Abstract
Radio telemetry systems are a useful way to continuously monitor broad electrical neuronal activity in behaving animals. It can also be used to study sleep disturbances or monitor seizure activity. Many different telemeter styles are available, but the more versatile and cost-efficient ones are the head mounted systems. They permit long-term recordings and allow more flexibility in the recordings. However, there are currently no such system available for non-human primate (NHP). In fact, the choices for NHP telemetry solutions are very limited. Here, we present a chronically implantable 3D printed chamber specifically designed to accommodate a rodent head-mounted system (RodentPACK) onto a NHP’s head. We recorded EEG signal for more than a year, confirmed quality of the signal, and the ability to use the data to monitor sleep activity. We also used two of our epileptic animals to validate the embedded alarm system for real time seizure monitoring. While initially not designed for NHP, but with a minimum number of adaptions, this telemeter is in fact perfectly suitable for NHP experiments. Since early medical intervention during seizures is critical to avoid status epilepticus and to save the animal’s life, real time seizures monitoring is becoming a safety requirement in many NHP studies. This method refines the current seizure monitoring methods for NHP and creates a flexible telemetry solution.
Keywords: Seizures, telemetry, Non-human primate, Sleep, EEG
1. Introduction:
Radio telemetry systems are a useful way to continuously monitor broad electrical neuronal activity, like local field potentials or electroencephalogram activity, in behaving animals. This technique has been previously used in non-human primate (NHP) to understand changes in sleep architecture (Barraud, Lambrecq et al. 2009, Rachalski, Authier et al. 2014, Goonawardena, Morairty et al. 2019) and in toxicology/safety studies to evaluate the pro-convulsant risk of new drugs (Durmuller, Guillaume et al. 2007, Authier, Paquette et al. 2009, Bassett, Troncy et al. 2014). In these studies, there were many constraints in order to utilize the telemetry systems. The telemeters must be subcutaneously implanted into the animals, and the data analysis was performed off-line. Another major constraint of these implantable system is their battery life, which should be taken into careful consideration when designing studies requiring several months of recording. In addition, these telemeters often have a manufacturer-designated data acquisition software that may not be compatible with other electrophysiological or behavioral acquisition system, thus prohibiting signals to be accurately synchronized (Lundt, Wormuth et al. 2016). Finally, subcutaneous implantation can cause skin irritation and picking by the animal, which may lead to chronic infection.
One alternative to an implanted subcutaneous telemeter is to use a head mounted system composed of a standardized, implanted connector and a plug-in external telemeter. This method could overcome the limit on the recording duration, since the batteries can easily be recharged or replaced. In addition, the implanted connector could acts as an interface between the electrodes inside the brain and another data acquisition system (Goonawardena, Plano et al. 2011, Dupin, Garcia et al. 2019). Several head-mounted systems are currently available with slightly different technical specification, but they were all developed to accommodate rodent experiment. Given NHP similarities to human brain and their high translational potential, NHP represents an important model in pre-clinical trials and in neuroscience. As welfare and regulation for NHP studies evolve, it is critical to refine research methods to address the community’s concerns. Thus, it is critical to develop an online seizure monitoring system for NHP to facilitate prompt intervention in case of seizures. Indeed, early medical intervention is essential to prevent status epilepticus and to save the animal’s life (Gainza-Lein, Fernandez et al. 2019).
The RodentPACK (RodentPACK system, emkaTECHNOLOGIES, Paris, France, Figure 1A) is a small head-mounted system that could record up to four bio-potential through a standardized connector and has an embedded seizures detection and alarm triggering software. While this device has been previously used in rats (Gurbanova, Aker et al. 2008) and dogs (Froget, Peyon et al. 2017) to the best of our knowledge it had not yet been used in NHP. Here, we report the long-term use of a rodent head mounted telemeter (RodentPACK) for sleep and seizures monitoring in NHP. We designed enclosures that would accommodate the telemeter and protect the telemeter against repeated impact from an NHP. These 3D printed enclosures were fixed into the cranial implant of three NHPs. We validated the quality of the recording for up to 12 months (data collection still in progress) and used the EEG recordings for sleep scoring. We also tested the integrated alarm system for real-time seizures monitoring in two of our epileptic NHPs.
Figure 1: Overall view of the RodentPACK and the enclosure.
