analytes were separated in a 5.5 min, isocratic run with a flow rate of 300 μl/min. Retention times were 2.1 min for venlafaxine and 1.7 min for O-desmethylvenlafaxine. Venlafaxine concentrations were assessed against a calibration curve linear from 1 to 1000 ng/mL with a 1/x weighting (assay CV < 10%). The following transitions were monitored in positive ESI mode: Venlafaxine: 278→121, Venlafaxine-D6: 284→121, O-desmethylvenlafaxine: 264→107. Because venlafaxine and O-desmethylvenlafaxine are both psychoactive compounds, the sum of the two concentrations was used in the analysis as the total active moiety.
The blood sample and the ECGs were typically obtained during the same visit. For logistical reasons, this was not possible for a minority of participants. In these cases, the ECG and blood sample were taken at similar times of the day. The blood samples were not required to be taken at venlafaxine trough time (i.e. immediately before the daily dose) because the serum levels were not being used as therapeutic drug monitoring. Therefore, the venlafaxine total active moiety serum levels were not compared with the reference ranges that have been suggested for potential venlafaxine therapeutic drug monitoring (e.g. 195–400 ng/ml). Similarly, serum levels were therefore not compared to dosages, but only to ECG changes.
Statistical Analysis
Analyses were performed using SAS 9.4. (SAS Institute Inc., Cary, North Carolina) and Tableau 2019.3 (Tableau Software, Seattle, WA). All reported p-values are 2-tailed, with significance level for all tests set a priori at p ≤ 0.05. ECG parameters included the heart rate, QTc, QT, QRS, and PR intervals, collected pre- and post- treatment. In a first set of analyses, we assessed the relationship between the change in QTc and the venlafaxine dosages. We also separately assessed the relationship between QTc change and serum venlafaxine, serum O-desmethylvenlafaxine, and serum venlafaxine plus O-desmethylvenlafaxine concentrations. We repeated these analyses for the heart rate and the other ECG parameters. For these analyses, paired t-tests were used to compare the QTc, heart rate, and other ECG parameters pre- and post- treatment with venlafaxine XR. The association between changes in these measures and venlafaxine XR dosages or serum concentrations was assessed with Pearson correlation coefficients. Linear regression models were applied to estimate the strength of these associations before and after controlling for relevant covariates (age, sex, and history of cardiac disease as measured with the CIRS-G score of the cardiac system).
We also examined the mean change in QTc from pre-post treatment with venlafaxine in those whose depression did remit with treatment (i.e., with a MADRS score of 10 or less post-treatment), compared to those whose depression did not remit. This comparison was conducted because depression has been demonstrated to be related to cardiovascular health36. We used a restricted maximum likelihood-based mixed model for repeated measures (MMRM) including timepoints, subgroups, and group-by-timepoint interaction. When the group-by-timepoint interaction was significant, contrasts were used to compare the mean change over time between two subgroups based on the least squares (LS) means for the subgroup’s group-by-timepoint interaction in the MMRM-model.
Results
Of the 468 participants who started open-label venlafaxine XR, 274 (59%) did not have complete ECG data at baseline and when completing 12–14 weeks of venlafaxine XR treatment: 91 due to participant withdrawal or administrative reasons for not completing this phase of the study, 147 because they responded to venlafaxine XR and did not complete the final ECG due to the logistics of the study, and 35 were non-responders but did not have both pre- and post-treatment ECG. There was also one death by suicide leading to incomplete ECG data. Of the remaining 194 participants with complete ECG data, 25 participants were excluded for either being on venlafaxine at baseline or having a venlafaxine serum concentration of zero (suggesting complete lack of adherence). The remaining 169 participants were included in this analysis and their demographic and clinical characteristics are described in Table 1.
Table 1.
