Acute asthma exacerbations account for 750,000 pediatric emergency department (ED) visits, 200,000 hospitalizations and 5.2 billion in excess healthcare costs in the US annually.1 Current ED treatment of acute exacerbations is based on severity at initial presentation as determined by clinical scoring tools, which are limited by subjectivity and poor association with actual airway function.2 Traditional methods to objectively assess lung function, including spirometry and peak flow, are not feasible or reproducible in younger children.3,4 Peak flow also tends to underestimate worsening lung function.5 Consequently, ED treatment is variable leading to inefficient use of asthma therapies, prolonged ED length of stay, and unnecessary hospitalizations.6
Oscillometry, a noninvasive method to assess lung function, has potential as an objective marker of acute asthma exacerbation severity and treatment response in the ED setting. Oscillometry is performed using a handheld device that applies external oscillatory signals at the mouth during normal tidal breathing to measure respiratory system impedance.7 Studies have shown oscillometry to be feasible and reproducible in pre-school aged children in the outpatient setting, but evaluations of oscillometry in the ED are limited.8,9 The aim of our study was to assess the feasibility and responsiveness of oscillometry, using tremoFlo c-100 (Thorasys, Montreal, QC, Canada), in the ED management of children with acute asthma exacerbations. TremoFlo c-100, or airwave oscillometry, is a new device that uses a combination of sine waves at multiple discrete frequencies to generate volume perturbations to measure respiratory impedance. TremoFlo is also portable and fast, making it ideal for clinical application in the ED setting.
We performed a feasibility study of 20 children aged 4–18 years presenting to an urban tertiary-care children’s hospital ED for an acute asthma exacerbation. Children with co-morbid illnesses were excluded. Vital signs, Pulmonary Asthma Score (15-point clinical exacerbation severity score), and respiratory impedance by oscillometry were collected three times: 1) prior to receiving any treatment (in 19 of 20), 2) after completion of initial therapies (combined nebulized albuterol/ipratropium bromide and oral corticosteroids); and 3) 2 hours post-initial therapies or at disposition. Treating physicians were blinded to oscillometry measurements to minimize bias in management decisions.
Respiratory impedance, as measured by the tremoFlo c-100, has two components, resistance (R) and reactance (X), that are measured at a range of oscillation frequencies (5–37 Hz and 7–41 Hz). Low frequency R (5 or 7 Hz) indicates total airway resistance, and low frequency X indicates lung tissue stiffness that has been correlated with peripheral airway obstruction.7 We performed 3–5 oscillometry measurements at each timepoint; the mean values from three acceptable measurements were recorded. An acceptable assessment includes three measurements with a low frequency R coefficient of variation (CV), a marker of test reproducibility, ≤15%.9 Respiratory impedance data were normalized using z-scores from previously published reference equations based on height and gender.10
We initially obtained 20 second measurements at a frequency range of 5–37 Hz. In order to improve the feasibility of oscillometry in the ED setting, we reduced the measurement duration to 13 seconds for the last 7 participants. We also changed the frequency range to 7–41 Hz to evaluate for less interference in children with higher respiratory rates. We analyzed respiratory impedance data at 11 Hz for consistency, as this frequency was measured in all participants.
Participants were 9.3±3.9 years, 50% male and 30% African American. Initial oxygen saturation was 95.1±2.6 percent, initial PAS was 8.4±2.1 indicating moderate exacerbation severity, ED length of stay was 4.2±1.6 hours, 35% required continuous albuterol, and 15% were admitted. There were 31.0 (15.8, 40.0) minutes from time of ED triage to initial oscillometry assessment, 50.5±19.6 minutes between timepoints 1 and 2, and 118.3±35.3 minutes between timepoints 2 and 3.
The majority (75%) of participants successfully completed the initial oscillometry assessment. Two participants were unable to perform the initial oscillometry assessment due to severity of exacerbations; both were able to complete subsequent assessments. Three completed the initial assessment but did not have acceptable measurements with CV > 15%. The mean number of measurements required at each assessment was 3.9±0.8. Across all timepoints, low frequency R CV was 10.9 (6.6,15.4). There was no difference in percentage of children able to successfully complete the initial oscillometry assessment in those evaluated with 20 second/5–37 Hz compared to 13 second/7–41 Hz measurements (p=1.00). No adverse events were reported.
