Abstract
Thyroid transcription factors (TTFs) - NKX2-1, FOXE1, PAX8 and HHEX - regulate multiple genes involved in thyroid development in mice but little is known about TTF regulation of thyroid-specific genes - thyroglobulin (TG), thyroid peroxidase (TPO), deiodinase type 2 (DIO2), sodium/iodide symporter (NIS) and TSH receptor (TSHR) - in adult, human thyrocytes. Thyrotropin (thyroid-stimulating hormone, TSH) regulation of thyroid-specific gene expression in primary cultures of human thyrocytes is biphasic yielding an inverted U-shaped dose-response curve (IUDRC) with upregulation at low doses and decreases at high doses. Herein we show that NKX2-1, FOXE1 and PAX8 are required for TSH-induced upregulation of the mRNA levels of TG, TPO, DIO2, NIS, and TSHR whereas HHEX has little effect on the levels of these thyroid-specific gene mRNAs. We show that TSH-induced upregulation is mediated by changes in their transcription and not by changes in the degradation of their mRNAs. In contrast to the IUDRC of thyroid-specific genes, TSH effects on the levels of the mRNAs for NKX2-1, FOXE1 and PAX8 exhibit monophasic decreases at high doses of TSH whereas TSH regulation of HHEX mRNA levels exhibits an IUDRC that overlaps the IUDRC of thyroid-specific genes. In contrast to findings during mouse development, TTFs do not have major effects on the levels of other TTF mRNAs in adult, human thyrocytes. Thus, we found similarities and important differences in the regulation of thyroid-specific genes in mouse development and TSH regulation of these genes in adult, human thyrocytes.
Keywords: Thyrocytes, Thyrotropin, Thyroid Transcription Factors, Differentiation
Introduction
It is known that four thyroid transcription factors (TTFs) must be expressed simultaneously to allow for the differentiation of stem/precursor cells to thyrocytes during mouse development (Fernandez et al., 2015). These are homeobox protein NKX2-1 (also known as TTF-1), forkhead box protein FOXE1 (also known as TTF-2), paired box protein PAX8 and hematopoietically expressed homeobox protein HHEX. A dominant role has been shown for PAX8 and NKX2-1 for the differentiation of mouse embryonic stem cells in vitro into mature thyroid follicular cells (Antonica et al., 2012). FOXE1 is important for the maintenance of the differentiated thyroid regulating the thyroid specific genes thyroglobulin (TG), thyroperoxidase (TPO) and sodium iodide symporter (NIS) as shown in rat FRTL-5 cells (Ortiz et al., 1997). HHEX is expressed during early thyroid development and in the adult thyroid but its specific role has only been addressed in a few studies and is still not well understood (Fernandez et al., 2015). It has been demonstrated in FRTL-5 cells that HHEX is a repressor of the TG promoter and that it is able to abolish the activating effects of PAX8 and NKX2-1 (Pellizzari et al., 2000).
In contrast to the well-described roles of the TTFs in mouse development, their roles and targets in adult, differentiated thyroid cells are less well elucidated. There have been numerous reports of the effects of deleting individual TTFs on expression of genes in rodent thyroid cell lines, for example, rat FRTL-5 and PC Cl3 cells (reviewed in 2015 Fernandez), and few in human cell lines (Taki et al., 2002,Sahin et al., 2005,Espadinha et al., 2007), but cell lines are not “normal” thyrocytes because cells in permanent culture are transformed. Moreover, there have been few observations in any of these reports of the roles of TTFs in maintenance of the differentiated state and regulation of the levels of thyroid-specific genes including TG, TPO, NIS, deiodinase type 2 (DIO2), and TSH receptor (TSHR) by thyrotropin (thyroid-stimulating hormone, TSH) in human thyroid cells in culture.
In this report, we present findings of the roles of NKX2-1, FOXE1, PAX8 and HHEX in regulation of thyroid-specific gene expression by TSH in normal, adult human thyrocytes in primary culture. We show that upregulation of the levels of thyroid-specific gene mRNAs are mediated by regulation of their transcription by NKX2-1, FOXE1 and PAX8 whereas HHEX differs from the other TTFs by having little effect on the levels of thyroid-specific gene mRNAs.
Materials and Methods
Primary culture of adult, human thyrocytes
We used our previously developed method to obtain primary cultures of human thyrocytes (Neumann et al., 2009,Morgan et al., 2018). Normal human thyroid samples were harvested from patients undergoing surgery for thyroid tumors at the National Institutes of Health Clinical Center under NIDDK Institutional Review Board approved protocols with the patients’ consent. Specimens were kept in Hank’s balanced salt solution (HBSS) (Mediatech, Inc., Manassas, VA) on ice, and isolation of thyrocytes proceeded within four hours after surgery under sterile conditions. Tissue samples were minced into small pieces in a petri dish with ice-cold HBSS followed by centrifugation at 150 g for 5 minutes and removal of the supernatant. The minced tissues were resuspended in sterile HBSS containing 3 mg/mL of collagenase type IV (Gibco™/Thermo Fisher Scientific, Inc., Waltham, MA) and incubated for 30 minutes at 37°C in a rotating incubator. After the digestion, centrifugation was performed to remove the supernatant, and isolated cells were resuspended in 10 mL Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Inc.) with 10% fetal bovine serum (FBS) (HyClone, Cytiva, Marlborough, MA) and plated in tissue culture dishes, and incubated at 37 °C in a humidified 5% CO2 incubator. Primary thyrocytes formed a confluent monolayer within 5-7 days and were maintained in DMEM containing 10% FBS, 100 IU/ml penicillin, and 10μg/mL streptomycin (Life Technologies Corp., Carlsbad, CA). Cultures of thyrocytes are viable, maintain easily measurable levels of thyroid-specific genes and are responsive to TSH for up to 12 passages after their isolation. We used passages 3 to 7 of human thyrocytes for the experiments in this report.