Demographic and clinical characteristics of the 169 participants.
| Frequency (%) | Mean (± SD, range) | ||
|---|---|---|---|
| Female | 104 (61.5%) | Age | 67.9 (± 6.5, 60–91) |
| Race | BMI | 30.2 (± 6.7, 17.3–57.4) | |
| White | 150 (88.8%) | Cumulative Illness Rating Scale for Geriatrics*- Count | 5.9 (± 2, 1–11) |
| Black | 15 (8.9%) | Cumulative Illness Rating Scale for Geriatrics*- Total Score | 9.4 (± 4, 1–21) |
| Asian | 3 (1.8%) | Cumulative Illness Rating Scale for Geriatrics*- Cardiac System Score | 0.66 (± 1.1, 0–3) |
| Native American | 1 (0.6%) | Baseline Montgomery-Asberg Depression Scale** | 27.9 (± 5.7, 15–43) |
| Comorbid pre-existing diagnoses | Final Montgomery-Asberg Depression Scale** | 18.9 (± 9.8, 0–39) | |
| Heart disease | 28 (16.6%) | Venlafaxine serum concentration | 225 (± 228, 8 – 1546) |
| Arrhythmia | 14 (8.3%) | O-desmethylvenlafaxine serum concentration | 344 (± 150, 16 – 741) |
| Hypertension | 88 (53.3%) | Total active moiety serum concentration*** | 569 (± 268, 62 – 1820) |
| Diabetes | 21 (12.7%) | ||
| Use of concomitant QTc-prolonging medication† | 69 (40.8%) |
Cumulative Illness Rating Scale for Geriatrics includes 14 organ systems rated on 0–4 for severity, count = number of organ systems (out of 14), total score = sum of severity scores of 14 systems (with score of 0 to 4 for each system and a total of 0–56).
Montgomery-Asberg Depression Scale, depression score out of 60 with higher scores indicating more severe depression (e.g.. ≥ 18 moderate depression, ≥ 35 severe depression).
Sum of venlafaxine and O-desmethylvenlafaxine serum concentration.
Participants that used a QTc-prolonging medication throughout the entire trial (at pre and post-treatment ECGs).
ECG Parameters
QTc significantly shortened by a mean (SD) of 3.7 ms (±16.7; 95% CI: 1.2–6.3) from pre- to post-treatment (Table 2). Using our QTc cutoffs of ≥ 450 ms for males and ≥ 460 ms for females, there were 13 participants (7.7%) with a prolonged QTc pre-treatment and 8 (4.7%) post-treatment. The QT interval also significantly shortened by a mean of 11.1 ms (±25.9; 95% CI: 7.2–15.0) while the heart rate significantly increased by a mean of 3.6 beats per minute (±9.7; 95% CI: 2.1–5.0). The PR interval also significantly shortened by a mean of 3.4 ms (±11.1; 95% CI: 1.7–5.1). The QRS width did not change significantly. Changes in QTc were not associated with venlafaxine dosages (r= −0.12, p=0.13) (Figure 1). Pre and post treatment QTc values for males and females are demonstrated in Supplemental Figure S1.
Table 2.
ECG parameters before (pre) and after (post) treatment with venlafaxine XR.
| Pre-Treatment | Post-treatment | Change from pre- to post-treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECG parameter | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | t-value | p-value |
| QTc | 416.5 | 25.4 | 361.9 | 493.2 | 412.8 | 23.8 | 346.0 | 486.9 | − 3.74 | 16.69 | −2.91 | 0.0041 |
| QT | 400.9 | 39.3 | 308 | 496 | 389.8 | 33.1 | 309 | 474 | − 11.11 | 25.88 | −5.58 | <0.0001 |
| QRS width | 90.7 | 18.5 | 65 | 180 | 90.8 | 20.1 | 60 | 192 | 0.16 | 6.71 | 0.31 | 0.7573 |
| PR | 167.5 | 23.6 | 110 | 258 | 164.1 | 24.2 | 110 | 278 | − 3.39 | 11.07 | −3.98 | 0.0001 |
| Heart rate | 69.1 | 13.0 | 45 | 107 | 72.6 | 11.8 | 48 | 106 | 3.55 | 9.74 | 4.74 | <0.0001 |
Figure 1.
