Abstract
Linoleic acid (LA) is the most abundant polyunsaturated fatty acid found in the western diet. Cytochrome P450-derived LA metabolites 9,10-epoxyoctadecenoic acid (9,10-EpOME), 12,13-epoxyoctadecenoic acid (12,13-EpOME), 9,10-dihydroxy-12Z-octadecenoic acid (9,10-DiHOME) and 12,13-dihydroxy-9Z-octadecenoic acid (12,13-DiHOME) have been studied for their association with various disease states and biological functions. Previous studies of the EpOMEs and DiHOMEs have focused on their roles in cytotoxic processes, primarily in the inhibition of the neutrophil respiratory burst. More recent research has suggested the DiHOMEs may be important lipid mediators in pain perception, altered immune response, and brown adipose tissue (BAT) activation by cold and exercise. The purpose of this review is to summarize the current understanding of the physiological and pathophysiological roles and modes of action of the EpOMEs and DiHOMEs in health and disease.
1. Introduction
Polyunsaturated fatty acids (PUFAs) are the backbone of numerous lipid signaling molecules that broadly serve as homeostatic regulators for inflammation, vasotension and other physiologic processes (Zha et al. 2014; Horrillo et al. 2010; Virtue et al. 2015; Markaverich, Alejandro, et al. 2002; Nieman et al. 2013; Levan et al. 2019). In addition to undergoing common reactions of all fatty acids, such as chain elongation and fatty acid beta-oxidation, PUFA metabolism is known to involve cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) enzymes, leading to the production of eicosanoids and numerous other lipid metabolites (Buczynski, Dumlao, and Dennis 2009). Linoleic acid (LA) is the most abundantly consumed PUFA in the human diet, which is mostly derived from vegetable oils, nuts, seeds, meats, and eggs (Whelan and Fritsche 2013). Through a CYP-dependent metabolism, LA is converted to linoleic epoxides 9,10-epoxyoctadecenoic acid (9,10-EpOME) and 12,13-epoxyoctadecenoic acid (12,13-EpOME), also known as leukotoxin and isoleukotoxin, respectively (Newman, Morisseau, and Hammock 2005).
The primary CYP isoforms responsible for this conversion are CYP2J2, CYP2C8 and CYP2C9; however, other inducible CYP isoforms, including CYP1A1, can generate epoxy-fatty acids and may be relevant when pharmacologically induced (Yang et al. 2013; Diani-Moore et al. 2014; Goswami et al. 2015). These epoxides are then metabolized principally by soluble epoxide hydrolase (sEH) to 9,10-dihydroxyoctadecenoic acid (9,10-DiHOME) and 12,13-dihydroxyoctadecenoic acid (12,13-DiHOME), also named leukotoxin diol and isoleukotoxin diol, respectively (Figure 1) (Newman, Morisseau, and Hammock 2005). These diols can be further metabolized by oxidation, generating THF diols (Markaverich, Alejandro, et al. 2002; Moghaddam et al. 1996) and DiHOME-glucuronides (Jude et al. 2000). sEH is widely expressed throughout the human body and has been reported to be expressed in tissue isolated from the liver, kidney, adrenals, pancreatic islets, pituitary gland, lymphoid tissues, muscles, specific vascular smooth muscles, epithelial cells, prostatic ducts, and gastrointestinal tract (Enayetallah et al. 2004). Interestingly, CYP2C9 expression is closely associated with sEH distribution, showing a coincidence of epoxide production and hydrolysis within the tissue (Enayetallah et al. 2004). sEH mRNA and/or protein expression can also be induced by various stimulations, including pharmacological agents such as the peroxisome proliferator clofibrate and PPARγ agonist rosiglitazone (Hammock and Ota 1983; De Taeye et al. 2010).
Figure 1.
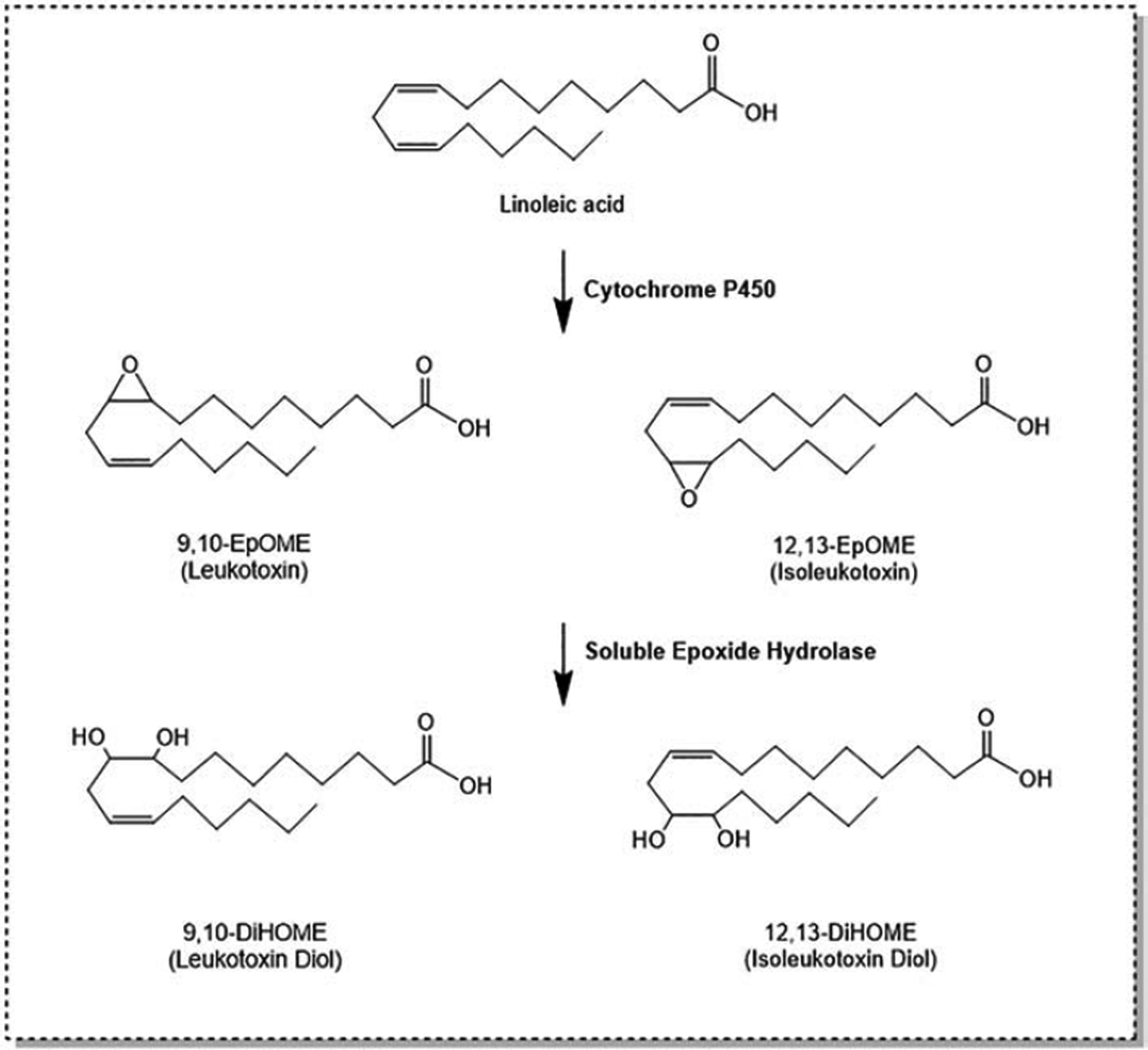
Linoleic acid is metabolized by cytochrome P450s to produce 9,10- and 12,13-EpOME. Subsequent hydrolysis by soluble epoxide hydrolase yields 9,10- and 12,13-DiHOME, respectively.
Physiologic concentrations of EpOMEs and DiHOMEs may be dependent both on the regulation of biosynthetic pathways (CYP450 and sEH) and dietary intake of their parent fatty acid, LA. Mice studies with soybean oil- or margarine containing high-fat diets have demonstrated increased LA consumption leads to increased concentrations of EpOMEs and DiHOMEs in the livers (Deol et al. 2017; Fan et al. 2020) and plasma (Fan et al. 2020). Interestingly, high LA consumption in these studies was generally associated with increased weight gain and a worse metabolic phenotype (Deol et al. 2017) (Fan et al. 2020). Treating volunteers with intralipid, an intravenous fat emulsion that is high in linoleate-rich soybean oil, has also been shown to increase concentrations of 12,13-DiHOME (Edwards et al. 2012). Since 12,13-DiHOME is not present in Intralipid, this physiologic increase is likely due to endogenous production.
In addition to their production from endogenous metabolism, the EpOMEs may be available from food sources. 9,10- and 12,13-EpOME have been identified in seed oil as well as the rice plant Oryza Sativa L. (Ansari et al. 1987; Powell, Smith, and Wolff 1967; Kato et al. 1983). In the rice plant, the EpOMEs were characterized as self-defense substances produced by the rice plant against rice blast disease (Kato et al. 1983). The oils high in EpOME, also known as vernolates, were used in varnish and, in theory, could be valuable synthetically since each vernolate carbon could be used in nylon production.
Recently, one of these linoleate metabolites, 12,13-DiHOME, has been demonstrated as a novel lipokine that regulates brown adipocyte biology in response to cold (Lynes et al. 2017) and exercise (Stanford et al. 2018). Given the emerging roles of this metabolite, it is important to understand how these metabolites relate to health and disease. In this review, we summarize early findings on the cytotoxicity of the LA derived EpOMEs and DiHOMEs and recent studies elucidating their diverse roles with a focus on immune response, pain perception, brown adipose tissue activation by cold and exercise (Table 1).