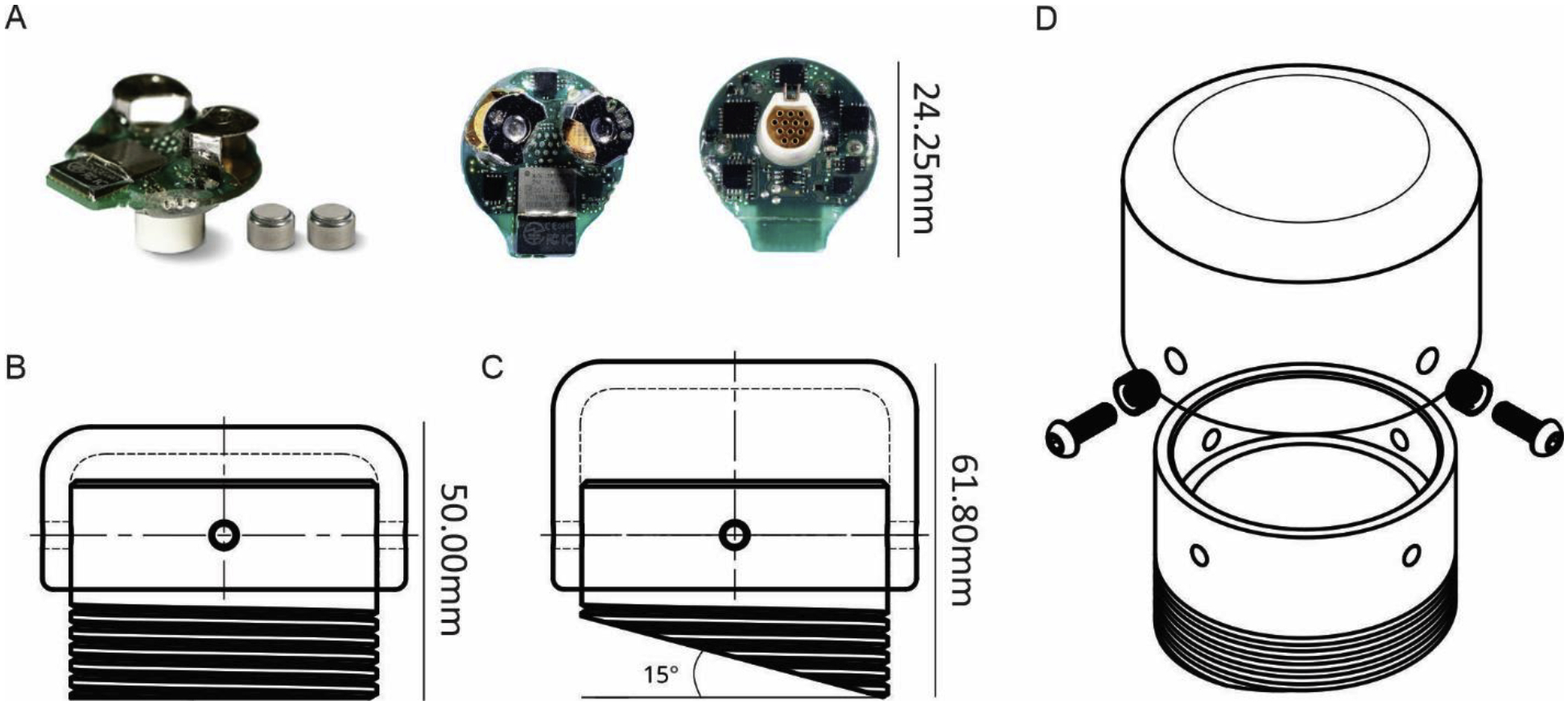
A. Photographs of the RodentPACK with the 2 zinc-air batteries from different angles. B. Drawing of a straight base enclosure with the short lid. C. Drawing of a 15° angle base enclosure with the tall lid. D. Exploded view of the enclosure, the lid, the brass nuts and the screws.
2. Methods:
Telemetry system:
The RodentPACK is a head-mounted telemetry system initially developed for rodents. The RodentPACK telemeter weighs 5.2 g with dimensions of 14.3 mm in height and 24.25 mm in width (Figure 1A). It is powered by two zinc-air, size 13 batteries and can radio up to 8 physiological signals: 4 biopotential signals, 3 axis acceleration and global acceleration. In our set-up, the telemeter sends radio signals to a computer station outside of the animal housing area. The acquired data can be streamed and analyzed in real-time for seizures detection (see below). For EEG acquisition, a circular 12-pin connector (A22017, Omnetics connector co, MN, USA) is permanently implanted in the skull. This connector is the interface between the electrodes inside the brain and the RodentPACK telemeter. One major advantage of this system is that the same telemeter is usable on any subject equipped with the same connector. Because the telemeter uses a commercially available connector, one can be flexible in how we record EEG activity. For instance, this connecter could be used to record EEG activity with an alternative electrophysiological data acquisition system (Figure 4A). In order to adapt the RodentPACK for NHP study, we designed an enclosure box that could accommodate and protect the connector and telemeter.