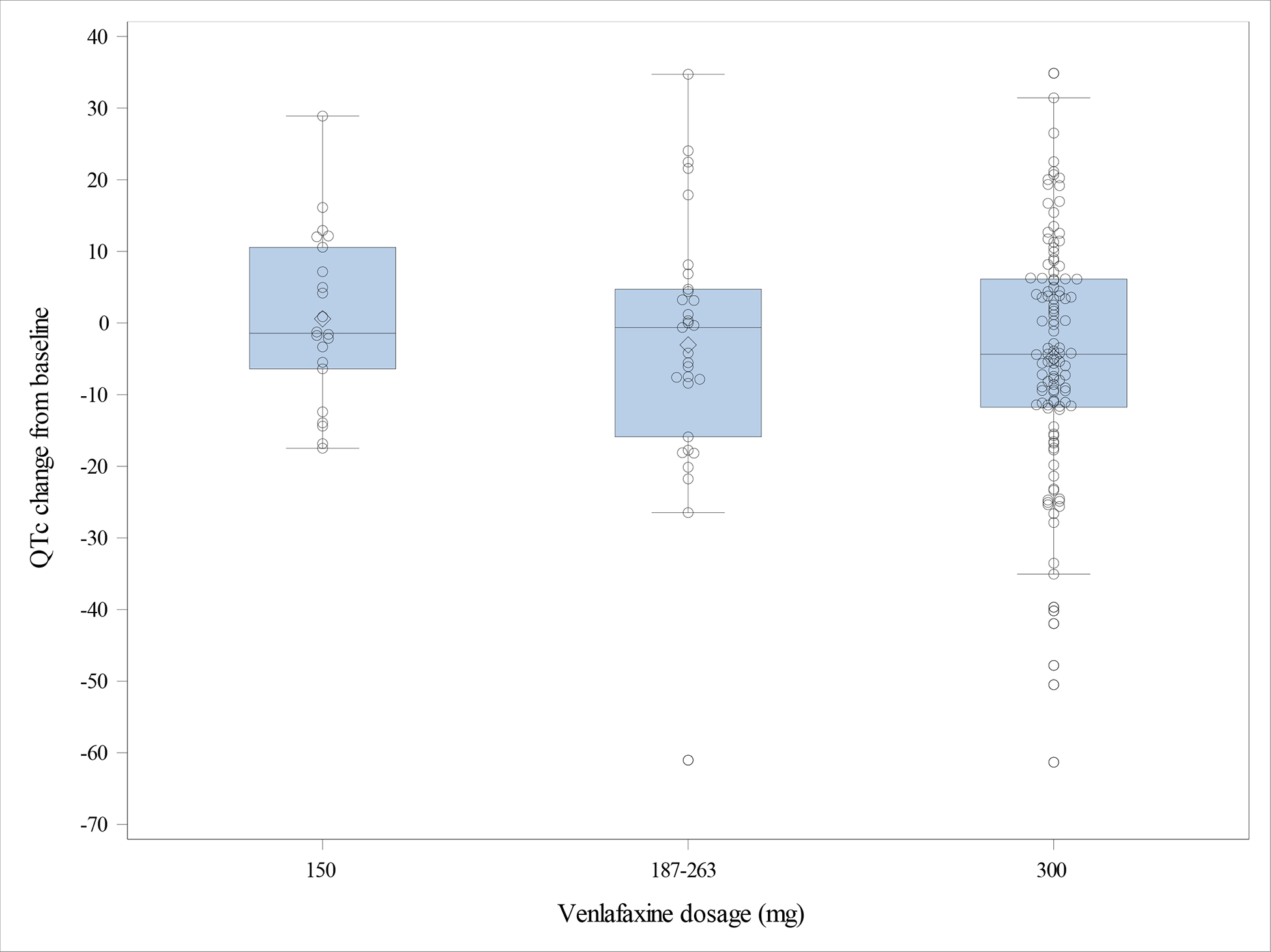
Change in QTc from pre- to post-treatment as a function of final venlafaxine dosage – Low: 150 mg/day; moderate: 187–263 mg/day; high: 300 mg/day.