Table 1.
Biological functions of EpOMEs and DiHOMEs
| Function | Model | Lipid | Key Findings | Mechanism | Reference |
|---|---|---|---|---|---|
| Inflammation and immune response | In vitro - endothelial cells from porcine pulmonary arteries | 9,10-EpOME and 9,10-DiHOME (60 and 90μM) | Induced oxidative stress | Activated NF-κB and AP-1 transcription factors | (Viswanathan et al. 2003) |
| In vitro – human neutrophils | 9,10-EpOME (0.1–1μM) and 9,10-DiHOME (1nM-0.1μM) | Induced chemotaxis of human neutrophils: 9,10-DiHOME with 100x greater potency | Undetermined: chemotaxis activation pathway different from that of fMLP that does not include the induction of expression of adhesion molecules or peroxides | (Totani et al. 2000) | |
| In vitro – HL-60 cells | Methylated EpOMEs (10–200μM) and DiHOMEs (200μM) | DiHOMEs inhibited the respiratory burst while the EpOMEs were weak | Undetermined: inhibition of the respiratory burst by a mechanism different from that of cyclosporin H or lipoxin A4 that involves modulation of NADPH oxidase activity | (Thompson and Hammock 2007) | |
| In vivo – adult men | 12,13-DiHOME | plasma levels were elevated during Intralipid infusion | Undetermined: proposed to lead to impaired neutrophil function and thereby immunosuppression | (Edwards et al. 2012) | |
| In vivo and in vitro – neonates | 12,13-DiHOME (75, 130, and 200μM) | Fecal concentrations were elevated in individuals with NGM3 and its application to dendritic cells and autologously purified naïve CD4+ cells resulted in a reduced percentage of CD4+CD25+Foxp3+ T cells | (Fujimura et al. 2016) | ||
| In vivo and in vitro – neonates, mice, human dendritic cells | 12,13-DiHOME (30 mg/kg (mice) and 75, 130, and 200μM (cells) | Increased neonate fecal concentrations were associated with risk of asthma at age four, treatment of mice exacerbated pulmonary inflammation and decreased lung regulatory T cells, and treatment of cells decreased immune tolerance | Undetermined: proposed immune dysfunction via PPARγ signaling in dendritic cells | (Levan et al. 2019) | |
| Endocrine Disruption | In vivo – adult rats | 9,10- and 12,13-DiHOME (2μg/mL) | A 1:1 mixture of the DiHOMEs disrupted the estrous cycle of female rats but did not affect the sexual behavior of male rats | Undetermined: proposed modulation of prolactin release similar to that of the arachidonic and steric acid epoxyderivatives | (Markaverich et al. 2005) |
| In vitro – U-33/ γ2 cells | 9,10-DiHOME (120μM) | Stimulated adipogenesis but inhibited osteoblastogenesis | Acted as a PPARγ2 ligand | (Lecka-Czernik et al. 2002) | |
| Mitogenesis | In vitro – MCF-7 cells | 9,10- and 12,13-DiHOME (0.32–1.6μM) | Stimulated cell proliferation | Undetermined: mechanisms not involving estrogen receptor or nuclear type II binding sites | (Markaverich et al. 2005) |
| In vivo – zebrafish, and mice | 12,13-DiHOME (10μM) | Modulated proliferation and mobilization of hematopoietic progenitor cells (HPC) in zebrafish embryos and the spleen of mice for the vascular development and repair process | Activated the canonical Wnt signaling | (Frömel et al. 2012) | |
| Pain | In vitro – mice | 12,13-DiHOME | Increased calcium flux in sensory neurons through TRPV1 | Activated PKC | (Zimmer et al. 2018) |
| In vivo - rats | 12,13-EpOME, 9,10-and 12,13-DiHOME | Endogenous localized levels increased with painful stimuli | Undetermined: proposed to act as an endogenous TRPV1 agonist | (Eskander et al. 2015) | |
| In vivo and in vitro – mice and cultured murine dorsal root ganglions | 9,10-EpOME (250nM - 10μM) | Induced mechanical and thermal pain in wild-type but not TRPV1-deficient mice; Increased calcium influx in DRGs which was blocked by the TRPV1 antagonist | Sensitization of TRPV via PKA pathway | (Sisignano et al. 2016) | |
| BAT Activation | In vivo – humans | 12,13-DiHOME | Plasma levels were increased by acute cold exposure and were negatively correlated with BMI, insulin resistance, fasting plasma insulin, and glucose concentrations, and circulating triglyceride and leptin levels | (Lynes et al. 2017) | |
| In vivo – mice | 12,13-DiHOME (1 and 10μg/kg) | Increased BAT activity and BAT-specific fatty acid and glucose uptake in mice | Promoted translocation of fatty acid transporters to the membrane | (Lynes et al. 2017) | |
| Exercise and skeletal muscle | In vivo - humans | 12,13-DiHOME | Plasma levels were increased by acute exercise and were positively correlated with cardiorespiratory fitness | (Stanford et al. 2018) | |
| In vivo - mice | 12,13-DiHOME (1μg/kg) | Decreased respiratory exchange ratio and increased fatty acid uptake in skeletal muscle and removal of interscapular BAT fully blunted the exercise-induced increase of 12,13-DiHOME | Induced expression of genes involved in mitochondrial activity and biogenesis and fatty acid uptake in the muscle | (Stanford et al. 2018) | |
| In vitro - C2C12 muscle cells and 3T3-L1 adipocytes | 12,13-DiHOME (1.5μM) | Increased fatty acid uptake, oxidation, and mitochondria respiration in the C2C12 but not 3T3-L1 adipocytes | (Stanford et al. 2018) |
2. Cytotoxicity of EpOMEs and DiHOMEs
2.1. Identification of Leukotoxin as a Major Factor in Burn and ARDS Patients
The first studies on CYP metabolites of LA came from investigations on mitochondrial toxic factors from burn victims. This work stemmed from the observation that many burn victims still had a “late death” after surviving the initial shock of the burn injury. This late death and later development to acute respiratory distress syndrome (ARDS) and sepsis were thought to be due to a toxic substance produced in the skin that entered general circulation (Aoyama et al. 1982). Rodent skin samples from experimentally burned animals were extracted, and a single lipid was isolated (Suzuki et al. 1981) that was eventually identified as 9,10-EpOME by GC/MS and NMR analysis (Yokoo et al. 1986). This metabolite was named “leukotoxin” since it was presumed that these metabolites were produced in the leukocytes (Ozawa et al. 1986; Hayakawa et al. 1986; Hayakawa et al. 1989). The same metabolites were found to be produced by neutrophils in the lung after hyperoxic exposure (Ozawa et al. 1986). This production in neutrophils was enhanced by a Ca2+ ionophore, inhibited by carbon monoxide and was enhanced by epoxide hydrolase inhibition in liver microsomes, demonstrating leukotoxin was produced by consecutive reactions with phospholipase A2 and CYP but could be metabolized by epoxide hydrolase (Ozawa, Sugiyama, Hayakawa, Taki, et al. 1988).
2.2. Pulmonary toxicity of the EpOMEs and DiHOMEs
After identification of leukotoxin as the toxic substance produced in the skin of the burn patients, leukotoxin was found in the lung lavages from patients with ARDS (Ozawa, Sugiyama, Hayakawa, Satake, et al. 1988). Subacute doses of leukotoxin administered to rats intravenously caused severe pulmonary edema in as little as 10 minutes that lasted at least 12 hours, demonstrating a causal effect in vivo (Jia-ning et al. 1988). It should be noted that although the doses were high in these experiments (50–150 μmol/kg, which corresponds to approximately 15–45 mg/kg), the blood concentrations of leukotoxin detected in patients with sepsis or severe burns were found to be as high as 100 μM (Kosaka et al. 1994) to 580 μM (Hanaki et al. 1991; Kosaka et al. 1994) (Hammock, B.D. et al Unpublished data).
Several perfusion studies have been conducted in isolated lungs to investigate the mechanisms responsible for the leukotoxin-induced pulmonary edema (Ishizaki et al. 1995a; Ishizaki et al. 1995b; Ishizaki et al. 1995c; Ishizaki et al. 1995d). In this isolated system, relatively high doses of leukotoxin (200 μM) were required to elicit edematous injury (Ishizaki et al. 1995b). This injury was associated with the release of lactate dehydrogenase (a measure of cellular damage) and increases in effluent nitrite, both of which could be blocked by a nitric oxide synthase (NOS) inhibitor or superoxide dismutase (Ishizaki et al. 1995a). This edematous injury in the isolated lung can be synergized by cotreatment of the vasoconstrictor endothelin-1 (ET-1) and can be blocked by treatment with an endothelin receptor (ETA) antagonist (Ishizaki et al. 1995c). At lower doses (2–20 μM) where edema is not observed, leukotoxin caused changes in pulmonary vasotension and capillary filtration. During hypoxic vasoconstriction, leukotoxin but not linoleate was able to elicit a transient increase in vasoconstriction followed by vasodilation. This relaxation was observed from a variety of vasoconstrictors including angiotensin II, phenylephrine and KCl and could be blocked by a methylene blue (a soluble guanylate cyclase inhibitor) and NG-monomethyl-L-arginine (a NOS inhibitor) (Ishizaki et al. 1995d). These studies demonstrate that high doses of leukotoxin induce pulmonary cell damage whereas low doses induce vasoconstriction followed by vasodilation in the lung, both of which are mediated by nitric oxide.