Figure 4:
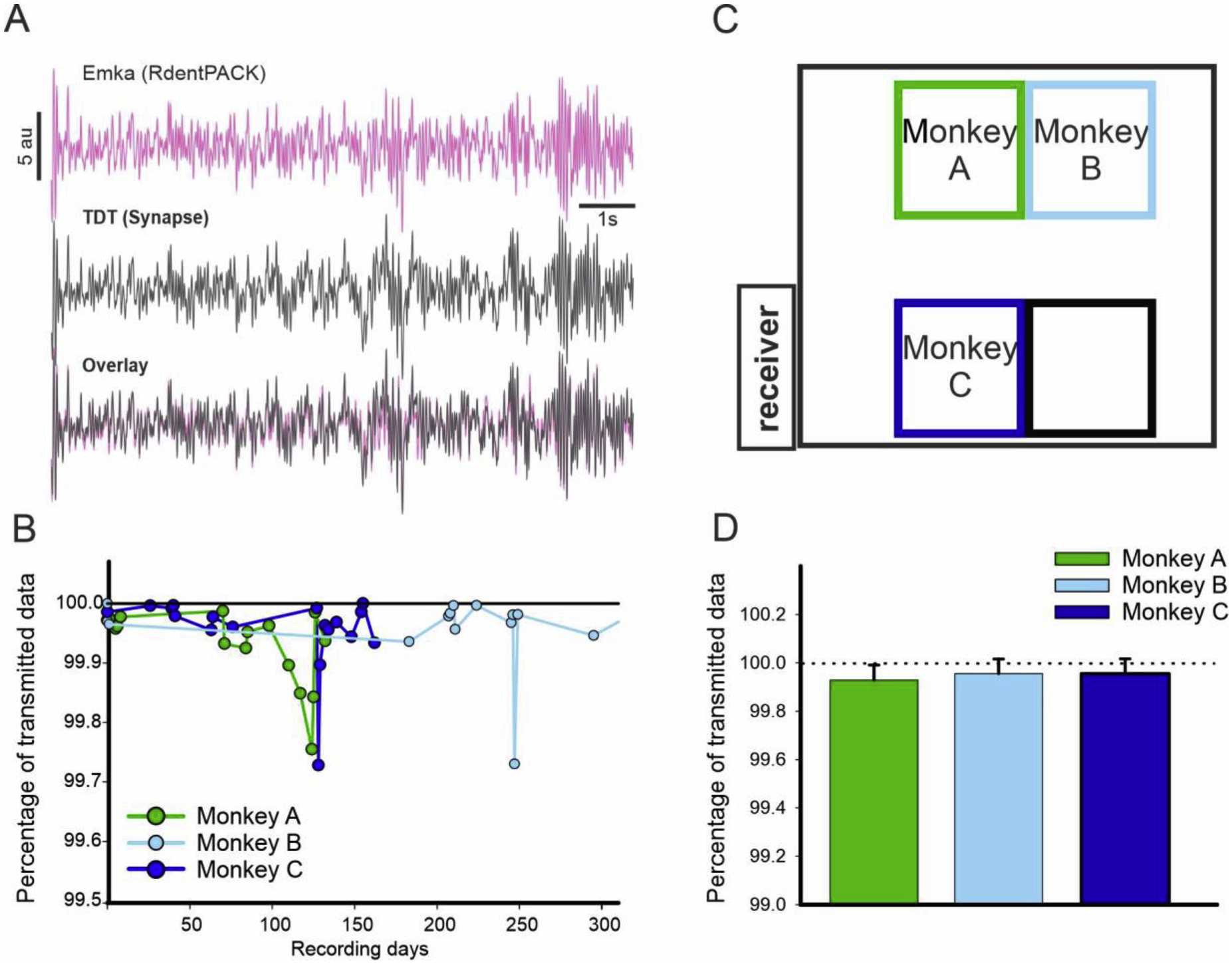
A. Example of overlap between the EEG signal recorded with the Emka software (pink) and the Synapse software (grey). B. Quality of the recording overtime for the three animals (100 being the maximum). C. Representation of the animal housing where the recordings were performed. The distances from the receiver to the center of the cages were 3.5, 3.9 and 4.3 meters respectively for Monkey A, B and C. D. Average percentage of data transmitted for the 3 animals using the same telemeter (no significant difference was found between the 3 animals, Kruskal-Wallis One Way ANOVA).
2.1. Design of the telemeter chamber
Unlike rodents, monkeys have incredible dexterity and strength. In order to utilize the RodentPACK for NHP, we needed an enclosure that could withstand chronic impact for several months. However, we were also limited by the surface area on the monkey’s cranial implant (see animals and surgical procedures). Current commercial enclosures were unable to fulfill these criteria; therefore, we designed and created a custom enclosure using Onshape, an online 3D CAD software (Cambridge, MA).
The enclosure chamber, base and lid combined, has an external diameter of 33.5 mm and a maximum height of 61.8 mm (Figure 1C). While the base was designed to be permanently implanted into the acrylic cap of the animal, the Lid is removable to permit access to the inside of the chamber. The base wall has two thicknesses, 3.1 mm for the lower part and 1.6 mm for the upper part of the base (Figure 2 B). The difference in thickness was motivated by the shape of the telemeter and the need to reinforce the part of base anchored in the acrylic. To accommodate the NHPs’ skull curvature and provide some flexibility during surgery, the bottom of the bases were designed with various angles (0°, 5°, 10°, and 15° angles) (Figure 1 B,C). Furthermore, to increase the contact surface area for dental acrylic adhesion, we added threads on the lower portion of the bases. The threads have a pitch of 1.9 mm and a height of 10.0 mm from the bottom of the base. This height was fixed regardless of the different angled base bottoms (Figure 1 B, C).