QTc change was not linearly correlated with serum concentration of venlafaxine plus O-desmethylvenlafaxine (Figure 2). QTc change was also not linearly correlated with serum venlafaxine or serum O-desmethylvenlafaxine concentrations examined separately (data not shown). While total active moiety concentrations demonstrated statistically significant correlations with increasing heart rate (r2 = 0.05, p = 0.003) and decreasing QT interval (r2 = 0.04, p = 0.009), the associated R-squared values are extremely small, suggesting these correlations are not clinically significant. There was no linear association between any serum concentrations and changes in QRS or PR intervals. The regression analysis (including age, sex, and history of cardiac disease) did not show an independent association between QTc and total active moiety serum concentration (F= −0.60, df=1, p=0.44), and none of the potential confounders were significant mediators.
Figure 2.

Serum total active moiety concentration (venlafaxine + O-desmethylvenlafaxine) and changes in ECG parameters. A. QTc interval. B. QT interval. C. QRS interval. D. Heart rate. E. PR interval.
The subgroup analysis examined whether depression remission status impacted venlafaxine’s effect on QTc by comparing remitters and non-remitters. There was no impact of depression remission status on QTc prolongation, as there was no statistical difference (p=0.47) in QTc pre to post-treatment change between depression remitters (−2.1ms, SE=2.61, p=0.0045) and non-remitters (−4.26ms, SE=1.48, p=0.42).
Potential Cardiac-Related Adverse Events
There was one completed suicide during the open-label phase of the study. There were no sudden cardiac deaths, other deaths, or known occurrences of Torsades de Pointes. One participant had an arrhythmia following a syncopal event while on venlafaxine XR. Evaluation revealed PVCs and recurrent supraventricular tachycardia. Electrophysiology study revealed the existence of an atrio-ventricular accessory pathway, which was successfully ablated. Beta blocker medication changes closely preceding the event was also thought to be a contributing factor. The participant’s QTc on admission was 484 (baseline measured 442), and venlafaxine was tapered and discontinued as a precautionary measure by the cardiology team. There were no ventricular arrhythmias.
Discussion
To our knowledge, this is the first study that prospectively evaluated the relationship between venlafaxine dosage and serum concentrations with ECG changes in a geriatric population. We had three main findings. First, the mean QTc did not increase and actually shortened slightly after 12–14 weeks of treatment with venlafaxine XR. Second, venlafaxine and total active moiety serum concentrations did not correlate with QTc changes. Third, there was no effect of sex, cardiac disease, or depression remission status on these results. These findings suggest that venlafaxine can be used in a group of older depressed patients at relatively high risk for QTc prolongation because of age, depression status, and co-morbid cardiovascular disease.
In our study, even high dosage of venlafaxine XR (i.e., 300 mg/day) did not prolong the QTc. Severity of cardiac comorbidities (based on CIRS-G scores of the cardiac system) did not predict the effect of venlafaxine on the QTc. This negative finding is consistent with some previous studies of older patients with stable medical conditions taking venlafaxine21,25. In a previous small study, two cases of prolonged QTc were reported (defined as ≥ 440 ms in this study) among 40 older depressed patients, for an incidence of 5% (2/40)6: QTc increased from 403 ms to 456 ms in one patient and from 433 ms to 469 ms in another one. Different criteria can be used to define a clinically meaningful QTc prolongation; in the current study we used a QTc ≥ 450 for males, ≥ 460 for females35. However, more lenient criterion requiring an increase from baseline by 60 ms and QTc > 480 ms has also been used in studies examining QTc prolongation in antidepressants37. Neither of these two patients from the earlier study would have met these more lenient criteria for QTc prolongation. Additionally, in this earlier study Bazett’s formula was used to calculate QTc, and this equation is known to overcorrect at higher heart rates35.