The hydration of the leukotoxins to leukotoxin diols by sEH was thought to be a means of detoxification (Kosaka et al. 1994) until it was revealed that this conversion to DiHOMEs enables toxicity of the EpOMEs (Greene and Hammock 1999). To study this, insect-derived Sf-21 cells with low endogenous epoxide hydrolase activity were transfected with sEH or microsomal epoxide hydrolase (mEH). DiHOMEs but not EpOMEs were toxic in the non-transfected naïve cells while both DiHOMEs and EpOMEs were toxic in the sEH transfected cells (Borhan et al. 1995; Moghaddam et al. 1997). To support this conclusion, administration of 9,10-DiHOME (35 mg/kg) to rats by cardiac puncture initiated respiratory stress and death within 2 hours, while no symptoms or mortality occurred with 9,10-EpOME at doses up to 100 mg/kg (Moghaddam et al. 1997). Consistently, treatment of mice with 300 mg/kg of a 1:1 mixture of methyl leukotoxin/isoleukotoxin esters was not lethal while the same dose of the corresponding diols resulted in the death of all mice (Zheng et al. 2001). Histopathologic analysis showed that the lungs of the DiHOME-treated mice had massive alveolar edema and hemorrhage with interstitial edema around blood vessels in the lungs, while EpOME-treated mice had only perivascular edema and a small change in alveolar spaces (Zheng et al. 2001). Moreover, treatment with an sEH inhibitor, 4-phenylchalcone oxide, decreased mortality induced by EpOME, but not DiHOME (Zheng et al. 2001).
Structure-activity relationship (SAR) studies using the insect-derived Sf-21 cell line have shown cytotoxicity can be observed in a number of analogous chemical structures with altered hydrocarbon length and functional groups (Greene et al. 2000). Although not tested, these experiments suggest that a large variety of dihydroxy-fatty acids, including dihydroxyeicosatrienoic acids (DiHETs) and dihydroxyoctadecadienoic acids (DiHODEs), may be similarly cytotoxic. Interestingly, some of the reported epoxides were toxic independent of sEH hydrolysis. It is unknown whether the sEH-independent cytotoxicity of these epoxides is mechanistically related to the cytotoxicity of DiHOME. The doses reported in these cytotoxicity experiments were generally high (~100 μM), but may still represent relevant concentrations during fatal sepsis where concentrations of DiHOMEs are massively elevated (Hamaguchi et al. 2019).
Moreover, EpOMEs and DiHOMEs also seem to be implicated in chronic lung conditions caused by environmental insults. One study investigating responses to subway air exposure found decreased levels of both regioisomers of DiHOME in bronchoalveolar lavage fluid after a subway air exposure in asthmatic individuals in comparison to increased levels in healthy individuals (Lundstrom et al. 2011). Healthy volunteers exposed to biodiesel exhaust exposure showed increased levels of plasma 9,10-DiHOME compared to filtered air controls (Gouveia-Figueira et al. 2018). Further, both EpOMEs and DiHOMEs were increased in bronchoalveolar lavage fluid of female, but not male, smokers with COPD relative to smokers with normal lung function (Balgoma et al. 2016). Together, these studies suggest the EpOMEs and DiHOMEs may be part of the inflammatory response to environmental insults in the lung.
A likely underlying mechanism of 9,10-DiHOME toxicity is its ability to disrupt mitochondrial function (Sisemore et al. 2001). Treatment of human HeLa cells with methylated 9,10-DiHOME at concentrations corresponding to those seen in ARDS patients (180 to 210 μM) was shown to cause mitochondrial swelling (i.e., increase of mitochondrial volume), cytochrome c release, and leakage of mitochondria-specific dye Mitotracker Green, all of which are indicative of 9,10-DiHOME’s ability to compromise mitochondrial inner membrane permeability and, consequently, disrupt mitochondrial function (Sisemore et al. 2001). The release of cytochrome c in these cells then triggers cell death (Yang et al. 1997). In contrast, the treatment of these cells with methylated 9,10-EpOME, linoleic acid, and structurally similar compounds did not result in mitochondrial swelling. Moreover, exposure to the threo configuration of 9,10-DiHOME induced swelling to a greater extent than the erythro configuration, suggesting 9,10-DiHOME’s effects are moiety- and regio-specific in the Hela cells (Sisemore et al. 2001).
Relative toxicity and mechanisms of LA, EpOMEs, and DiHOMEs were directly compared in the rabbit renal proximal tubule. It was shown that both methyl LA (1 mM) and an equimolar mixture of the methyl EpOMEs (1 mM) were not toxic, while an equimolar mixture of the methyl DiHOMEs (1mM) induced mitochondrial dysfunction and cell death (Moran et al. 1997). Conversely, 500 μM of the free acid forms of LA, 9,10-EpOME, 9,10-DiHOME, 12,13-EpOME, and 12,13-DiHOME all induced mitochondrial dysfunction and cell death in the renal proximal tubules (Moran et al. 2000). The free acid forms of LA, 9,10-EpOME, and 12,13-EpOME were most toxic. Moreover, the free acid forms of 9,10-DiHOME and 12,13-DiHOME were more toxic than their methyl ester derivatives (Moran et al. 2000; Moran et al. 1997). The toxicity of LA and the EpOMEs was attributed to their ability to induce the uncoupling of oxidative phosphorylation (Moran et al. 2000).
Furthermore, in rabbit renal cortical mitochondria, treatment with the free acid form of 12,13-EpOME at 50 μM resulted in reduced ADP-stimulated respiration (State 3 respiration) and increased ADP-depleted respiration (State 4 respiration) (Moran, Nowak, and Grant 2001). Additionally, respiration sensitive to the ATP synthase inhibitor, oligomycin, was decreased by 12,13-EpOME while oligomycin insensitive respiration was increased. Oligomycin-sensitive oxygen consumption can serve as a marker of oxidative phosphorylation, and oligomycin-insensitive respiration represents the oxygen being consumed that is not associated with ATP synthesis in mitochondria, i.e., mitochondrial uncoupling (Schnellmann 1994; Schnellmann and Manning 1990). Compared to 12,13-EpOME, 12,13-DiHOME did not affect these variables, suggesting that hydrolysis to 12,13-DiHOME is a mechanism of detoxification of the linoleic acid metabolite for the prevention of mitochondrial dysfunction in renal cortical mitochondria (Moran, Nowak, and Grant 2001). Divergent effects of the EpOMEs and DiHOMEs demonstrate species- and tissue-specific toxic effects of these metabolites.
2.3. Cardiotoxicity of the EpOMEs and DiHOMEs
One component of the toxic response to leukotoxin is the ability to disrupt cardiovascular function. In dogs, leukotoxins were more cardio-depressive than linoleates were by reducing aortic flow and blood pressure (Fukushima et al. 1988; Sugiyama et al. 1987). Treating leukotoxin or isoleukotoxin to isolated papillary muscles from cats decreased developed force, an index of myocardial contractility. In addition, both leukotoxin and isoleukotoxin caused vasoconstriction in isolated perfused carotid arteries from cats (Siegfried et al. 1990).
Arachidonic acid-derived epoxides or epoxyeicosanoids (EETs), and sEH are known to be important factors in regulating cardiovascular function (Chiamvimonvat et al. 2007; Imig 2006; Deng, Theken, and Lee 2010; Imig 2018). Thus, most of the cardio-protective effects of sEH inhibitors, including protection against cardiac hypertrophy (Xu et al. 2006), ischemia-reperfusion injury (Islam et al. 2017), and fibrosis post-myocardial infarction (Sirish et al. 2013) have been attributed to their ability to increased epoxy-fatty acids (EpFA) and EETs in particular. However, there is evidence of an alternative hypothesis in which dihydroxy-fatty acids (DiHFAs) partially contribute to these effects and that limiting the production of DiHFAs by sEH inhibitors may be beneficial. Mice that had endothelial cell-specific overexpression of CYP2C8 (CYP2C8-Tie2), but not CYP2J2 or sEH, had reduced recovery of left ventricular developed pressure and increased infarct size after ischemia-reperfusion injury (Edin et al. 2011). This reduced recovery was associated with increased concentrations of 9,10- and 12,13-DiHOMEs in the heart perfusates. Dosing Langendorff-perfused WT hearts with either 9,10-DiHOME or 12,13-DiHOME was able to recapitulate the reduced recovery and increased coronary resistance found in CYP2C8-Tie2 mice (Edin et al. 2011; Bannehr et al. 2019). In addition, in a comparison of young and aged sEH-null and cardiac myocyte specific CYP2J2 overexpressing mouse hearts, sEH-null mice had postischemic protection in both young and aged hearts while CYP2J2 overexpression was only protective in young but not the aged hearts (Chaudhary et al. 2013). The amount of protective EETs present in both genetic backgrounds was comparable, and no increase was found in sEH expression and activities in aged CYP2J2 overexpressing hearts; however, the authors found CYP2J2 overexpression resulted in increased 12,13-DiHOME, which correlated with increased reactive oxygen species (ROS) markers. Consistently, the authors found that sEH inhibition improved cardioprotection in the aged CYP2J2 overexpressing hearts. Thus, in addition to enhancing the cardioprotective role of EETs, sEH inhibition may also block the harmful effects of DiHOMEs.
In contrast to the postischemic toxic effects of 12,13-DiHOME observed in the hearts with ischemia/reperfusion, other studies have found modest increases in contractile function within 10–20 min were observed in LA, 12,13-EpOME, and 12,13-DiHOME perfused rat hearts, with 12,13-DiHOME’s positive effects lasted until washout. No arrhythmias and negative inotropic effects were observed (Mitchell et al. 2002). Moreover, the administration of a mixture of 9,10- and 12,13-EpOMEs to rats in vivo showed only a small decrease in blood pressure with no significant effect on heart rate or pulse (Mitchell et al. 2002). It seems that the effects of EpOMEs and DiHOMEs on cardiovascular function are complex and may be dose and species-dependent.