Knockdown of thyroid transcription factors (NKX2-1, FOXE1, HHEX, and PAX8)
Human thyrocytes were seeded in DMEM containing 10% FBS into 12-well culture plates at 1.2×105 cells/well. After 24 hours, cells were transfected with ON-TARGET plus human NKX2-1 (TTF1), FOXE1 (TTF2), HHEX, PAX8, or non-targeting pool siRNA (scrambled siRNA) using DharmaFECT 1 transfection reagent (Dharmacon, Inc., Lafayette, CO) according to the manufacturer’s instructions. Forty-eight hours after transfection, the medium was changed to DMEM containing 0.1% bovine serum albumin (BSA) (MP Biomedicals, Solon, OH) and again transfected with ON-TARGET plus siRNA one day prior to stimulation with bovine thyrotropin (TSH) (Millipore Sigma, Burlington, MA). Cells were stimulated with 0-300 mU/mL (0-5400 nM) bovine TSH for 48 hours and then lysed and subjected to quantitative reverse transcriptase PCR (RT-PCR).
Quantitative reverse transcriptase PCR (RT-PCR)
Thyrocytes were lysed, and total RNA was extracted using RNeasy Mini Kits (Qiagen, Hilden, Germany) followed by reverse transcription to synthesize the first-strand cDNA using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Prepared cDNA was subjected to quantitative RT-PCR in 25 μL reaction volume containing iTaq™ Universal Probe Supermix (Bio-Rad Laboratories, Hercules, CA), primers (Applied Biosystems), and probes for TG (TaqMan assay ID: Hs00174974_m1), TPO (TaqMan assay ID: Hs00892519_m1), DIO2 (TaqMan assay ID: Hs00988260_m1), NIS (TaqMan assay ID: Hs00166567_m1), TSHR (TaqMan assay ID: Hs01053846_m1), NKX2-1 (TaqMan assay ID: Hs00968940_m1), FOXE1 (TaqMan assay ID: Hs00916085_s1), HHEX (TaqMan assay ID: Hs00242160-M1), and PAX8 (TaqMan assay ID: Hs00242160_m1) (Applied Biosystems). Results of quantitative RT-PCR were normalized to GAPDH (Taqman assay ID: Hs99999905_m1) to correct for differences in RNA input and calculated using the 2−ΔΔCT method. Subsequently, results were normalized to each donor’s response to bovine TSH.
Measurement of the rates of degradation of stable mRNA for thyroid-specific genes
Human thyrocytes were seeded at 0.8×105 cells/well in 24-well culture plates in DMEM supplemented with 10% FBS. After one day, the medium was changed to DMEM with 0.1% BSA 24 hours before the experiment. Cells were stimulated with 0, 1, or 100 mU/mL (0, 18, or 1800 nM) of bovine TSH. After 48 hours, cells were treated with 10 μg/mL of actinomycin D (Sigma-Aldrich, Inc., Burlington, MA) and 0, 1, or 100 mU/mL (0, 18, or 1800 nM) of bovine TSH and lysed at each time points (0, 4, 8, and 16 hours). Total RNA was purified, and quantitative RT-PCR was utilized for measurement of TG, TPO, DIO2, and TSHR gene expression.
Measurement of newly transcribed mRNA
Newly synthesized mRNA was measured using incorporation of 4-thiouridine (4sU) (Garibaldi et al., 2017). Human thyrocytes were cultured in 15 cm culture dishes until 80% confluency in DMEM containing 10% FBS. The medium was changed to DMEM containing 0.1% BSA, and 0, 1, or 100 mU/mL (0, 18, or 1800 nM) bovine TSH was added. After 72 hours, cells were treated with 200 μM 4-thiouridine (4sU) Sigma Aldrich, St. Louis, MO, catalog #T4509. After 4 hours the medium was aspirated, and the cells were lysed. Total RNA was purified, and thyroid gene expression was analyzed using quantitative RT-PCR. The 4sU-incorporated newly transcribed mRNA was biotinylated by EZ-Link™ HPDP-Biotin (Thermo Fisher Scientific) per the manufacturer’s directions. Subsequently, 4sU-labeled mRNA was separated from unlabeled mRNA using μMACS and MultiMACS Streptavidin Kits (Miltenyi Biotec Inc., Auburn, CA) catalog #130-074-101 according to the manufacturer’s instructions. Dithiothreitol (DTT) Crystalgen, Commack, N.Y. Catalog # 300-262-001 was used to elute the RNA from the beads. The DTT in the RNA sample was lost from the sample during RNeasy micro kit (Qiagen, Hilden, Germany) purification and followed by quantitative RT-PCR to measure thyroid gene expressions.