An important aspect of the current study is the measurement of venlafaxine and O-desmethylvenlafaxine serum concentrations to account for differences in venlafaxine adherence and metabolism. Polymorphisms in CYP2D6, the major metabolizer of venlafaxine to O-desmethylvenlafaxine38 have been shown to impact serum venlafaxine concentrations in a geriatric population27. A theoretical concern with venlafaxine is that poor metabolizers might have high enough venlafaxine concentrations to cause QTc prolongation. Thus, even if there is no overall increase in QTc, a few patients may still be at risk because the same dosage can yield various concentrations. Our study does not support this concern: despite a 10-fold variation in its range, serum venlafaxine concentration was not correlated with QTc changes (see Figure 2A). Participants with higher serum concentrations of venlafaxine, O-desmethylvenlafaxine, or total active moiety concentrations did not have an increase in QTc interval from baseline.
In a recent study, Hefner et al reported a positive correlation between serum venlafaxine concentrations and QTc in 34 older patients18. There were two methodologic differences that may account for our different results. First, our study was prospective, allowing us to account for baseline QTc. Second, it is possible these different results are due the use of Fridericia’s formula, whereas Hefner et al did not report the QTc formula they used. The specific QTc formula is important in determining true cardiac risk, particularly within drugs that increase the heart rate. Venlafaxine has been shown to increase the heart rate in a small but statistically significant manner24,39,40, as it did in our study. Thus, using the appropriate QTc formula is particularly important with venlafaxine to avoid over-correcting the QT interval. The most commonly used formula (Bazett’s, QTcB = QT/ √RR) is known to overcorrect at high and low heart rates, causing inappropriately elevated QTc interval values and Fridericia’s formula (QTcF = QT/(RR)1/3) is superior in this regard41. Other studies have demonstrated that evaluation of drug-induced QTc changes is affected by the choice of QTc formula42. These methodologic differences may also account for some previous reports that venlafaxine prolongs the QTc both at therapeutic doses17 and in overdose19. However, the effect in overdose has been recently questioned, underscoring the importance of which QTc formula is used43. Typically, prospective studies have not observed an effect of venlafaxine on the QTc interval21–25.
There are important limitations to our study. First, the study did not have a placebo group, limiting our ability to control for clinical variables that may confound the relationship between QTc, venlafaxine dosage, and serum concentrations. Although we accounted for baseline QTc interval and some potential confounders in the regression model, there could be additional confounders that were not investigated. Second, there was no positive control in the study design (e.g. participants prescribed a known QTc-prolonging compound, such as moxifloxacin), so we are unable to conclude with certainty that this study would detect drug-induced QTc prolongation. Third, there was a large number of participants excluded for our analysis, including participants who discontinued participation in the clinical trial (19%) and those that continued with the trial but did not have both pre-treatment and post-treatment ECGs (39%). This significant exclusion percentage could have impacted our ability to accurately detect QTc changes in a more generalizable population. The majority of participants who continued in the trial but were missing a follow-up ECG were in remission from depression at the end of venlafaxine trial, which could have introduced a confound for those who were not included in our analysis.
A fourth limitation is that complete ECG descriptions were unavailable in two-thirds of participants due to the secondary nature of our analysis. Thus, we were unable to account for rhythms such as atrial fibrillation or bundle branch blocks, which can impact the accuracy of QTc calculations. However, some studies suggest that the QTc Fridericia formula we used is an appropriate formula for determining QTc in atrial fibrillation44,45. Finally, the generalizability of the results is limited to older individuals in their 60’s and 70’s whose medical comorbidities were stable enough to have participated in and completed our clinical trial. The results cannot be directly applied to very old patients (i.e., those in their 80’s or 90’s) or those with comorbidities such as unstable (e.g., ischemic) cardiac disease. However, the inclusion of patients with a history of cardiac disease allows for a greater level of generalizability than many previous studies.