3. Biological Functions of the EpOMEs and DiHOMEs
3.1. Inflammation and Immune Response
It has been reported that LA-induced endothelial cell activation or dysfunction in atherosclerosis may be mediated through oxidative stress (Hennig and Toborek 2001). Consistently, in endothelial cells isolated from porcine pulmonary arteries, 90 μM LA induced oxidative stress (as measured by DCF fluorescence), while both 9,10-EpOME and 9,10-DiHOME induced oxidative stress at high concentrations (90 μM) but not at low concentrations (up to 30 μM) (Viswanathan et al. 2003). Moreover, as measured by electrophoretic mobility shift assays, LA (90 μM) and high concentrations of 9,10-EpOME (90 μM) and 9,10-DiHOME (60 μM) activated NF-κB and AP-1 transcription factors, both of which mediate inflammation (Viswanathan et al. 2003).
DiHOMEs are synthesized by activated neutrophils and induce chemotaxis of other neutrophils at relatively low doses (~10 nM) (Ishizaki, Ozawa, and Voelkel 1999; Totani et al. 2000). This induction was not through the expression of adhesion molecules or peroxide production, as is the case of the well-known chemoattractant fMLP, but rather through an independent pathway (Totani et al. 2000). At relatively higher doses (20–200 μM), both DiHOME isomers inhibited the neutrophil respiratory burst in HL-60 cells, which are neutrophil-like cells derived from human promyelocytic-leukemia (Thompson and Hammock 2007). Given the relatively significant difference in doses required for the chemotactic and inhibitory effects, DiHOMEs may serve as a type of negative feedback that limits the inflammation. DiHOME-mediated inhibition occurs by a mechanism different from that of cyclosporin H or lipoxin A4, both of which are respiratory burst inhibitors that prevent both superoxide production and degranulation. It is thought that the DiHOMEs stimulate the use of NADPH oxidase substrates or induce physiochemical alterations in the membrane microenvironment of NADPH oxidase, thereby modulating the activity of NADPH oxidase, which is responsible for the production of the respiratory burst (Thompson and Hammock 2007). Because of its ability to inhibit the respiratory burst, 12,13-DiHOME may inhibit the immune response. Plasma levels of 12,13-DiHOME were found to be significantly elevated in healthy adult men with normal BMIs during Intralipid infusion; therefore, it is thought that 12,13-DiHOME may contribute to immunosuppression seen in patients receiving Intralipid infusion for parenteral nutrition (Edwards et al. 2012). This may be particularly true in the case of total parenteral nutrition.
12,13-DiHOME has recently been found to be associated with the gut microbiome of young children who develop asthma (Fujimura et al. 2016). This study showed a particular community of gut bacteria, referred to as the neonatal gut microbiome 3 (NGM3), was associated with an elevated relative risk of developing atopy, a heightened immune response to allergens, by two years of age and asthma by four years of age. 12,13-DiHOME was found to be the major metabolite identified from these samples that could shift the regulatory T cell populations to cause adaptive immune cell dysfunction and were found to be associated with an increased relative risk of developing asthma (Fujimura et al. 2016; Levan et al. 2019). Exploration of the sources of 12,13-DiHOME in the neonate gut microbiome identified three bacterial epoxide hydrolase genes that were thought to be responsible for mediating the effects on atopy and asthma (Levan et al. 2019). Each of these genes was able to convert 12,13-EpOME to 12,13-DiHOME, and the combination of the three genes was associated with atopy and asthma. Furthermore, treating mice with 12,13-DiHOME before a challenge with cockroach antigen increased the allergic response, including an increase in peribronchial and perivascular inflammatory infiltrates and increased expression of inflammatory cytokines, compared to vehicle controls (Levan et al. 2019). Activation of PPARγ was presumed to be the primary mechanism responsible for these biological effects; however, other receptors that are activated by 12,13-DiHOME, such as transient receptor potential vanilloid 1 (TRPV1), could not be ruled out (Levan et al. 2019).
3.2. Endocrine Disruption
The endocrine-disrupting effects of DiHOMEs were initially investigated from the observation that corncob bedding disrupted normal mating behavior and reproductive capabilities in rats (Markaverich, Mani, et al. 2002). A few years prior, Moghaddam et al. reported that when hydrolyzed the diepoxides of linoleate yielded trivial amounts of the expected tetra hydroxy products and that the major products were cis- and trans-tetrahydrofuran diols (THF-diols) (Moghaddam et al. 1996). DiHOMEs and their corresponding THF-diols byproducts were later identified as major components of corncob bedding responsible for these effects (Markaverich et al. 2005; Markaverich, Alejandro, et al. 2002). Interestingly, dosing rats with a 1:1 mixture of 9,10- and 12,13-DiHOME was able to cause disruption of the estrous cycle in female rats but was unable to cause changes in male reproductive function or mating behavior in either sex (Markaverich et al. 2005). Based on the disruption of the estrous cycle in female rats, it is presumed that both components of corncob bedding could dysregulate normal estrogen signaling pathways. These endocrine-disrupting effects could be particularly relevant to the growth of hormone-sensitive cancers, including breast and ovarian cancers. When tested in vitro, 12,13-DiHOME was able to stimulate the proliferation of MCF-7 cells (estrogen receptor-positive breast cancer cells) but was unable to compete [3H]estradiol binding to the estrogen receptor (Markaverich, Alejandro, et al. 2002). It was not tested whether 12,13-DiHOME could enhance tumor growth in vivo. It is possible these reproductive effects may be relevant to human health due to the ubiquity of corn oil in western diets; however, to the best of our knowledge, no studies have followed the relationship between 12,13-DiHOME in the blood and reproductive function.
Both 9,10-DiHOME and 9,10-EpOME have been shown to act as PPARγ ligands, as determined by their displacement of the PPARγ ligand [3H] T0900393 from the recombinant PPARγ ligand-binding domain (Lecka-Czernik et al. 2002). Activation of PPARγ2 by the potent synthetic ligand rosiglitazone is known to stimulate adipogenesis but inhibit osteoblastogenesis (Benvenuti et al. 2007; Patel, Butters, and Arnett 2014). Consistently, 9,10-DiHOME was shown to stimulate adipogenesis but inhibit osteoblastogenesis, measured by lipid accumulation, mineral deposition, and gene expression in U-33/γ2 cells, a murine marrow-derived mesenchymal progenitor cell line with exogenous expression of PPARγ2 (Lecka-Czernik et al. 2002). However, 9,10-EpOME prevented osteoblast differentiation but did not stimulate adipogenesis (Lecka-Czernik et al. 2002).
3.3. Mitogenesis
In zebrafish and mice, it was discovered that 12,13-DiHOME is a critical modulator of progenitor cell proliferation and mobilization for the vascular development and repair process, which is accomplished by activation of the canonical Wnt signaling cascade (Frömel et al. 2012). Knockdown or inhibition of sEH resulted in defects in the caudal vein plexus (CVP), a transient hematopoietic tissue, and decreased numbers of cmyb/lmo2 double-positive cells, a subpopulation of hematopoietic cells in the CVP, in zebrafish embryos. 12,13-DiHOME, but not 12,13-EpOME, was able to restore numbers of cmyb/lmo2 double-positive cells. In mice, sEH knockout led to decreased proliferation and colony formation of hematopoietic progenitor cells (HPC) in the spleen of irradiated wild-type animals, decreased mobilization of HPC into circulation from the bone marrow in response to G-CSF, and decreased vascular repair after hindlimb ischemia. 12,13-DiHOME, but not 12,13-EpOME, restored the recovery of blood flow in sEH knockout mice, but not wild-type mice, with hind limb ischemia (Frömel et al. 2012).
3.4. Pain
Many eicosanoids are implicated in the regulation of pain. Prostaglandins are positive regulators of pain that mediate thermal and mechanical hyperalgesia during inflammation (Inceoglu et al. 2006). In contrast, many EpFAs are negative regulators of pain by blocking inflammation-induced hyperalgesia and other forms of pain, such as diabetes-caused peripheral neuropathy (Morisseau et al. 2010; Wagner et al. 2014; Wagner et al. 2017). To increase the endogenous levels of pain-relieving EpFAs in vivo, sEH inhibitors have been used, which appeared to be highly effective in multiple types of pain (Schmelzer et al. 2006; Terashvili et al. 2008). sEH inhibitors appear to reduce pain through a number of mechanisms, including cannabinoid signaling (Wagner et al. 2011) and ER stress (Inceoglu et al. 2015). These small molecules are currently being taken into the clinic for the treatment of pain in man and in companion animals (Kodani and Hammock 2015).
Care should be taken not to equate sEH inhibition with the activity of EpFAs. Although EpFAs alone have established pain-relieving effects, these studies do not rule out the possibility of alternative hypotheses. One alternative hypothesis is that diol metabolites of sEH may be pronociceptive, and sEH inhibition blocks the formation of these painful metabolites. In support of this hypothesis, Zimmer et al. (2018) recently showed in sensory neurons that 12,13-DiHOME increased calcium flux by TRPV1 through a PKC mediated mechanism, leading to increased thermal hyperalgesia in vivo, which is not evident in TRPV1 knockout mice. Additionally, treatment with painful stimuli such as complete Freund’s Adjuvant or nerve growth factor increased endogenous concentrations of 12,13-DiHOME (Eskander et al. 2015; Zimmer et al. 2018). This work indicates that chronic pain increases endogenous concentrations of DiHOMEs, and sEH inhibition blocks the production of these proalgesic metabolites while increasing concentrations of analgesic and possibly other EpFAs.