Immunoblotting
Thyrocytes (1×105 cells/well) were seeded into 12-well plates in DMEM containing 10%FBS. The media was changed 24 h prior to the experiment to 0.1% BSA-containing DMEM. Cells were stimulated with TSH (10 mU/mL (180 nM)) for 7 days. Cells were washed on ice with cold phosphate-buffered saline and lysed by addition of 1% SDS sample buffer. Lysates were boiled for 10 min, and 40 μL of cell lysate was electrophoresed on a standard 10% SDS-polyacrylamide gel (Bio-Rad Laboratories). After electrophoresis, proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories) and subsequently probed with a rabbit polyclonal TPO antibody (Invitrogen, Carlsbad, CA) and secondary antibody conjugated with infrared fluorophores for detection and quantification on the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE).
Statistical analysis
Statistical analyses were performed using the Prism 8 (version 8.4.0) software (GraphPad software, La Jolla, CA). Results are presented as mean±SE from at least three independent experiments with thyrocytes from three different donors. Student’s t-test was used to assess differences between measurements, and differences were considered statistically significant at P< 0.05.
Results
In previous reports, we observed that TSH regulation of thyroid-specific gene expression in human thyrocytes in primary culture was biphasic causing an inverted U-shaped dose-response curve (IUDRC) (Jang et al., 2020, Morgan et al., 2016). We reported marked increases in TG and NIS protein but were unable to reliably quantify DIO2 protein. Herein, we show 11-fold stimulation of TPO protein by Western Blot (Supplementary Figure 1). mRNA levels of TG, TPO, DIO2 and TSHR were progressively increased at low TSH concentrations and inhibited at high TSH concentrations. Herein we determined the roles of TTFs on TSH regulation of expression of TG, TPO, DIO2, NIS, and TSHR mRNAs. Using specific siRNAs, we knocked down PAX8, NKX2-1, and FOXE1 individually and examined expression of thyroid specific genes (Fig. 1). Knockdown (KD) efficiencies for gene expression of TTFs were 57% for PAX8, 73% for NKX2-1, and 78% for FOXE1. Knockdown of these TTFs did not prevent generation of IUDRCs for regulation of expression of the genes. However, we observed that the KD of PAX8, NKX2-1 and FOXE1 decreased the responses to TSH of TG, TPO, DIO2, NIS, and TSHR but to different degrees. KD of NKX2-1 and FOXE1 inhibited the TSH-induced increase in all four genes. KD of PAX8 inhibited the increase in TG, TPO, DIO2, and NIS but not of TSHR. In contrast, HHEX KD did not inhibit TSH responses of any of the thyroid-specific genes (Supplementary Fig. 2A–E) even though the KD efficiency for HHEX mRNA was 86%.
Figure 1. TSH-stimulated thyroid-specific gene regulation by thyroid transcription factors.
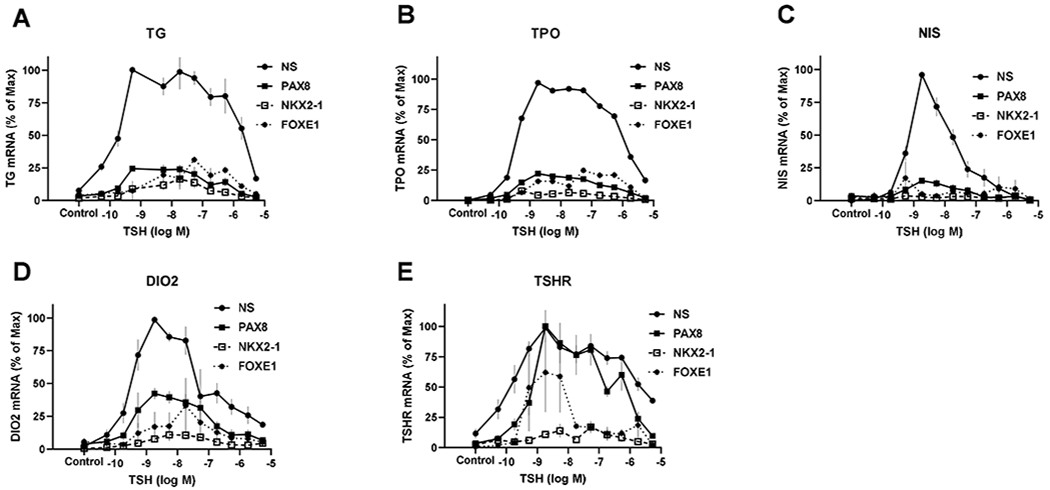
Human thyrocytes were transfected with non-specific (NS) or siRNA targeting PAX8, NKX2-1, and FOXE1 for 48 hours and incubated without (control) or with TSH at the indicated doses for an additional 48 hours. Subsequently, levels of mRNA for (A) thyroglobulin (TG), (B) thyroperoxidase (TPO), (C) sodium/iodide symporter (NIS), (D) iodothyronine deiodinase type 2 (DIO2), and (E) TSH receptor (TSHR) were measured by quantitative RT-PCR. Data are expressed as % of maximum response to TSH±SEM of thyrocytes from three different donors.