While our study was not powered to detect rare events (e.g., with an incidence of less than 1%), it is still important to note that there were no cases of Torsades de Pointes or sudden cardiac death with venlafaxine dosages up to 300 mg/day and serum active moiety concentrations up to 1800 ng/ml in a relatively high-risk population. This has important implications for clinical practice, as our findings suggest that venlafaxine could be initiated in older adults without ECG monitoring. In an era when telehealth is becoming increasingly utilized, and often necessary, these results suggest that venlafaxine could be initiated remotely without the need for an in-person baseline or follow-up ECG. Overall, our results suggest that therapeutic doses of venlafaxine do not prolong the QTc in older patients with late-life depression, or if it does, it is very rare.
Supplementary Material
Conflicts of Interest and Source of Funding:
Funding: National Institute of Mental Health, UPMC Endowment in Geriatric Psychiatry, Taylor Family Institute for Innovative Psychiatric Research, National Center for Advancing Translational Sciences, Center for Brain Research in Mood Disorders, and the Campbell Family Mental Health Research Institute.
This study was supported mainly by the National Institute of Mental Health (R01 MH083660 and P30 MH90333 to University of Pittsburgh, R01 MH083648 to Washington University, and R01 MH083643 to University of Toronto). Additional funding was provided by the UPMC Endowment in Geriatric Psychiatry, the Taylor Family Institute for Innovative Psychiatric Research (at Washington University), the Washington University Institute of Clinical and Translational Sciences grant (UL1 TR000448) from the National Center for Advancing Translational Sciences (NCATS), and the Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health, Toronto. Pfizer contributed venlafaxine extended release capsules for this study.
Disclosures:
EJL has received research support from Takeda, Lundbeck, Janssen, the Taylor Family Institute for Innovative Psychiatric Research, the Barnes-Jewish Foundation, the Patient-Centered Outcomes Research Institute, and the McKnight Brain Research Foundation. He has received consulting fees from Janssen and Jazz Pharmaceuticals.
JPM has received research support from the Patient-Centered Outcomes Research Institute.
JFK has received medication supplies for investigator-initiated studies from Pfizer and Indivior. He receives compensation for work on the editorial boards of the Journal of Clinical Psychiatry and American Journal of Geriatric Psychiatry. He has received an honoraria for developing and presenting an educational webinar (disease, not product-focused) for Otsuka.
CFR receives compensation from the American Association for Geriatric Psychiatry as editor of the American Journal of Psychiatry and receives royalty income for intellectual property (the Pittsburgh Sleep Quality Index) as well as occasional honoraria for giving invited lectures and consultation.
DMB has received research support from CIHR, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. He is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He also receives in-kind equipment support from Magventure for two investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior.
BHM reports research financial support from Brain Canada, CAMH Foundation, Canadian Institutes for Health Research, and Patient Centered Outcomes Research Institute; nonfinancial support from Pfizer (medication for an NIH-funded trial), Eli Lilly (medication and matching placebo for an NIH-funded trial), Capital Solution Design LLC (software for a trial funded by the CAMH Foundation), and HAPPYneuron (software for a trial funded by Brain Canada).
For the remaining authors, there were no relevant disclosures.