However, the EpFAs may not be analgesic in all scenarios; it has been reported that 9,10-EpOME may mediate paclitaxel-induced neuropathic pain by sensitizing TRPV1 and increasing activities of nociceptive neurons (Sisignano et al. 2016). Also, other types of oxidized linoleic metabolites that are not regulated by sEH have also been implicated in pain progression (Patwardhan et al. 2010; Patwardhan et al. 2009); thus, it seems that EpOMEs and DiHOMEs represent only part of concerted oxidation of LA to mediate pain.
3.5. Brown Adipose Tissue Activation by Cold
Brown adipose tissue (BAT) has recently emerged as a novel target for obesity treatment and prevention (Lidell, Betz, and Enerback 2014; Cannon and Nedergaard 2004). In contrast to white adipose tissue (WAT), BAT is responsible for non-shivering thermogenesis by uncoupling ATP synthesis from respiration, leading to heat production (Cannon and Nedergaard 2004).
Studies investigating the role of 12,13-DiHOME’s in regulating BAT function started with a lipidomics screen to identify bioactive lipids that were increased in humans subjected to acute cold exposure (Lynes et al. 2017). Moreover, plasma levels of 12,13-DiHOME were found to be positively correlated with BAT activities but negatively correlated with BMI, insulin resistance, fasting plasma insulin and glucose concentrations, and circulating triglyceride and leptin levels (Lynes et al. 2017). The finding that plasma levels of 12,13-DiHOME were negatively associated with obesity was consistent with a previous report (Pickens et al. 2017) and has since been validated in a larger sample of volunteers (Vasan et al. 2019). Similarly, cold exposed mice (4°C) had increased serum levels of 12,13-DiHOME and increased expression of sEH in the BAT, but not in other tissues that express sEH (Lynes et al. 2017). Interestingly, cold-exposed mice that have a defect in normal BAT development (Myf5CreBmpr1af/f mice) produced 12,13-DiHOME from the compensated sWAT instead of the BAT; suggesting 12,13-DiHOME secretion may be a component of all thermogenic adipocytes rather than just brown adipocytes. Thus, it was concluded that 12,13-DiHOME could be a thermogenic BATokine activated by cold-exposure.
Pharmacologic experiments testing the effects of 12,13-DiHOME in mice further confirmed they play a role in thermogenesis. Injection of 1 μg/kg 12,13-DiHOME increased oxygen consumption, carbon dioxide production, and cold resistance. Moreover, injection of 1 μg/kg 12,13-DiHOME resulted in an increase in BAT-specific lipids, FA, and glucose uptake in mice. Furthermore, in diet-induced obese mice injected daily with 10 μg/kg 12,13-DiHOME for 2 weeks, a decrease in circulating triglycerides and an increase in expression of lipoprotein lipase in BAT was observed with no significant change in weight, glucose tolerance, or circulating non-esterified fatty acids (Lynes et al. 2017). Together, these results suggested that 12,13-DiHOME may activate BAT by increasing the availability and oxidation of free fatty acids.
The increased fatty acid uptake in BAT observed with 12,13-DiHOME treatment has been attributed to the increases of membrane translocation of the low-glycosylated form of fatty acid transporters CD36 and oligomeric fatty acid transport protein 1 (FATP1) (Lynes et al. 2017). 12,13-DiHOME did not have a significant effect on maximal respiratory capacity or uncoupling in the BAT, but basal respiration was increased, indicating increased basal fuel uptake and metabolism (Lynes et al. 2017). Taken together, 12,13-DiHOME serves as a link between cold exposure and thermogenesis by promoting fatty acid uptake in brown adipocytes via an autocrine or paracrine manner. Increased fatty acids provide more substrates for CYP and sEH, consequently increasing more 12,13-DiHOME production as a feedforward mechanism (Lynes et al. 2017).
3.6. Exercise and Skeletal Muscle Regulation
Several studies have reported that 12,13-DiHOME is increased in response to exercise. In a series of studies investigating exercise-regulated metabolites of athletes, blood levels of 12,13-DiHOME were increased immediately post the exercise in both long-distance runners (Nieman et al. 2013) and cyclists (Nieman et al. 2014). It was found that both 9,10- and 12,13-DiHOME were increased post the cycling exercise and were subsequently reduced back to baseline once the exercise was over. Only post-exercise 12,13-DiHOME levels were positively correlated with post-exercise oxidized LA derivative 13- and 9-hydroxy-octadecadienoic acid (13-HODE + 9-HODE), a new oxidative stress biomarker for acute exercise (Nieman et al. 2014). Similar changes in 12,13-DiHOMEs were observed over the course of a 45-minute bout of exercise in young or old male healthy subjects of varying levels of physical fitness (Stanford et al. 2018). Additionally, it was found that routinely active subjects had significantly higher pre-exercise 12,13-DiHOME levels compared to subjects who did not regularly exercise (Stanford et al. 2018). Resting 12,13-DiHOME concentration was positively correlated with cardiorespiratory fitness, as measured by peak oxygen uptake, and negatively correlated with total fat mass, BMI, body weight, and triglycerides. However, covariate analysis showed that when percentage fat mass is accounted for, the only significant correlation was with triglycerides (Stanford et al. 2018).
Plasma levels of 12,13-DiHOME are similarly increased in either trained or untrained male mice subjected to acute exercise (Stanford et al. 2018). Chronic exercise training also significantly increased 12,13-DiHOME and decreased body and fat mass, consistent with the negative correlation between resting 12,13-DiHOME levels and body and fat mass observed in humans. The surgical removal of interscapular BAT from the mice blunted the effects of exercise on 12,13-DiHOME levels, despite no difference in basal 12,13-DiHOME compared to mice that underwent sham surgery. A single bout of exercise and 3 weeks of exercise training increased mRNA expression of Ephx1 in BAT but not in other tissues. No changes in Ephx2 gene expression were found in any of the tissues. Taken together, it was concluded that even though various tissues produce 12,13-DiHOME, BAT is responsible for the exercise-induced increase in 12,13-DiHOME (Stanford et al. 2018).
Similar to brown adipose tissue, mice injected with 12,13-DiHOME had increased fatty acid uptake in skeletal muscle and a decrease in the respiratory exchange ratios. This increase in fatty acid uptake was also observed in vitro in differentiated C2C12 myotubes but not in differentiated 3T3-L1 white adipocytes (Stanford et al. 2018). Further, 12,13-DiHOME increased basal and maximal respiration in C2C12 cells. Thus, 12,13-DiHOME may support active skeletal muscle function by increasing lipid uptake and mitochondrial activities.
4. Conclusion
Recent studies have brought new interest in the roles of CYP-derived LA metabolites EpOMEs and DiHOMEs in health and disease. Despite the toxic effects observed at high concentrations, pharmacologic strategies that target 12,13-DiHOME may hold promise as a therapeutic approach towards treating of pain, obesity, and related metabolic dysfunction. Both cold and exercise induce secretion of 12, 13-DiHOME from the BAT into circulation. At low concentrations that appear to be safe, 12,13-DiHOME has been shown to effectively increase fatty acid uptake in the BAT and skeletal muscle and decrease circulating triglycerides in mice. Moreover, this low dose of 12,13-DiHOME had no effect on blood pressure or pulse (except for a brief increase in diastolic pressure) (Lynes et al. 2017). Therefore, 12,13-DiHOME can mimic the beneficial effects of cold exposure and exercise on improving lipid metabolism.
On the other hand, the therapeutic use of sEH inhibitors, which can block the production of DiHOMEs, is currently being explored for many different disease states, including obesity (Lopez-Vicario et al. 2015), insulin resistance (Luria et al. 2011), and metabolic syndrome (Iyer et al. 2012). Therefore, it is important to have a better understanding of the biological activities of CYP-derived LA metabolites, including the EpOMEs and DiHOMEs, in health and disease.
Many questions remain with respect to the roles of EpOMEs and DiHOMEs in physiology. One of these is whether a change in LA content in the diet would affect the processes in which EpOMEs or DiHOMEs are involved, such as immune response, pain perception, BAT activation by cold and exercise. LA is the most abundant PUFA in the human diet and consumption has increased over the past century (Blasbalg et al. 2011). Since increased dietary intakes of LA lead to increased plasma and tissue levels of EpOMEs and DiHOMEs, it seems plausible there may be physiological consequences of increased DiHOMEs on health.
An additional remaining question is whether common molecular mechanisms exist that mediate the mitochondrial toxicity of 12,13-DiHOME at high doses and the beneficial effects on metabolism that occurs at lower doses. Common to both effects are an increase in uncoupling of the electron transport chain (ETC). In the case of mitochondrial toxicity, uncoupling of the ETC can reduce efficiency of oxidative phosphorylation, leading to reduced cellular function, increased oxidative stress and eventually cell death, as observed with high doses of 12,13-DiHOME. These effects likely underly many of the toxics effects that have been observed in a variety of organs, including cardiovascular, pulmonary and renal systems. By comparison, the “inefficient” use of chemical fuel is a feature of BAT and uncoupling of the ETC drives non-shivering thermogenesis. It is unclear whether 12,13-DiHOME-mediated uncoupling observed in other cell types occurs in brown adipocytes and contributes to the thermogenic phenotype observed in BAT. If this is true, understanding how dose regulates uncoupling responses in BAT relative to other organ systems may be the key to the pharmacologic application of 12,13-DiHOME for treating metabolic diseases, such as hyperlipidemia and type 2 diabetes.