There were statistically significant differences in z-scores for R11 and X11 between timepoints 1 and 2 and 1 and 3, but not between timepoints 2 and 3 (Figure 1). After completion of initial therapies, R decreased and X increased: ΔR11= −16% (−37%,−6%) and ΔX11=48% (18%,64%), indicating improved lung function. Between the second and third timepoints, ΔR11= −5% (−14%,7%) and ΔX11=−10% (−19%,14%). There was also a significant difference in PAS between timepoints 1 and 2 (difference −1.8; p=0.001), but not between timepoints 2 and 3. There was no significant correlation between PAS and either R11/X11 at any timepoint, or percent change in PAS and R11/X11 between timepoints.
Figure 1.
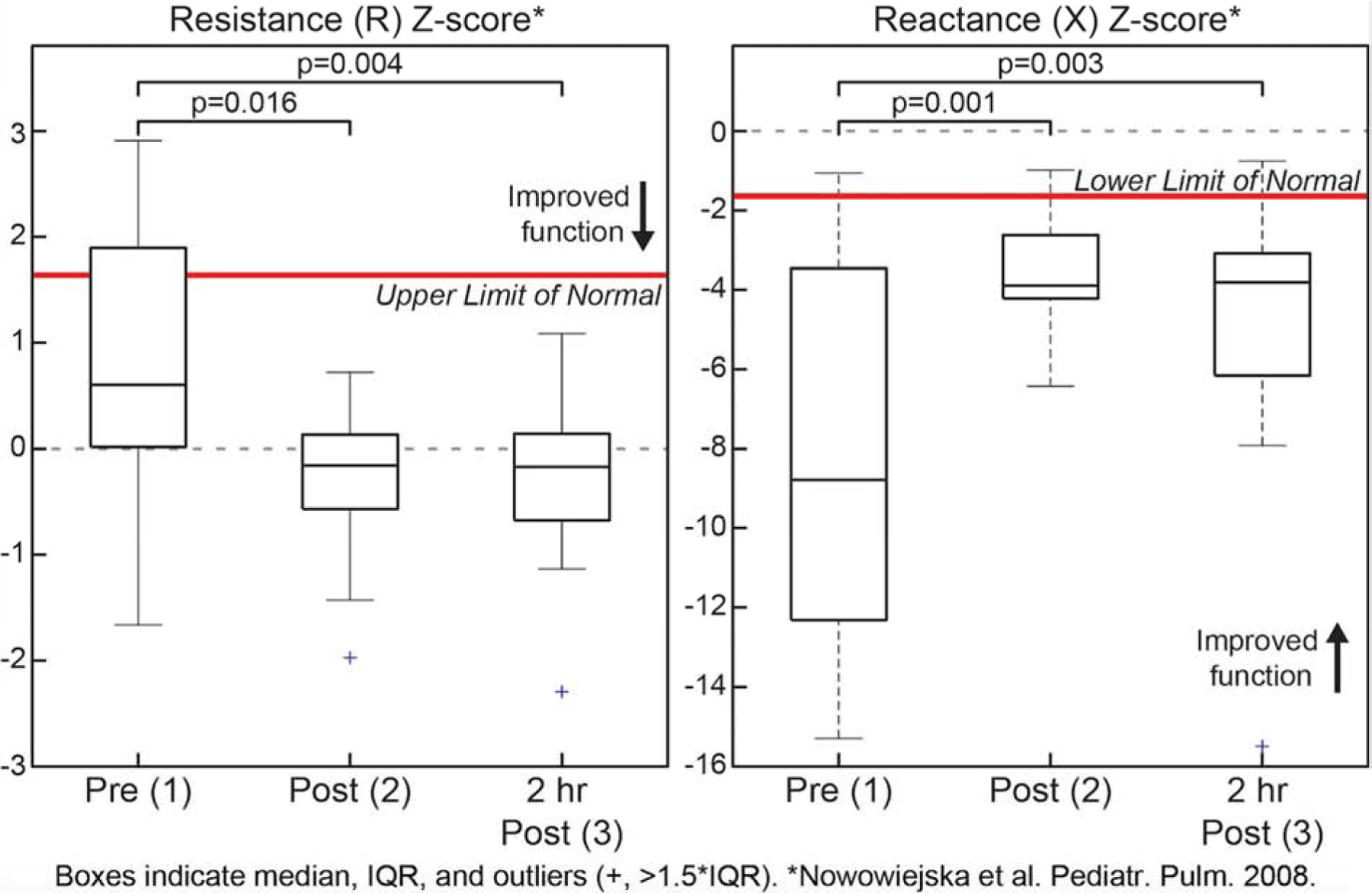
Change in Z-score of R11 and X11 across ED course timepoints.