Contributor Information
J. Philip Miller, Division of Biostatistics, Washington University School of Medicine, St Louis, MO, USA
Timothy W. Smith, Cardiovascular Division, Department of Medicine, Washington University School of Medicine, St Louis, MO, USA
Yasmina Saade, Department of Psychiatry, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Benoit H. Mulsant, Centre for Addiction and Mental Health, Department of Psychiatry, University of Toronto, Toronto, ON, Canada
References
- 1.Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30:517–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staab JP, Evans DL. Efficacy of venlafaxine in geriatric depression. Depression and anxiety. 2000;12:63–8. [DOI] [PubMed] [Google Scholar]
- 3.Owen JR, Nemeroff CB. New antidepressants and the cytochrome P450 system: focus on venlafaxine, nefazodone, and mirtazapine. Depress Anxiety. 1998;7 Suppl 1:24–32. [PubMed] [Google Scholar]
- 4.Marshe VS, Maciukiewicz M, Rej S, et al. Norepinephrine Transporter Gene Variants and Remission From Depression With Venlafaxine Treatment in Older Adults. Am J Psychiatry. 2017;174:468–75. [DOI] [PubMed] [Google Scholar]
- 5.Debonnel G, Saint-Andre E, Hebert C, et al. Differential physiological effects of a low dose and high doses of venlafaxine in major depression. Int J Neuropsychopharmacol. 2007;10:51–61. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EM, Whyte E, Mulsant BH, et al. Cardiovascular changes associated with venlafaxine in the treatment of late-life depression. Am J Geriatr Psychiatry. 2006;14:796–802. [DOI] [PubMed] [Google Scholar]
- 7.Wathra R, Mulsant BH, Thomson L, et al. Hypertension and Orthostatic Hypotension with Venlafaxine Treatment in Depressed Older Adults. Journal of Psychopharmacology, 2020, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algra A, Tijssen JG, Roelandt JR, et al. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. [DOI] [PubMed] [Google Scholar]
- 10.Roden DM. Drug-induced prolongation of the QT interval. New England Journal of Medicine. 2004;350:1013–22. [DOI] [PubMed] [Google Scholar]
- 11.Beach SR, Kostis WJ, Celano CM, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry. 2014;75:e441–9. [DOI] [PubMed] [Google Scholar]
- 12.Jasiak NM, Bostwick JR. Risk of QT/QTc prolongation among newer non-SSRI antidepressants. Ann Pharmacother. 2014;48:1620–8. [DOI] [PubMed] [Google Scholar]
- 13.Drye LT, Spragg D, Devanand DP,et al. Changes in QTc interval in the citalopram for agitation in Alzheimer’s disease (CitAD) randomized trial. PLoS One. 2014;9:e98426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zivin K, Pfeiffer PN, Bohnert AS, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry. 2013;170:642–50. [DOI] [PubMed] [Google Scholar]
- 15.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. [DOI] [PubMed] [Google Scholar]
- 16.Fossa AA, Gorczyca W, Wisialowski T, et al. Electrical alternans and hemodynamics in the anesthetized guinea pig can discriminate the cardiac safety of antidepressants. J Pharmacol Toxicol Methods. 2007;55:78–85. [DOI] [PubMed] [Google Scholar]
- 17.Bavle A Venlafaxine induced QTc interval prolongation in a therapeutic dose. Asian J Psychiatr. 2015;16:63–4. [DOI] [PubMed] [Google Scholar]
- 18.Hefner G, Hahn M, Hohner M, et al. QTc Time Correlates with Amitriptyline and Venlafaxine Serum Levels in Elderly Psychiatric Inpatients. Pharmacopsychiatry. 2019;52:38–43. [DOI] [PubMed] [Google Scholar]
- 19.Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unterecker S, Pfuhlmann B, Kopf J, et al. Increase of Heart Rate and QTc by Amitriptyline, But Not by Venlafaxine, Is Correlated to Serum Concentration. J Clin Psychopharmacol. 2015;35:460–3. [DOI] [PubMed] [Google Scholar]
- 21.Emul M, Dalkiran M, Samim S, et al. The influences of depression and venlafaxine use at therapeutic doses on atrial conduction. J Psychopharmacol. 2009;23:163–7. [DOI] [PubMed] [Google Scholar]