Acknowledgment
The work was supported by NIH T32GM099608 to SDK, NIEHS (RIVER Award) R35 ES030443-01 and NIEHS Superfund Research Program P42 ES04699 to BDH, and NIH R15 1R15DK114790-01A1 to LZ
The authors apologize to authors whose work has not been included in the review due to space limitation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ansari MH, Ahmad Suhail, Ahmad F, Ahmad M, and Osman SM. 1987. ‘Co-occurrence of Coronaric and Vernolic Acids in Compositae Seed Oils’, Lipid / Fett, 89: 116–18. [Google Scholar]
- Aoyama H, Suzuki K, Izawa Y, Kobashashi M, and Ozawa T. 1982. ‘Mitochondria-toxic activity in burned human skin: relation to severity of burn and period after burn’, Burns Incl Therm Inj, 9: 13–6. [DOI] [PubMed] [Google Scholar]
- Balgoma D, Yang M, Sjodin M, Snowden S, Karimi R, Levanen B, Merikallio H, Kaarteenaho R, Palmberg L, Larsson K, Erle DJ, Dahlen SE, Dahlen B, Skold CM, Wheelock AM, and Wheelock CE. 2016. ‘Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD’, Eur Respir J, 47: 1645–56. [DOI] [PubMed] [Google Scholar]
- Bannehr M, Lohr L, Gelep J, Haverkamp W, Schunck WH, Gollasch M, and Wutzler A. 2019. ‘Linoleic Acid Metabolite DiHOME Decreases Post-ischemic Cardiac Recovery in Murine Hearts’, Cardiovasc Toxicol, 19: 365–71. [DOI] [PubMed] [Google Scholar]
- Benvenuti S, Cellai I, Luciani P, Deledda C, Baglioni S, Giuliani C, Saccardi R, Mazzanti B, Dal Pozzo S, Mannucci E, Peri A, and Serio M. 2007. ‘Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells’, J Endocrinol Invest, 30: Rc26–30. [DOI] [PubMed] [Google Scholar]
- Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, and Rawlings RR. 2011. ‘Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century’, Am J Clin Nutr, 93: 950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, and Hammock BD. 1995. ‘Improved Radiolabeled Substrates for Soluble Epoxide Hydrolase’, Analytical Biochemistry, 231: 188–200. [DOI] [PubMed] [Google Scholar]
- Buczynski Matthew W., Dumlao Darren S., and Dennis Edward A.. 2009. ‘Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology’, Journal of Lipid Research, 50: 1015–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J. 2004. ‘Brown adipose tissue: function and physiological significance’, Physiol Rev, 84: 277–359. [DOI] [PubMed] [Google Scholar]
- Chaudhary KR, Zordoky BN, Edin ML, Alsaleh N, El-Kadi AO, Zeldin DC, and Seubert JM. 2013. ‘Differential effects of soluble epoxide hydrolase inhibition and CYP2J2 overexpression on postischemic cardiac function in aged mice’, Prostaglandins Other Lipid Mediat, 104–105: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, Ho CM, Tsai HJ, and Hammock BD. 2007. ‘The soluble epoxide hydrolase as a pharmaceutical target for hypertension’, J Cardiovasc Pharmacol, 50: 225–37. [DOI] [PubMed] [Google Scholar]
- De Taeye, Bart M, Morisseau Christophe, Coyle Julie, Covington Joseph W., Luria Ayala, Yang Jun, Murphy Sheila B., Friedman David B., Hammock Bruce B., and Vaughan Douglas E.. 2010. ‘Expression and Regulation of Soluble Epoxide Hydrolase in Adipose Tissue’, Obesity (Silver Spring, Md.), 18: 489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Theken KN, and Lee CR. 2010. ‘Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation’, J Mol Cell Cardiol, 48: 331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol P, Fahrmann J, Yang J, Evans JR, Rizo A, Grapov D, Salemi M, Wanichthanarak K, Fiehn O, Phinney B, Hammock BD, and Sladek FM. 2017. ‘Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice’, Sci Rep, 7: 12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diani-Moore S, Ma Y, Gross SS, and Rifkind AB. 2014. ‘Increases in levels of epoxyeicosatrienoic and dihydroxyeicosatrienoic acids (EETs and DHETs) in liver and heart in vivo by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and in hepatic EET:DHET ratios by cotreatment with TCDD and the soluble epoxide hydrolase inhibitor AUDA’, Drug Metab Dispos, 42: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, Foley JF, Torphy R, Ronnekleiv OK, Tomer KB, Lee CR, and Zeldin DC. 2011. ‘Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart’, Faseb j, 25: 3436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Lindsay M., Lawler Nathan G., Nikolic Sonja B., Peters James M., Horne James, Wilson Richard, Davies Noel W., and Sharman James E.. 2012. ‘Metabolomics reveals increased isoleukotoxin diol (12,13-DHOME) in human plasma after acute Intralipid infusion’, Journal of Lipid Research, 53: 1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayetallah AE, French RA, Thibodeau MS, and Grant DF. 2004. ‘Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues’, J Histochem Cytochem, 52: 447–54. [DOI] [PubMed] [Google Scholar]
- Eskander MA, Ruparel S, Green DP, Chen PB, Por ED, Jeske NA, Gao X, Flores ER, and Hargreaves KM. 2015. ‘Persistent Nociception Triggered by Nerve Growth Factor (NGF) Is Mediated by TRPV1 and Oxidative Mechanisms’, J Neurosci, 35: 8593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Kim J, You M, Giraud D, Toney AM, Shin SH, Kim SY, Borkowski K, Newman JW, and Chung S. 2020. ‘alpha-Linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation’, J Nutr Biochem, 76: 108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömel Timo, Jungblut Benno, Hu Jiong, Trouvain Caroline, Eduardo Barbosa-Sicard Rüdiger Popp, Liebner Stefan, Dimmeler Stefanie, Hammock Bruce D., and Fleming Ingrid. 2012. ‘Soluble epoxide hydrolase regulates hematopoietic progenitor cell function via generation of fatty acid diols’, Proceedings of the National Academy of Sciences of the United States of America, 109: 9995–10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Kei E., Sitarik Alexandra R., Havstad Suzanne, Lin Din L., Levan Sophia, Fadrosh Douglas, Panzer Ariane R., Brandon LaMere Elze Rackaityte, Lukacs Nicholas W., Wegienka Ganesa, Boushey Homer A., Ownby Dennis R., Zoratti Edward M., Levin Albert M., Johnson Christine C., and Lynch Susan V.. 2016. ‘Neonatal gut microbiota associates with childhood multi–sensitized atopy and T–cell differentiation’, Nature medicine, 22: 1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Akihiko, Hayakawa Mika, Sugiyama Satoru, Ajioka Masayoshi, Ito Takayuki, Satake Tatsuo, and Ozawa Takayuki. 1988. ‘Cardiovascular effects of leukotoxin (9,10-epoxy-12-octadecenoate) and free fatty acids in dogs’, Cardiovascular Research, 22: 213–18. [DOI] [PubMed] [Google Scholar]
- Goswami SK, Inceoglu B, Yang J, Wan D, Kodani SD, da Silva CA, Morisseau C, and Hammock BD. 2015. ‘Omeprazole increases the efficacy of a soluble epoxide hydrolase inhibitor in a PGE(2) induced pain model’, Toxicol Appl Pharmacol, 289: 419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia-Figueira S, Karimpour M, Bosson JA, Blomberg A, Unosson J, Sehlstedt M, Pourazar J, Sandstrom T, Behndig AF, and Nording ML. 2018. ‘Mass spectrometry profiling reveals altered plasma levels of monohydroxy fatty acids and related lipids in healthy humans after controlled exposure to biodiesel exhaust’, Anal Chim Acta, 1018: 62–69. [DOI] [PubMed] [Google Scholar]
- Greene JF, and Hammock BD. 1999. ‘Toxicity of linoleic acid metabolites’, Adv Exp Med Biol, 469: 471–7. [DOI] [PubMed] [Google Scholar]
- Greene Jessica F., Newman John W., Williamson Kristin C., and Hammock Bruce D.. 2000. ‘Toxicity of Epoxy Fatty Acids and Related Compounds to Cells Expressing Human Soluble Epoxide Hydrolase’, Chemical Research in Toxicology, 13: 217–26. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M, Wu HN, Tanaka M, Tsuda N, Tantengco OAG, Matsushima T, Nakao T, Ishibe T, Sakata I, and Yanagihara I. 2019. ‘A case series of the dynamics of lipid mediators in patients with sepsis’, Acute Med Surg, 6: 413–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock BD, and Ota K. 1983. ‘Differential induction of cytosolic epoxide hydrolase, microsomal epoxide hydrolase, and glutathione S-transferase activities’, Toxicol Appl Pharmacol, 71: 254–65. [DOI] [PubMed] [Google Scholar]
- Hanaki Y, Kamiya H, Ohno M, Hayakawa M, Sugiyama S, and Ozawa T. 1991. ‘Leukotoxin, 9, 10-epoxy-12-octadecenoate: a possible responsible factor in circulatory shock and disseminated intravascular coagulation’, Jpn J Med, 30: 224–8. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Ogawa T, Sugiyama S, and Ozawa T. 1989. ‘Hydroxyl radical and leukotoxin biosynthesis in neutrophil plasma membrane’, Biochem Biophys Res Commun, 161: 1077–85. [DOI] [PubMed] [Google Scholar]
- Hayakawa Mika, Sugiyama Satoru, Takamura Tadanobu, Yokoo Kazuhisa, Iwata Masaru, Suzuki Kiyoshi, Taki Fumio, Takahashi Seiji, and Ozawa Takayuki. 1986. ‘Neutrophils biosynthesize leukotoxin, 9, 10-epoxy-12-octadecenoate’, Biochemical and Biophysical Research Communications, 137: 424–30. [DOI] [PubMed] [Google Scholar]
- Hennig Bernhard, and Toborek Michal. 2001. ‘Nutrition and endothelial cell function: implications in atherosclerosis’, Nutrition Research, 21: 279–93. [DOI] [PubMed] [Google Scholar]