Figure 2:
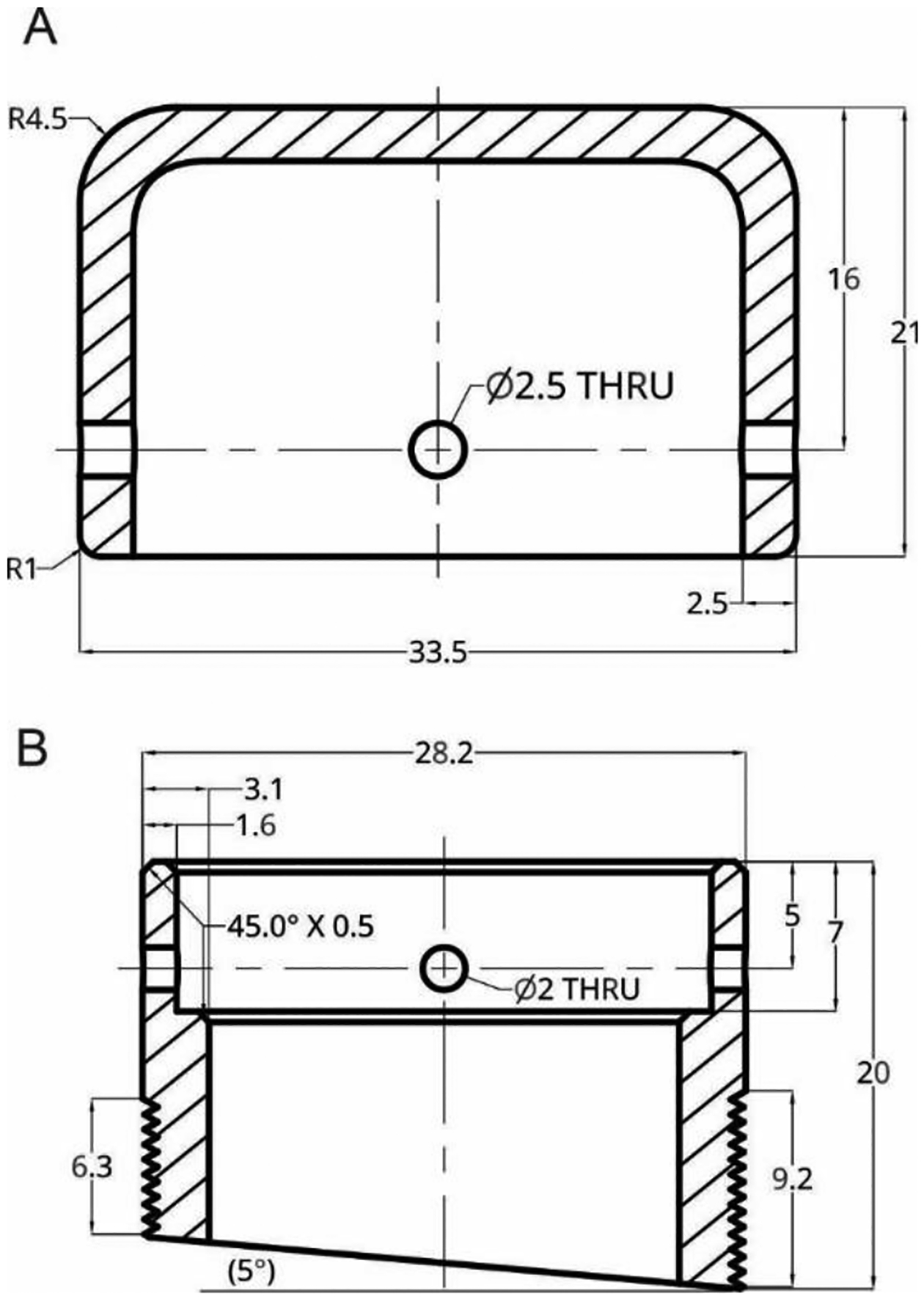
Detailed schematic of the lid (A) and base (B) of the telemeter chamber. A correspond to the short lid and B to a 5-degree base. All dimensions are in millimeters.
The lid has an internal diameter of 28.5 mm which provides a 0.3 mm gap from the external diameter of the base. This clearance can vary based on the properties of the plastic and is adjustable during/after processing. The bases and the lid have each four equally spaced, concentric holes for latter attachment. The centers of the holes were placed 5.0 mm from the lower edge of the lid and from the upper edge of the base. Their diameter was 2.0 mm for the base and 2.5 mm for the lid. In an effort to mitigate the encumbrance on the head cap when the telemeter was unused, we designed a short and tall lid of respectively 15.0 mm and 21.0 mm in height. For Monkey A, the enclosure was printed in Acrylonitrile Butadiene Styrene (ABS), however, the chamber cracked due to wear and tear 132 days after the first recording. Because of this, for Monkey B and C, we printed in nylon using selective laser sintering (SLS) technique (Shapeways, New York, NY). The nylon enclosure have been in place for more than a year so far (recording still in progress for Monkey C). Because the chamber was not in direct contact with the skull, biocompatibility of the material was not an issue. We used Nylon PA11 (Arkema, Innovative Chemistry, Paris, France), a bio-based engineering plastic that has an excellent resistance to chemicals. It is characterized by high elongation at break, good elasticity, and high impact resistance.