We studied whether changes in the rates of mRNA degradation were involved in the biphasic response to TSH, especially the downregulation phase at higher TSH doses. We used actinomycin D to inhibit gene transcription and then measured the levels of mRNAs over time in cells incubated with 0, 1, or 100 mU/ml of TSH (Fig. 2). The rates of loss of mRNAs for TG, TPO, DIO2 and TSHR were similar with half-lives of approximately 8 hours and none were affected by TSH.
Figure 2. Degradation rates of mRNAs for thyroid-specific genes.
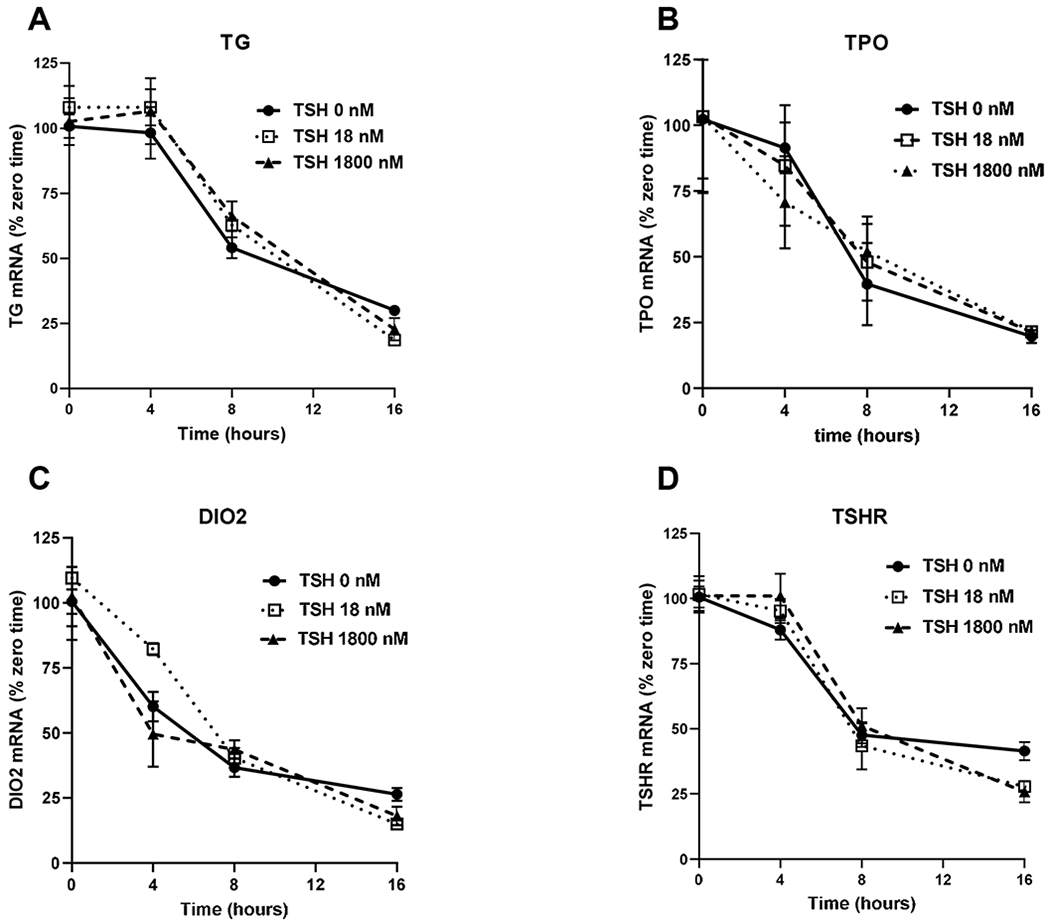
Human thyrocytes were incubated with 0, 1, or 100 mU/mL (1, 18, or 1800 nM) TSH for 48 hours. After 48 hours, 10 μg/mL of actinomycin D was added to block transcription, and mRNA levels for (A) TG, (B) TPO, (C) DIO2, and (D) TSHR were measured by quantitative RT-PCR at the indicated time points. Data are expressed as the mean±SEM of thyrocytes from three different donors.
To confirm that effects on gene transcription were the cause of both phases of the IUDRC, we measured 4-thiouridine (4sU) incorporation into newly synthesized mRNA stimulated by low and high concentrations of TSH (Fig. 3). Incorporation of 4sU into the mRNAs for TG, TPO, DIO2 and TSHR followed the same biphasic pattern of TSH effects as was observed with measurements of steady-state mRNA level. Thus, we conclude that the IUDRC of regulation by TSH of thyroid-specific genes expression is caused by changes in their transcription without any effects on mRNA degradation.
Figure 3. Effect of TSH on synthesis of thyroid-specific gene mRNAs.