- Horrillo Raquel, Ana González-Périz, Marcos Martínez-Clemente, Marta López-Parra, Natàlia Ferré, Esther Titos, Eva Morán-Salvador, Ramon Deulofeu, Vicente Arroyo, and Joan Clària. 2010. ‘5-Lipoxygenase Activating Protein Signals Adipose Tissue Inflammation and Lipid Dysfunction in Experimental Obesity’, The Journal of Immunology, 184: 3978. [DOI] [PubMed] [Google Scholar]
- Imig JD 2006. ‘Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors’, Cardiovasc Drug Rev, 24: 169–88. [DOI] [PubMed] [Google Scholar]
- ———. 2018. ‘Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics’, Pharmacol Ther, 192: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, and Hammock BD. 2015. ‘Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain’, Proc Natl Acad Sci U S A, 112: 9082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, and Hammock BD. 2006. ‘Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain’, Life Sci, 79: 2311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Ozawa T, and Voelkel NF. 1999. ‘Leukotoxins and the Lung’, Pulmonary Pharmacology & Therapeutics, 12: 145–55. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Shigemori K, Nakai T, Miyabo S, Hayakawa M, Ozawa T, Voelkel NF, and Chang SW. 1995c. ‘Endothelin-1 potentiates leukotoxin-induced edematous lung injury’, Journal of Applied Physiology, 79: 1106–11. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Shigemori K, Nakai T, Miyabo S, Ozawa T, Chang SW, and Voelkel NF. 1995a. ‘Leukotoxin, 9,10-epoxy-12-octadecenoate causes edematous lung injury via activation of vascular nitric oxide synthase’, The American journal of physiology, 269: L65–70. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Shigemori K, Yamamura Y, Matsukawa S, Nakai T, Miyabo S, Hayakawa M, Ozawa T, and Voelkel NF. 1995b. ‘Increased nitric oxide biosynthesis in leukotoxin,9,10-epoxy-12-octadecenoate injured lung’, Biochemical and Biophysical Research Communications, 210: 133–37. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Takahashi H, Ozawa T, Chang SW, and Voelkel NF. 1995d. ‘Leukotoxin, 9,10-epoxy-12-octadecenoate causes pulmonary vasodilation in rats’, American Journal of Physiology-Lung Cellular and Molecular Physiology, 268: L123–L28. [DOI] [PubMed] [Google Scholar]
- Islam O, Patil P, Goswami SK, Razdan R, Inamdar MN, Rizwan M, Mathew J, Inceoglu B, Stephen Lee KS, Hwang SH, and Hammock BD. 2017. ‘Inhibitors of soluble epoxide hydrolase minimize ischemia-reperfusion-induced cardiac damage in normal, hypertensive, and diabetic rats’, Cardiovasc Ther, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, and Brown L. 2012. ‘Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats’, Exp Diabetes Res, 2012: 758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia-ning Hu, Taki Fumio, Sugiyama Satoru, Asai Junpei, Izawa Yohei, Satake Tatsuo, and Ozawa Takayuki. 1988. ‘Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema’, Lung, 166: 327–37. [DOI] [PubMed] [Google Scholar]
- Jude AR, Little JM, Freeman JP, Evans JE, Radominska-Pandya A, and Grant DF. 2000. ‘Linoleic acid diols are novel substrates for human UDP-glucuronosyltransferases’, Arch Biochem Biophys, 380: 294–302. [DOI] [PubMed] [Google Scholar]
- Kato Tadahiro, Yamaguchi Yoshihiro, Uyehara Tadao, Yokoyama Toshiro, Namai Tsuneo, and Yamanaka Susumu. 1983. ‘Self defensive substances in rice plant against rice blast disease’, Tetrahedron Letters, 24: 4715–18. [Google Scholar]
- Kodani SD, and Hammock BD. 2015. ‘The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: drug metabolism to therapeutics for chronic pain’, Drug Metab Dispos, 43: 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka Kazuhiro, Suzuki Kohji, Hayakawa Mika, Sugiyama Satoru, and Ozawa Takayuki. 1994. ‘Leukotoxin, a linoleate epoxide: Its implication in the late death of patients with extensive burns’, Molecular and Cellular Biochemistry, 139: 141–48. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik Beata, Moerman Elena J., Grant David F., Lehmann Jürgen M., Manolagas Stavros C., and Jilka Robert L.. 2002. ‘Divergent Effects of Selective Peroxisome Proliferator-Activated Receptor-γ2 Ligands on Adipocyte Versus Osteoblast Differentiation’, Endocrinology, 143: 2376–84. [DOI] [PubMed] [Google Scholar]
- Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, Fujimura KE, McKean M, Ownby DR, Zoratti EM, Boushey HA, Cabana MD, Johnson CC, and Lynch SV. 2019. ‘Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance’, Nat Microbiol, 4: 1851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, and Enerback S. 2014. ‘Brown adipose tissue and its therapeutic potential’, J Intern Med, 276: 364–77. [DOI] [PubMed] [Google Scholar]
- Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, and Claria J. 2015. ‘Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides’, Proc Natl Acad Sci U S A, 112: 536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom SL, Levanen B, Nording M, Klepczynska-Nystrom A, Skold M, Haeggstrom JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, Eklund A, Wheelock AM, and Wheelock CE. 2011. ‘Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure’, PLOS ONE, 6: e23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, and Hammock BD. 2011. ‘Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance’, Proc Natl Acad Sci U S A, 108: 9038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, Baer LA, May FJ, Gao F, Narain NR, Chen EY, Kiebish MA, Cypess AM, Bluher M, Goodyear LJ, Hotamisligil GS, Stanford KI, and Tseng YH. 2017. ‘The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue’, Nat Med, 23: 631–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O’Malley B, and Faith R. 2002. ‘A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells’, Environ Health Perspect, 110: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markaverich Barry M., Mary Ann Alejandro, Markaverich David, Zitzow Lois, Casajuna Nancy, Camarao Nathan, Hill Jamal, Bhirdo Ken, Faith Robert, Turk John, and Crowley Jan R.. 2002. ‘Identification of an Endocrine Disrupting Agent from Corn with Mitogenic Activity’, Biochemical and Biophysical Research Communications, 291: 692–700. [DOI] [PubMed] [Google Scholar]
- Markaverich Barry M., Crowley Jan R., Alejandro Mary A., Shoulars Kevin, Casajuna Nancy, Mani Shaila, Reyna Andrea, and Sharp John. 2005. ‘Leukotoxin Diols from Ground Corncob Bedding Disrupt Estrous Cyclicity in Rats and Stimulate MCF-7 Breast Cancer Cell Proliferation’, Environmental Health Perspectives, 113: 1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell Lex A., Grant David F., Melchert Russell B., Petty Nathan M., and Kennedy Richard H.. 2002. ‘Linoleic acid metabolites act to increase contractility in isolated rat heart’, Cardiovascular Toxicology, 2: 219–29. [DOI] [PubMed] [Google Scholar]
- Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, and Hammock BD. 1997. ‘Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase’, Nat Med, 3: 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam M, Motoba K, Borhan B, Pinot F, and Hammock BD. 1996. ‘Novel metabolic pathways for linoleic and arachidonic acid metabolism’, Biochimica et biophysica acta, 1290: 327–39. [DOI] [PubMed] [Google Scholar]
- Moran Jeffery H., Mitchell Lex A., Bradbury J. Alyce, Qu Wei, Zeldin Darryl C., Schnellmann Rick G., and Grant David F.. 2000. ‘Analysis of the Cytotoxic Properties of Linoleic Acid Metabolites Produced by Renal and Hepatic P450s’, Toxicology and Applied Pharmacology, 168: 268–79. [DOI] [PubMed] [Google Scholar]
- Moran Jeffery H., Nowak Grazyna, and Grant David F.. 2001. ‘Analysis of the Toxic Effects of Linoleic Acid, 12,13-cis-Epoxyoctadecenoic Acid, and 12,13-Dihydroxyoctadecenoic Acid in Rabbit Renal Cortical Mitochondria’, Toxicology and Applied Pharmacology, 172: 150–61. [DOI] [PubMed] [Google Scholar]
- Moran Jeffery H., Weise Rick, Schnellmann Rick G., Freeman JP, and Grant David F.. 1997. ‘Cytotoxicity of Linoleic Acid Diols to Renal Proximal Tubular Cells’, Toxicology and Applied Pharmacology, 146: 53–59. [DOI] [PubMed] [Google Scholar]
- Morisseau Christophe, Inceoglu Bora, Schmelzer Kara, Tsai Hsing-Ju, Jinks Steven L., Hegedus Christine M., and Hammock Bruce D.. 2010. ‘Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids’, Journal of Lipid Research, 51: 3481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman John W., Morisseau Christophe, and Hammock Bruce D.. 2005. ‘Epoxide hydrolases: their roles and interactions with lipid metabolism’, Progress in Lipid Research, 44: 1–51. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Shanely RA, Gillitt ND, Pappan KL, and Lila MA. 2013. ‘Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners’, J Proteome Res, 12: 4577–84. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Shanely RA, Luo B, Meaney MP, Dew DA, and Pappan KL. 2014. ‘Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling’, Am J Physiol Regul Integr Comp Physiol, 307: R68–74. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Hayakawa M, Takamura T, Sugiyama S, Suzuki K, Iwata M, Taki F, and Tomita T. 1986. ‘Biosynthesis of leukotoxin, 9,10-epoxy-12 octadecenoate, by leukocytes in lung lavages of rat after exposure to hyperoxia’, Biochem Biophys Res Commun, 134: 1071–8. [DOI] [PubMed] [Google Scholar]