For Monkey B and C, we performed post-printing processing on the nylon enclosures. Brass nuts (Tri-Star Inc. chevron threaded inserts M20X100C) were manually heated with a soldering iron and pressed into the four 2.5 mm holes of the lid. The brass nuts provide the threads for the screws, which reduced the wear and tear on the lid. However, to maintain the MRI compatibility of our NHP acrylic cap, no brass nuts were added to the base. Anchoring screws (McMaster-Carr 92095A104) were used to secure the lid to the base and for the protection of the connector, we recommend to always secure the lid onto the base. Finally, because SLS parts have a slightly rough, grainy surface finish, we sanded the exposed areas of the chamber to reduce dirt adherence and thus reducing the risk of skin infection around the edge of the cap. All parts were gas sterilized before surgery.
2.2. Animals and surgical procedures
Three adults Rhesus monkeys (Macaca mulatta, 2 males and 1 female, 7–12 kg, approximately 3 years old at the beginning of the study) were used for these studies. The animals were pair housed, except during night monitoring, and had ad libitum access to food and water. All experiments were performed in accordance with the U.S. Public Health Service Policy on the humane care and use of laboratory animals, including the provisions of the “Guide for the Care and Use of Laboratory Animals”(Garber J, Barbee R et al. 2011). All studies were approved by the Biosafety and Institutional Animal Care and Use Committees of Emory University. Animals were first conditioned to being handled by the experimenter and to sit in a primate chair. They then underwent an aseptic surgical procedure under isoflurane anesthesia (1–3%) for placement of seven epidural screw electrodes (diameter: 0.25 mm, length 0.4 mm). Four electrodes were inserted bilaterally through small holes drilled in the skull over the area 6 (6DR Dorsal premotor area) for bipolar recording, two additional screws were placed around one of the monkey’s eyes for EEG/EOG bipolar recording, and one reference electrode was placed in the occipital lobe (Saleem and Logothetis 2017). Wires from the electrodes were soldered to a 12-pin Omnetic circular connector (A22017–001, Omnetics, Minneapolis, MN, USA) also embedded in the acrylic. The connector was kept protected in the home-made designed 3D printed enclosure previously described. The base of the enclosure chamber was covered with acrylic along with several bone screws, which were inserted around the telemeter chamber to strengthen the anchor of the acrylic-covered base to the skull. Bone screws were not in direct contact with the base to prevent undue stress on the plastic.
A dummy telemeter was used during the surgery to verify that the circular connector was correctly located at the center of the chamber. Furthermore, all of the animals had a recording chamber implanted on the contralateral side of the telemeter chamber (for acute, deep electrophysiological recordings and seizures induction). The recording chamber was implanted following the same methodology as the telemeter enclosure chamber. A head holder was placed on the back of the skull and embedded into their acrylic cap (Crist Instrument, Hagerstown, MD; USA). After surgery, the animals were given analgesics and prophylactic antibiotic treatment for at least one week. Recordings started after the end of recovery period.
2.3. Data acquisition and set up
During set up, we plugged the RodentPACK telemeter onto the female connector embedded into the NHP cap and closed the chamber. Then, the animal was returned back to home cage before 17:30 to give adequate time for eating before lights-off at 19:00. We positioned a recording camera with infrared light in front of the home cage and stationed a moveable cart outside the housing room. The cart contains the receiver and laptop that collects the data (Figure 4B). Besides installing an Ethernet data port for the camera system, no other alteration was needed within the animal housing room. After night monitoring, the entire set-up is removed in the morning to permit cleaning of the animal cages and feeding of the monkeys. We recorded four signals: two EEG, one EEG/EOG, and one accelerometer. EEG recordings were stored at 250Hz and acceleration (x,y,z coordinates using , where is the gravitational acceleration) was recorded at 10Hz. Acquisitioned data were converted to EDF format in order to be imported and saved into CED (spike2 V.8, CED, Cambridge, UK) and Matlab format (Matlab R2019a, The MathWorks Inc, Natick, MA, USA) for further analysis. To evaluate the recording quality of RodentPACK signal, we simultaneously recorded EEG signal with a secondary electrophysiological recording system (Tucker Davis Technologies (TDT), Synapse software, Alachua, FL., USA). A three-way connector was used to simultaneously record the EEG signals of Monkey A with both systems. EEG signals were amplified, sampled at 250 Hz, and stored on a computer for further analysis. After extracting and importing both recordings into Matlab environment, data were normalized and band-pass filtered using 2th order Butterworth filtering (0.1–40 Hz). The mean-squared deviation between the two signals was calculated and we extracted from the RodentPACK the quality of the signal (QOS). The QOS is set up to be a binary value, sampled at 250Hz, where 1 correspond to a successful transmission and 0 to a failed transmission.