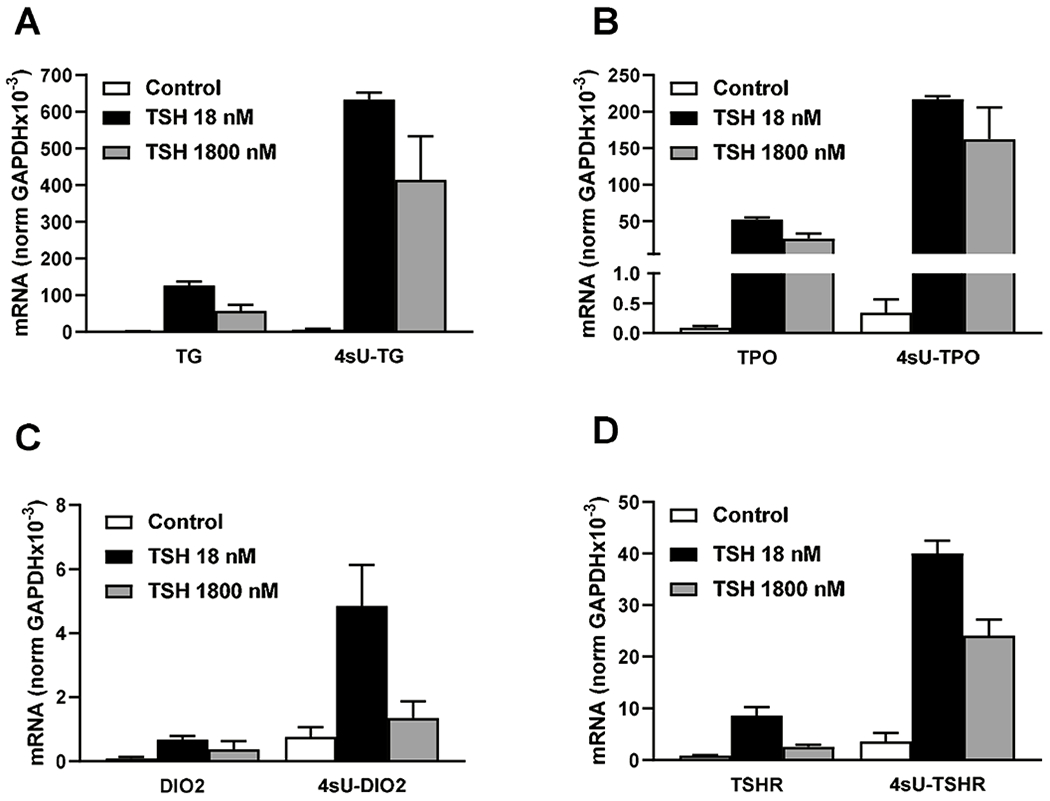
Human thyrocytes were incubated with 0, 1, or 100 mU/mL (1, 18, or 1800 nM) TSH for 48 hours followed by labeling of newly transcribed mRNA with 200 μM of 4-thiouridine (4sU) for 4 hours. Purified 4sU-labeled mRNA was biotinylated and isolated using streptavidin-coated magnetic beads. (A) TG, (B) TPO, (C) DIO2, and (D) TSHR expression levels were measured as total mRNA and newly transcribed 4sU-labeled mRNA. Data are expressed as the mean±SEM of thyrocytes from three different donors.
We examined the effects of TSH on TTF mRNA levels (Fig. 4). TSH regulation of NKX2-1, FOXE1 and PAX8 exhibited monophasic dose-response curves with downregulation at high doses of TSH. The responses, however, were small 25% to 50%. In contrast, surprisingly, HHEX exhibited the same IUDRC to TSH that we observed for thyroid-specific genes stimulated by TSH with a maximum increase in HHEX mRNA of approximately 4-fold.
Figure 4. Thyroid transcription factor regulations by TSH.
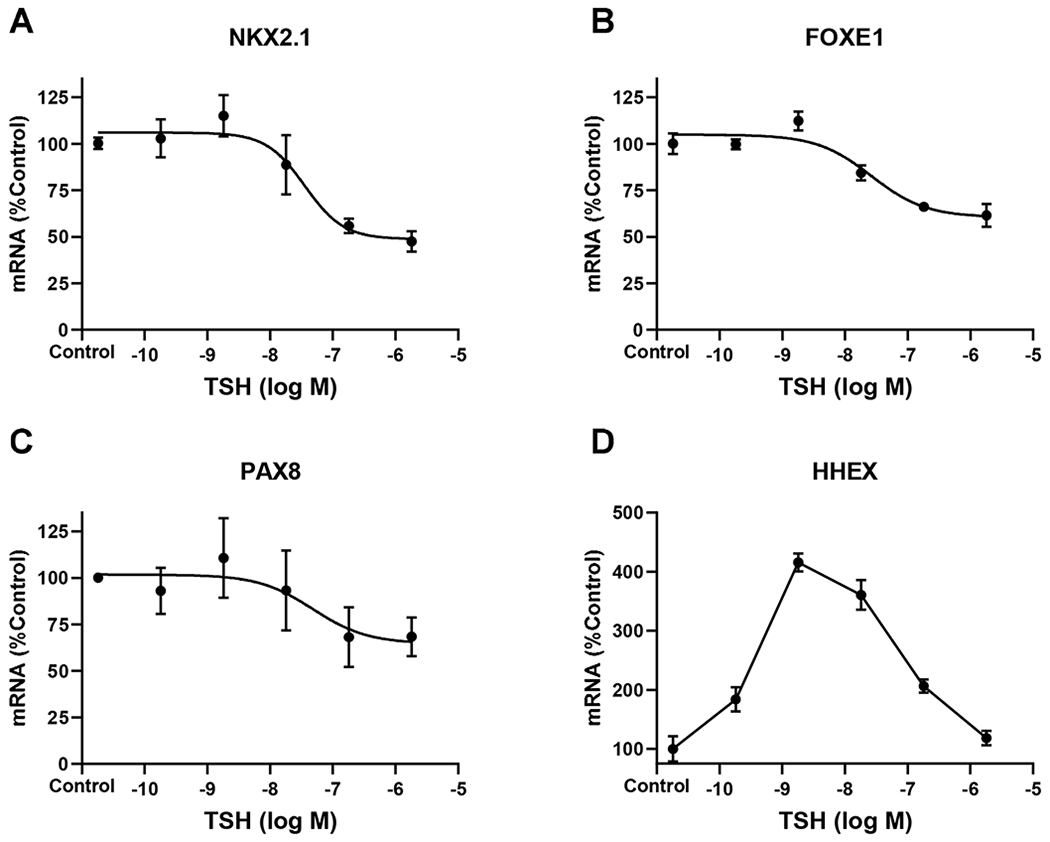