- Ozawa Takayuki, Sugiyama Satoru, Hayakawa Mika, Satake Tatsuo, Taki Fumio, Iwata Masaru, and Taki Kazumi. 1988. ‘Existence of Leukotoxin 9,10-Epoxy-12-Octadecenoate in Lung Lavages from Rats Breathing Pure Oxygen and from Patients with the Adult Respiratory Distress Syndrome’, American Review of Respiratory Disease, 137: 535–40. [DOI] [PubMed] [Google Scholar]
- Ozawa Takayuki, Sugiyama Satoru, Hayakawa Mika, Taki Fumio, and Hanaki Yoshihiro. 1988. ‘Neutrophil microsomes biosynthesize linoleate epoxide (9, 10-epoxy-12-octadecenoate), A biological active substance’, Biochemical and Biophysical Research Communications, 152: 1310–18. [DOI] [PubMed] [Google Scholar]
- Patel JJ, Butters OR, and Arnett TR. 2014. ‘PPAR agonists stimulate adipogenesis at the expense of osteoblast differentiation while inhibiting osteoclast formation and activity’, Cell Biochem Funct, 32: 368–77. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, and Hargreaves KM. 2009. ‘Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia’, Proc Natl Acad Sci U S A, 106: 18820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan Amol M., Akopian Armen N., Ruparel Nikita B., Diogenes Anibal, Weintraub Susan T., Uhlson Charis, Murphy Robert C., and Hargreaves Kenneth M.. 2010. ‘Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents’, The Journal of Clinical Investigation, 120: 1617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CA, Sordillo LM, Zhang C, and Fenton JI. 2017. ‘Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE)’, Metabolism, 70: 177–91. [DOI] [PubMed] [Google Scholar]
- Powell RG, Smith CR, and Wolff IA. 1967. ‘cis-5,cis-9,cis-12-octadecatrienoic and some unusual oxygenated acids inXeranthemum annuum seed oil’, Lipids, 2: 172–77. [DOI] [PubMed] [Google Scholar]
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, and Hammock BD. 2006. ‘Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors’, Proc Natl Acad Sci U S A, 103: 13646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellmann RG 1994. ‘Measurement of oxygen consumption’ in Eds. Tyson CA and Frazier JM (ed.), Methods in Toxicology, Part B In Vitro Toxicity Indicators (Academic Press: San Diego: ). [Google Scholar]
- Schnellmann RG, and Manning RO. 1990. ‘Perfluorooctane sulfonamide: a structurally novel uncoupler of oxidative phosphorylation’, Biochimica et biophysica acta, 1016: 344–48. [DOI] [PubMed] [Google Scholar]
- Siegfried MR, Aoki N, Lefer AM, Elisseou EM, and Zipkin RE. 1990. ‘Direct cardiovascular actions of two metabolites of linoleic acid’, Life Sci, 46: 427–33. [DOI] [PubMed] [Google Scholar]
- Sirish P, Li N, Liu JY, Lee KS, Hwang SH, Qiu H, Zhao C, Ma SM, Lopez JE, Hammock BD, and Chiamvimonvat N. 2013. ‘Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis’, Proc Natl Acad Sci U S A, 110: 5618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisemore Marlene F., Zheng Jiang, Yang Joy C., Thompson David A., Plopper Charles G., Cortopassi Gino A., and Hammock Bruce D.. 2001. ‘Cellular Characterization of Leukotoxin Diol-Induced Mitochondrial Dysfunction’, Archives of Biochemistry and Biophysics, 392: 32–37. [DOI] [PubMed] [Google Scholar]
- Sisignano M, Angioni C, Park CK, Meyer Dos Santos S, Jordan H, Kuzikov M, Liu D, Zinn S, Hohman SW, Schreiber Y, Zimmer B, Schmidt M, Lu R, Suo J, Zhang DD, Schafer SM, Hofmann M, Yekkirala AS, de Bruin N, Parnham MJ, Woolf CJ, Ji RR, Scholich K, and Geisslinger G. 2016. ‘Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain’, Proc Natl Acad Sci U S A, 113: 12544–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford Kristin I., Lynes Matthew D., Takahashi Hirokazu, Baer Lisa A., Arts Peter J., May Francis J., Lehnig Adam C., Middelbeek Roeland J. W., Richard Jeffrey J., So Kawai, Chen Emily Y., Gao Fei, Narain Niven R., Distefano Giovanna, Shettigar Vikram K., Hirshman Michael F., Ziolo Mark T., Kiebish Michael A., Tseng Yu-Hua, Coen Paul M., and Goodyear Laurie J.. 2018. ‘12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake’, Cell Metabolism, 27: 1111–20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Satoru, Hayakawa Mika, Nagai Shuichiro, Ajioka Masayoshi, and Ozawa Takayuki. 1987. ‘Leukotoxin, 9, 10-epoxy-12-octadecenoate, causes cardiac failure in dogs’, Life Sciences, 40: 225–31. [DOI] [PubMed] [Google Scholar]
- Suzuki Kohji, Aoyama Hisashi, Izawa Yohei, Kobayashi Masanao, and Ozawa Takayuki. 1981. ‘Isolation of a substance toxic to mitochondrial function from the burned skin of rats’, Burns, 8: 110–17. [Google Scholar]
- Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, and Harder DR. 2008. ‘Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray’, J Pharmacol Exp Ther, 326: 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson David Alan, and Hammock Bruce D.. 2007. ‘Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst’, Journal of biosciences, 32: 279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totani Y, Saito Y, Ishizaki T, Sasaki F, Ameshima S, and Miyamori I. 2000. ‘Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP’, European Respiratory Journal, 15: 75. [DOI] [PubMed] [Google Scholar]
- Vasan SK, Noordam R, Gowri MS, Neville MJ, Karpe F, and Christodoulides C. 2019. ‘The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study’, Diabetologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue S, Masoodi M, de Weijer BAM, van Eijk M, Mok CYL, Eiden M, Dale M, Pirraco A, Serlie MJ, Griffin JL, and Vidal-Puig A. 2015. ‘Prostaglandin profiling reveals a role for haematopoietic prostaglandin D synthase in adipose tissue macrophage polarisation in mice and humans’, International Journal of Obesity (2005), 39: 1151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan Saraswathi, Hammock Bruce D., Newman John W., Meerarani Purushothaman, Toborek Michal, and Hennig Bernhard. 2003. ‘Involvement of CYP 2C9 in Mediating the Proinflammatory Effects of Linoleic Acid in Vascular Endothelial Cells’, Journal of the American College of Nutrition, 22: 502–10. [DOI] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Gill SS, and Hammock BD. 2011. ‘Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction’, J Agric Food Chem, 59: 2816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Lee KS, Yang J, and Hammock BD. 2017. ‘Epoxy fatty acids mediate analgesia in murine diabetic neuropathy’, Eur J Pain, 21: 456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Yang J, Inceoglu B, and Hammock BD. 2014. ‘Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy’, J Pain, 15: 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J, and Fritsche K. 2013. ‘Linoleic acid’, Adv Nutr, 4: 311–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, and Chiamvimonvat N. 2006. ‘Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors’, Proc Natl Acad Sci U S A, 103: 18733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Solaimani P, Dong H, Hammock B, and Hankinson O. 2013. ‘Treatment of mice with 2,3,7,8-Tetrachlorodibenzo-p-dioxin markedly increases the levels of a number of cytochrome P450 metabolites of omega-3 polyunsaturated fatty acids in the liver and lung’, J Toxicol Sci, 38: 833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jie, Liu Xuesong, Bhalla Kapil, Kim Caryn Naekyung, Ibrado Ana Maria, Cai Jiyang, Peng Tsung-I., Jones Dean P., and Wang Xiaodong. 1997. ‘Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked’, Science, 275: 1129. [DOI] [PubMed] [Google Scholar]
- Yokoo Kazuhisa, Hayakawa Mika, Sugiyama Satoru, Ozawa Takayuki, Aoyama Hisashi, Izawa Yohei, Kondo Tadao, and Hayakawa Yoshihiro. 1986. ‘A Novel Uncoupler of Mitochondrial Respiration, 9, 10-Epoxy-12-octadecenoate, Exists in Human Burned Skin’, Journal of Clinical Biochemistry and Nutrition, 1: 121–27. [Google Scholar]
- Zha Weibin, Edin Matthew L., Vendrov Kimberly C., Schuck Robert N., Lih Fred B., Jat Jawahar Lal, Bradbury J. Alyce, DeGraff Laura M., Hua Kunjie, Tomer Kenneth B., Falck John R., Zeldin Darryl C., and Lee Craig R.. 2014. ‘Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity’, Journal of Lipid Research, 55: 2124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Jiang, Plopper Charles G., Lakritz Jeffery, Storms David H., and Hammock Bruce D.. 2001. ‘Leukotoxin-Diol’, American Journal of Respiratory Cell and Molecular Biology, 25: 434–38. [DOI] [PubMed] [Google Scholar]
- Zimmer B, Angioni C, Osthues T, Toewe A, Thomas D, Pierre SC, Geisslinger G, Scholich K, and Sisignano M. 2018. ‘The oxidized linoleic acid metabolite 12,13-DiHOME mediates thermal hyperalgesia during inflammatory pain’, Biochimica et biophysica acta, 1863: 669–78. [DOI] [PubMed] [Google Scholar]