2.4. Vigilance state analysis
Sleep scoring through the night (19:00–07:00) was performed in spike2 using an open access sleep scoring script (“sleep score”, Spike2script, CED, Cambridge, UK). Sleep scoring was based on video recording, accelerometer activity, EOG signal, and EEG spectral power (delta: 1–4Hz, theta: 4–8Hz, alpha: 8–13Hz, and beta: 13–30Hz). The EEG channel with the least movement artifacts was selected for the spectral analysis. Our sleep stage scoring followed the recommendations by the American Academy of Sleep Medicine Visual Scoring Task Force and used 30 second epochs (Silber, Ancoli-Israel et al. 2007). Awake periods were characterized by fast alpha waves with occasional 2–7Hz mixed activity (< 50% of epoch). As sleep takes hold, stage 1 was characterized by an increase progression of 2–7Hz activity (>50% of epoch) and less fast alpha activity (< 50% of epoch). K-complexes and sleep spindles were the defining electrophysiological signal hallmarks of stage 2. Slow wave sleep (SWS), defined as < 2Hz activity, characterized stage 3 and stage 4. Epochs with less than 50% of SWS activity were assigned as stage 3, while epochs with more than 50% of SWS activity were assigned as stage 4. Like the awake stage, rapid eye movement (REM) was characterized by low voltage and fast activity. However, the animal must have slept for greater than two minutes prior to the start of the first REM epoch. Epochs with brief awakenings and shifts in position during sleep were scored the same as the previous epoch. When finalized, the vigilance scores were exported in Matlab format, and the hypnogram was constructed using a home-made Matlab program compiling the manual sleep scores of 30 second epochs over a [7PM–7AM] period.
2.5. Seizure induction and automatic seizure detection
In Monkey A and C, seizures were induced by using penicillin (Penicillin G sodium, Panpharma) diluted in sterile water (Millipore Sigma, Merck, Germany) for a final concentration 2500u/ul. A dose of 500u was injected into the arm region of the motor cortex using a mechanical pump connected to a Hamilton syringe at a rate of 1 ul/min. Seizure events occurred for a period of 4–6 hours after injection. Seizures were behaviorally characterized by tonic-clonic contraction of the contralateral forearm. Details from the model have been previously described (Devergnas, Piallat et al. 2012, Prabhu, Piallat et al. 2014, Prabhu, Chabardes et al. 2015). The RodentPACK software has an embedded alarm system that can be used to detect seizures and will automatically prompt an email notification to experimenters and veterinary staff for immediate attention. To configure an alarm, one need to select a parameter to track, a threshold values and a set time. The threshold is the value to which your parameter will be compared and the set time is the duration for which the value of the parameter must be maintained above the threshold to trigger the alarm. The system offer a series of preloaded parameters to track. In this study, we choose to track the power in one specific frequency band thus limit needed to be specified. Thus, we first performed an offline spectral analysis of our typical seizures. Offline analysis was performed using the spectrogram function in Matlab (hamming window 256, 50% overlap, resulting in a spectral resolution of approximatively 1Hz). Based on this spectrogram, we selected the frequency band which was consistently increased during seizures and determined a power threshold (Figure 5A–B). In addition, the recording were manually reviewed of line by an expert to validate the event detected as seizures (success) or false alarm and detect seizures missed by the alarm system.