The results of our study demonstrate that oscillometry is feasible, responsive, and safe in children with acute asthma exacerbations in the ED. To our knowledge, this study is the first to investigate the use of the tremoFlo c-100 in the ED assessment of children with acute exacerbations. We made adaptations, which improved the feasibility of performing oscillometry assessments in the ED and did not affect the quality of measurements. We observed considerable improvement in raw and z-score values of R and X after initial ED asthma therapies. The change in z-score X was greater than R suggesting that reactance may be a better marker of airway obstruction in children with acute exacerbations. Interestingly, there was no significant change in R/X during the two hours after completion of initial therapies suggesting the majority of change in lung function occurs in the first hour, which may contribute to expedited ED decision-making. Lastly, we found no correlation between the PAS and either R/X, but we speculate that the clinical severity score and oscillometry measurements may represent different domains of an acute asthma exacerbation.
Limitations of our study include that interpretation of respiratory impedance values is relative to normative values in a healthy population and reference equations have not yet been developed for the tremoFlo. In addition, due to the small sample size we were unable to draw meaningful inferences regarding the association of oscillometry measures and ED clinical outcomes. A larger trial is needed to establish the validity of oscillometry in determining ED clinical outcomes. Oscillometry may be a valuable bedside measure of lung function to direct ED treatment and disposition decisions and reduce the clinical morbidity associated with asthma.
Funding source:
Supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations:
- ED
Emergency department
- FOT
Forced oscillation technique
- R
Resistance
- X
Reactance
- PAS
Pulmonary Asthma Score
- CV
Coefficient of variation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: S.J. Szefler has consulted for Astra Zeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Propeller Health, Regeneron and Sanofi and has received research support from the National Institutes of Health, the National Heart, Lung and Blood Institute, the National Heart, Lung and Blood Institute, Propeller Health, the Colorado Cancer, Cardiovascular and Pulmonary Disease Program.
Contributor Information
Nidhya Navanandan, Section of Pediatric Emergency Medicine, Children’s Hospital Colorado, University of Colorado, School of Medicine.
Katharine L. Hamlington, Pediatric Pulmonary and Sleep Medicine, Children’s Hospital Colorado, University of Colorado, School of Medicine.
Rakesh D. Mistry, Section of Pediatric Emergency Medicine, Children’s Hospital Colorado, University of Colorado, School of Medicine.
References
- 1.Camargo CA, Rachelefsky G, Schatz M. Managing asthma exacerbations in the emergency department: summary of the National Asthma Education and Prevention Program Expert Panel Report 3 guidelines for the management of asthma exacerbations. J Emerg Med. 2009;37(2 Suppl):S6–S17. [DOI] [PubMed] [Google Scholar]
- 2.Bekhof J, Reimink R, Brand PL. Systematic review: insufficient validation of clinical scores for the assessment of acute dyspnoea in wheezing children. Paediatr Respir Rev. 2014;15(1):98–112. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick MH, Stevens MW, Schultz T, Scribano PV. Difficulty in obtaining peak expiratory flow measurements in children with acute asthma. Pediatr Emerg Care. 2004;20(1):22–6. [DOI] [PubMed] [Google Scholar]
- 4.Arnold DH, Gebretsadik T, Abramo TJ, Hartert TV. Noninvasive testing of lung function and inflammation in pediatric patients with acute asthma exacerbations. J Asthma. 2012;49(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105(2):354–8. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor MG, Saville BR, Hartert TV, Arnold DH. Treatment variability of asthma exacerbations in a pediatric emergency department using a severity-based management protocol. Clin Pediatr (Phila). 2014;53(13):1288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komarow HD, Myles IA, Uzzaman A, Metcalfe DD. Impulse oscillometry in the evaluation of diseases of the airways in children. Ann Allergy Asthma Immunol. 2011;106(3):191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112(2):317–22. [DOI] [PubMed] [Google Scholar]
- 9.Ducharme FM, Davis GM. Measurement of respiratory resistance in the emergency department: feasibility in young children with acute asthma. Chest. 1997;111(6):1519–25. [DOI] [PubMed] [Google Scholar]
- 10.Nowowiejska B, Tomalak W, Radliński J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol. 2008;43(12):1193–7. [DOI] [PubMed] [Google Scholar]