Human thyrocytes were incubated without (control) or with TSH at the indicated doses for 48 hours. mRNA levels for (A) NKX2-1, (B) FOXE1, (C) PAX8, and (D) HHEX were measured using quantitative RT-PCR. Data are expressed as the mean±SEM of thyrocytes from three different donors.
As effects of gene regulation between TTFs have been reported during mouse development (Fernandez et al., 2015), we examined this phenomenon in differentiated human thyrocytes (Fig. 5). FOXE1, NKX2.1, PAX8 or HHEX KD did not affect the mRNA levels of other TTFs, however, FOXE1 KD upregulated mRNAs of PAX8 and HHEX by 50%. Knockdown efficiencies for gene expression of TTFs were 71% for FOXE1, 72% for NKX2-1, 63% PAX8, and 92% for HHEX.
Figure 5. Effect of each TTF on the gene expression levels of other TTFs.
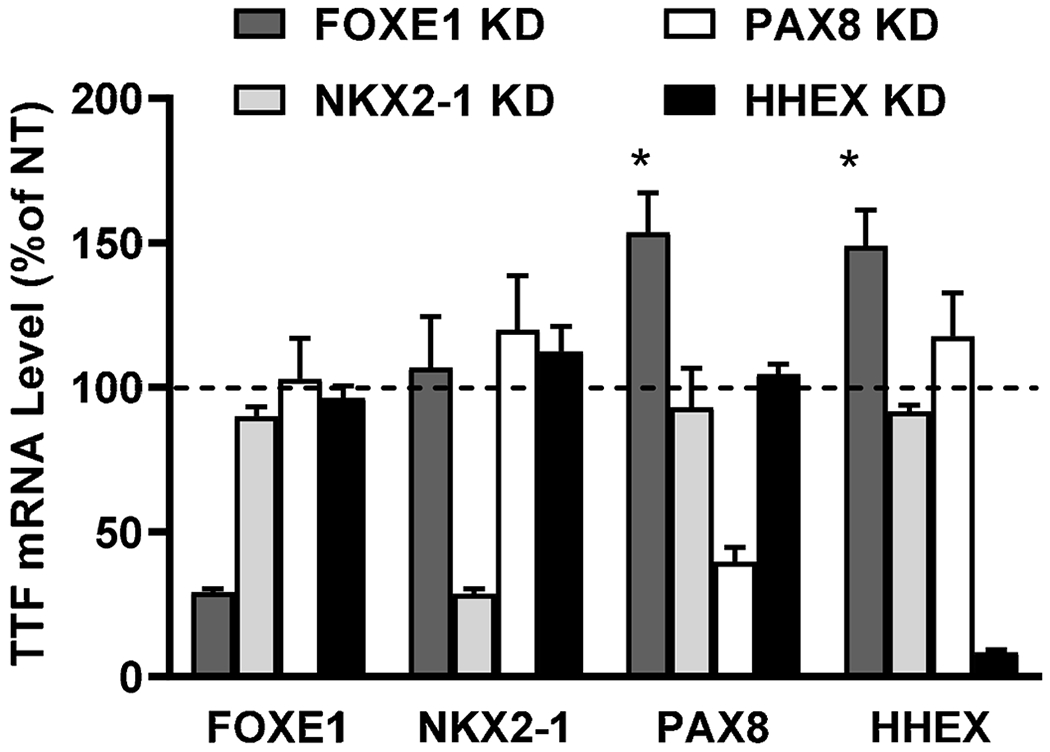
Human thyrocytes were transfected with non-targeting or targeting siRNA against thyroid transcription factors (TTFs). After 48 hours, mRNA levels for FOXE1, NKX2-1, PAX8, and HHEX were measured. Data are normalized to expression levels in cells treated with non-targeting siRNA (NT) and expressed as the mean±SEM of thyrocytes from three different donors.