Figure 5:
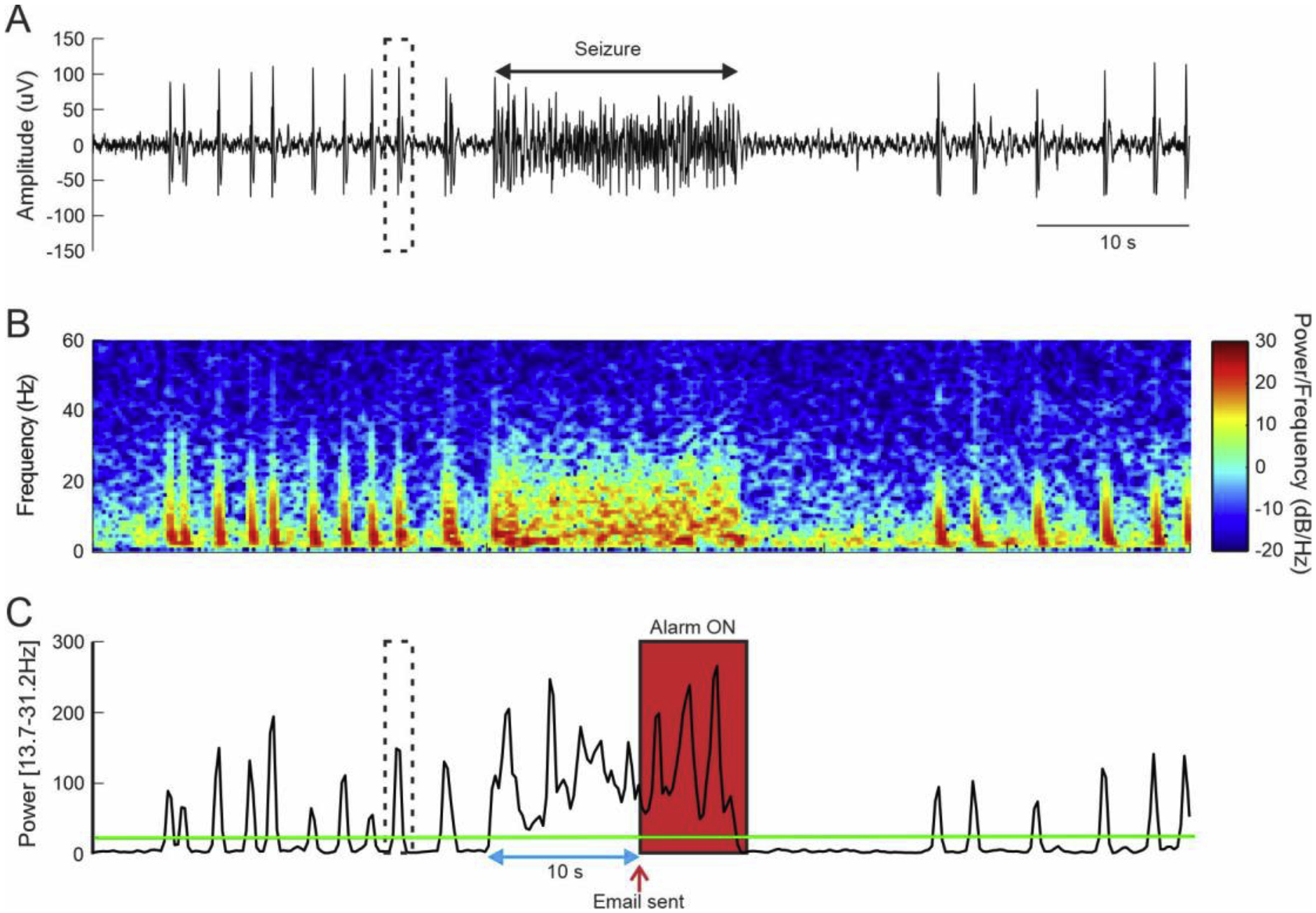
A. Typical seizure and interictal spike activity recorded on Monkey A. The dashed box shows an example of interictal spike. B. Corresponding spectrogram showing an increase of oscillatory activity during seizure as well as during interictal activity. C. Change in the power in the [13.7–31.2] Hz band. Green line indicates the power threshold used to trigger the alarm. The blue arrow illustrate the time threshold. The red arrow indicates when the email was sent, and the red box indicates when the alarm is ON. Threshold was set at 20db for a duration of 10s.
2.6. Statistics:
To evaluate QOS changes over time, we looked at the correlation between the QOS values and the recording days using the Spearman’s rank test. Comparison of the signal quality between animals was performed with a Kruskal-Wallis One Way ANOVA (SigmaPlot V14, Systat Software Inc, CA, USA). The inter-reader agreement was calculated with a Kappa test (GraphPad Prism, San Diego, CA). A P value of less than 0.05 was considered significant.
3. Results
3.1. Reliability and signal quality over time:
Figure 4A shows the overlap between the EEG signal recorded via the RodentPACK telemeter and the signal recorded via the Synapse software and the mean square error between the 2 signals was 0.0387, 0.0071 and 0.0231 respectively for channel 1, 2 and 3. We did not find a significant correlation between the QOS values and the numbers of days since the first recording (r=−0.4, p-value = 0.06; r = −0.08, p = 0.75; r = −0.37, p = 0.13 respectively for Monkey A, B, and C). We recorded 132 days, 381 days, 203 days respectively for Monkey A, B, and C. This indicates that the quantity of lost data did not change over time, even after more than a year of recording (Figure 4B). In addition, we did not find a significant difference in QOS values between the three animals despite a slight lower value for Monkey A, which was housed the furthest away from the receiver (Kruskal-Wallis One Way p=0.095, Figure 4C–D).
3.2. Sleep scoring
We used the RodentPACK acquisitioned data to score the sleep of our animals. For all of the animals, the five sleep patterns were identified (Figure 3). Two experimenters scored three baseline nights for Monkey A and C and two nights for Monkey B. The inter-reader agreement between the two experimenters was considered as strong (0.82 ±0.03, Inter-reader agreement, Kappa test). A hypnogram of a typical night with several clear sleep cycle, is shown in Figure 3B. Sleep cycles correspond to the time during which the animal goes from light sleep to deep sleep and finally REM sleep. These cycle are illustrated by the wave shapes seen on the hypnogram. The peaks on the hypnogram correspond to the wake times throughout the night. Monkey A often briefly awakens during the night to eat or groom oneself. Similar result was found for Monkey B and C (data not shown).