- 22.Gasto C, Navarro V, Marcos T, et al. Single-blind comparison of venlafaxine and nortriptyline in elderly major depression. J Clin Psychopharmacol. 2003;23:21–6. [DOI] [PubMed] [Google Scholar]
- 23.Mbaya P, Alam F, Ashim S, et al. Cardiovascular effects of high dose venlafaxine XL in patients with major depressive disorder. Hum Psychopharmacol. 2007;22:129–33. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph RL, Derivan AT. The safety and tolerability of venlafaxine hydrochloride: analysis of the clinical trials database. J Clin Psychopharmacol. 1996;16:54S–9S; discussion 9S-61S. [DOI] [PubMed] [Google Scholar]
- 25.Schweizer E, Weise C, Clary C, et al. Placebo-controlled trial of venlafaxine for the treatment of major depression. J Clin Psychopharmacol. 1991;11:233–6. [PubMed] [Google Scholar]
- 26.Zhan Y-Y, Liang B-Q, Wang H, et al. Effect of CYP2D6 variants on venlafaxine metabolism in vitro. Xenobiotica. 2016;46:424–9. [DOI] [PubMed] [Google Scholar]
- 27.Whyte EM, Romkes M, Mulsant BH, et al. CYP2D6 genotype and venlafaxine-XR concentrations in depressed elderly. Int J Geriatr Psychiatry. 2006;21:542–9. [DOI] [PubMed] [Google Scholar]
- 28.Vinetti M, Haufroid V, Capron A, et al. Severe acute cardiomyopathy associated with venlafaxine overdose and possible role of CYP2D6 and CYP2C19 polymorphisms. Clin Toxicol (Phila). 2011;49:865–9. [DOI] [PubMed] [Google Scholar]
- 29.Mikkelsen CR, Jornil JR, Andersen LV, et al. Distribution of Eight QT-Prolonging Drugs and Their Main Metabolites Between Postmortem Cardiac Tissue and Blood Reveals Potential Pitfalls in Toxicological Interpretation. J Anal Toxicol. 2018;42:375–83. [DOI] [PubMed] [Google Scholar]
- 30.Glassman AH, Bigger JT. Cardiovascular effects of therapeutic doses of tricyclic antidepressants. A review. Arch Gen Psychiatry. 1981;38:815–20. [DOI] [PubMed] [Google Scholar]
- 31.Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First M, Spitzer R, Gibbon M, et al. Structured clinical interview for Axis I DSM-IV disorders. New York: Biometrics Research; 1994. [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 34.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. [DOI] [PubMed] [Google Scholar]
- 35.Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–50. [DOI] [PubMed] [Google Scholar]
- 36.Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14:145–55. [DOI] [PubMed] [Google Scholar]
- 37.Thase ME, Larsen KG, Reines E, et al. The cardiovascular safety profile of escitalopram. Eur Neuropsychopharmacol. 2013;23:1391–400. [DOI] [PubMed] [Google Scholar]
- 38.Fogelman SM, Schmider J, Venkatakrishnan K, et al. O- and N-demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacology. 1999;20:480–90. [DOI] [PubMed] [Google Scholar]
- 39.Nemeroff CB, Thase ME. A double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatients. J Psychiatr Res. 2007;41:351–9. [DOI] [PubMed] [Google Scholar]
- 40.Sir A, D’Souza RF, Uguz S, et al. Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. J Clin Psychiatry. 2005;66:1312–20. [DOI] [PubMed] [Google Scholar]
- 41.Vandenberk B, Vandael E, Robyns T, et al. Which QT Correction Formulae to Use for QT Monitoring? J Am Heart Assoc. 2016, June 17; 5(6):e003264 (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noordam R, van den Berg ME, Niemeijer MN, et al. Assessing Prolongation of the Heart Rate Corrected QT Interval in Users of Tricyclic Antidepressants: Advice to Use Fridericia Rather Than Bazett’s Correction. J Clin Psychopharmacol. 2015;35:260–5. [DOI] [PubMed] [Google Scholar]
- 43.Isbister GK. Electrocardiogram changes and arrhythmias in venlafaxine overdose. Br J Clin Pharmacol. 2009;67:572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dash A, Torado C, Paw N, et al. QT correction in atrial fibrillation - Measurement revisited. J Electrocardiol. 2019;56:70–6. [DOI] [PubMed] [Google Scholar]
- 45.Musat DL, Adhaduk M, Preminger MW, et al. Correlation of QT interval correction methods during atrial fibrillation and sinus rhythm. Am J Cardiol. 2013;112:1379–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