Discussion
As noted above, there have been few reports of the roles of TTFs in mediating the effects of TSH on transcription of thyroid-specific genes in adult, differentiated thyroid cells. Many of these studies were performed in rodent cell lines that were derived from adult thyroid glands or from thyroid cancers. Studies in rat FRTL-5 cells found that NKX2-1 (Ohmori et al., 1998,Oguchi and Kimura, 1998) and HHEX (Pellizzari et al., 2000) and in rat PC Cl 3 cells that FOXE1 (Fernandez et al., 2013) were involved in TSH regulation. The majority of studies in human thyroid cells in culture were in cells derived from cancers that do not, in general, respond to TSH. We studied the roles of TTFs in primary cultures of human thyrocytes (Morgan et al., 2018). These cells maintain their responsivity to TSH for several passages in culture. TSH regulation of gene expression of thyroid-specific genes TG, TPO, DIO2, NIS and TSHR in these cells is biphasic with increases at low doses of TSH and inhibition at high doses; that is, TSH regulation exhibits an IUDRC (Jang et al., 2020,Morgan et al., 2016). IUDRCs have been found in a number of receptor/cell signaling systems and have been suggested to allow for a more varied responsiveness (Calabrese, 2016).
We found that TSH regulation of TG, TPO, DIO2, NIS, and TSHR gene expression in human thyrocytes was mediated by NKX2-1, FOXE1 and PAX8 but not by HHEX. The lack of effect of HHEX knockdown was the most unexpected finding and clearly underlines the difference in human thyrocytes in primary culture compared to the previous systems studied. Although knockdown of NKX2-1, FOXE1 or PAX8 markedly inhibited TSH-mediated upregulation of these genes it did not affect the biphasic effect, that is, decreased levels of mRNA at high TSH doses were still observed. In contrast, TSH caused monophasic effects on NKX2-1, FOXE1 and PAX8 mRNAs with moderate decreases in their levels at high TSH doses over a dose range similar to that of the decreases in TG, TPO, DIO2, NIS, and TSHR mRNAs. Regulation of HHEX mRNA levels by TSH was different than the other TTF mRNAs in that TSH caused biphasic effects in a manner similar to TSH effects on mRNAs of TG, TPO, DIO2, NIS and TSHR. Therefore, HHEX may have a regulatory role in TSH-mediated responses in adult, human thyrocytes without having a direct effect on TG, TPO, TSHR, NIS, and DIO2 gene expression. HHEX was found to inhibit TG gene expression in FRTL5 rat thyroid cells (Suzuki et al., 1998). The upregulation of TG, TPO, DIO2, NIS, and TSHR gene expression stimulated by TSH is dependent on NKX2-1, FOXE1 and PAX8, however, the mediator(s) of their decreased expression at high TSH doses is not clear. The decreases of TTFs at high TSH doses (Fig. 4) may lead to the decreases measured in the mRNA levels of the thyroid-specific genes but this was not proven in this report. It was conceivable that the regulation of gene expression was caused by changes in transcription and not by changes in mRNA degradation. We measured mRNA degradation by inhibiting transcription with actinomycin D. Although this is not the optimal method by which to measure mRNA turnover, we think it is valid in comparing the effects of TSH on mRNA degradation because the effects of actinomycin D on all genes would be similar in control cells and cells treated with TSH. Using this method, we found that TSH had no effect on mRNA degradation. We assessed transcription rates directly by measuring incorporation of 4-thiouridine into newly synthesized mRNA. The changes in the rates of newly synthesized mRNA caused by TSH were similar to the changes measured in the levels of the total mRNA for all thyroid-specific genes.
During development in mice, there are effects of one TTF to regulate the transcription of other TTFs (Fernandez et al., 2015). For example, NKX2-1 and PAX8 were found to increase transcription of FOXE1 and HHEX, and PAX8 to increase its own transcription. In contrast to these findings, we observed only small effects of FOXE1 KD to increase expression of PAX8 and HHEX in differentiated human thyrocytes. The mechanisms underlying these differences are not known.
TSH doses used in this report are higher than normal physiological levels found in the blood of humans. We hypothesized that these high doses were needed because the levels of TSHR expression in primary cultured human thyrocytes was 100-fold lower than in freshly isolated thyroid tissues. We recently reported that an IUDRC for cAMP stimulation by TSH in cultured adult, human thyrocytes is dependent on the level of TSHR expression and homodimerization of TSHRs (Boutin et al., 2020). We suggest that homodimerization of TSHRs is needed in the regulation of thyroid-specific genes by TSH in human thyrocytes also.
In conclusion, TSH upregulation of thyroid-specific gene expression is dependent on NKX2-1, FOXE1 and PAX8, but not HHEX, to increase their rates of transcription in differentiated human thyroid cells. TSH regulation of NKX2-1, FOXE1 and PAX8 mRNAs is monophasic whereas that of HHEX is biphasic. The mediators/mechanism of the decreased levels of thyroid-specific gene mRNAs at high doses of TSH remains unclear, however, we cannot exclude the possibility that decreases in TTFs mediate this effect and should be studied further.
Supplementary Material
Highlights.
TSH upregulation of thyroid-specific genes is dependent on NKX2-1, FOXE1 and PAX8, but not HHEX
TSH regulation of thyroid-specific genes is mediated by changes in their transcription and not by the degradation of mRNAs
TSH causes monophasic inhibition of NKX2-1, FOXE1 and PAX8 mRNA but an IUDRC for HHEX mRNA
TTFs do not have major effects on the levels of other TTF mRNAs in adult, human thyrocytes
Acknowledgments
We thank Oyeyemi Ruth Adewale and Stephanie Cardenas for helping to procure patients’ tissue samples.
This research was supported by 1 Z01 DK011007 from the Intramural Research Program of the NIDDK, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts to disclose
References
- [1].Fernandez LP, Lopez-Marquez A and Santisteban P, 2015. Thyroid transcription factors in development, differentiation and disease, Nat Rev Endocrinol. 11, 29–42. [DOI] [PubMed] [Google Scholar]
- [2].Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M and Costagliola S, 2012. Generation of functional thyroid from embryonic stem cells, Nature. 491, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ortiz L, Zannini M, Di Lauro R and Santisteban P, 1997. Transcriptional control of the forkhead thyroid transcription factor TTF-2 by thyrotropin, insulin, and insulin-like growth factor I, J Biol Chem. 272, 23334–9. [DOI] [PubMed] [Google Scholar]
- [4].Pellizzari L, D’Elia A, Rustighi A, Manfioletti G, Tell G and Damante G, 2000. Expression and function of the homeodomain-containing protein Hex in thyroid cells, Nucleic Acids Res. 28, 2503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Taki K, Kogai T, Kanamoto Y, Hershman JM and Brent GA, 2002. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3′,5′-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells, Mol Endocrinol. 16, 2266–82. [DOI] [PubMed] [Google Scholar]
- [6].Sahin M, Allard BL, Yates M, Powell JG, Wang XL, Hay ID, Zhao Y, Goellner JR, Sebo TJ, Grebe SK, Eberhardt NL and McIver B, 2005. PPARgamma staining as a surrogate for PAX8/PPARgamma fusion oncogene expression in follicular neoplasms: clinicopathological correlation and histopathological diagnostic value, J Clin Endocrinol Metab. 90, 463–8. [DOI] [PubMed] [Google Scholar]
- [7].Espadinha C, Cavaco BM and Leite V, 2007. PAX8PPARgamma stimulates cell viability and modulates expression of thyroid-specific genes in a human thyroid cell line, Thyroid. 17, 497–509. [DOI] [PubMed] [Google Scholar]
- [8].Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM and Gershengorn MC, 2009. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice, Proc Natl Acad Sci U S A. 106, 12471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morgan SJ, Neumann S and Gershengorn MC, 2018. Normal Human Thyrocytes in Culture, Methods Mol Biol. 1817, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garibaldi A, Carranza F and Hertel KJ, 2017. Isolation of Newly Transcribed RNA Using the Metabolic Label 4-Thiouridine, Methods Mol Biol. 1648, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jang D, Morgan SJ, Klubo-Gwiezdzinska J, Banga JP, Neumann S and Gershengorn MC, 2020. Thyrotropin, but Not Thyroid-Stimulating Antibodies, Induces Biphasic Regulation of Gene Expression in Human Thyrocytes, Thyroid. 30, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ohmori M, Endo T, Harii N and Onaya T, 1998. A novel thyroid transcription factor is essential for thyrotropin-induced up-regulation of Na+/I− symporter gene expression, Mol Endocrinol. 12, 727–36. [DOI] [PubMed] [Google Scholar]
- [13].Oguchi H and Kimura S, 1998. Multiple transcripts encoded by the thyroid-specific enhancer-binding protein (T/EBP)/thyroid-specific transcription factor-1 (TTF-1) gene: evidence of autoregulation, Endocrinology. 139, 1999–2006. [DOI] [PubMed] [Google Scholar]
- [14].Fernandez LP, Lopez-Marquez A, Martinez AM, Gomez-Lopez G and Santisteban P, 2013. New insights into FoxE1 functions: identification of direct FoxE1 targets in thyroid cells, PLoS One. 8, e62849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morgan SJ, Neumann S, Marcus-Samuels B and Gershengorn MC, 2016. Thyrotropin and Insulin-Like Growth Factor 1 Receptor Crosstalk Upregulates Sodium-Iodide Symporter Expression in Primary Cultures of Human Thyrocytes, Thyroid. 26, 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Calabrese EJ, 2016. The Emergence of the Dose-Response Concept in Biology and Medicine, Int J Mol Sci. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suzuki K, Lavaroni S, Mori A, Ohta M, Saito J, Pietrarelli M, Singer DS, Kimura S, Katoh R, Kawaoi A and Kohn LD, 1998. Autoregulation of thyroid-specific gene transcription by thyroglobulin, Proc Natl Acad Sci U S A. 95, 8251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boutin A, Krieger CC, Marcus-Samuels B, Klubo-Gwiezdzinska J, Neumann S and Gershengorn MC, 2020. TSH Receptor Homodimerization in Regulation of cAMP Production in Human Thyrocytes in vitro, Frontiers in Endocrinology. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