Figure 3:
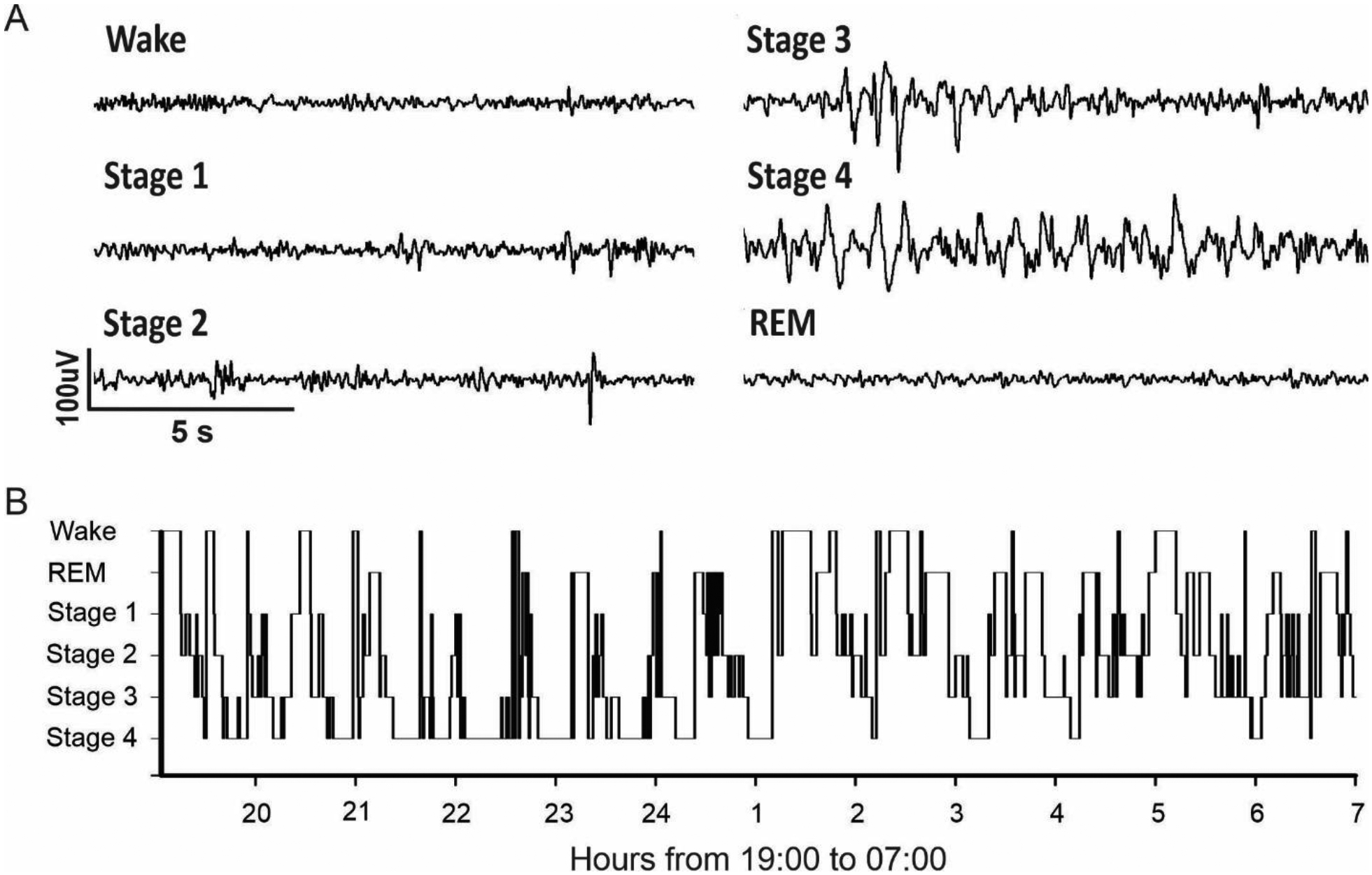
A. Sleep scoring criteria. Wake: low amplitude, high frequency. Stage 1: intermediate amplitude, mixed frequency. Stage 2: sleep spindles and K-complex. Stage 3: high amplitude delta waves are < 50% of epoch duration. Stage 4: high amplitude delta waves are > 50% of epoch duration. REM: low amplitude, high frequency, and asleep for at least 2 minutes. B. Typical hypnogram obtained from 19:00 to 07:00 (example obtained from Monkey A).
3.3. Automatic seizure detection:
To accurately set up the threshold for our seizure detection, we first performed an off-line spectral frequency analysis of typical seizures in Monkey A (Figure 5). At the beginning of the seizure, we observed an increase of the power in the frequency band [13.7 – 31.2 Hz] that stay elevated during all the duration of the ictal period. However, the power in this frequency band was also elevated during interictal spikes observed in between seizures (Figure 5). While considered as abnormal epileptic activity, we did not want to trigger the alarm when interictal activity was detected. Thus, to prevent false alarm, we set up a time duration at 12 s, time during which the power of the band needs to stay above the threshold (Figure 5C). Consequently, our alarm was set up to be triggered when the power in the [13.7 – 31.2 Hz] band was above 20db for at least 12 consecutive seconds. The same threshold configuration was used for both animals. For Monkey A, the sensitivity was 100% - all 14 seizures were detected and the false alarm rate was zero. However, despite a clear increase of power in the [13.7 – 31.2 Hz] frequency range at the beginning of the seizures, the power of this band fluctuated during the seizure which resulted in sending several alert emails per seizure (Figure 6A and Supplementary Figure 1). Thus, a total of 39 alert emails were sent for 14 seizures (in average of 2.9 alert emails per seizure). This redundancy of the alert email for the same seizure could be resolved by lowering the power threshold, but it can also be considered as an additional opportunity to pay attention to the animal behavior. For Monkey C, the sensitivity was 100% - all 23 seizures were correctly detected (see Supplementary figure 1 and 2 for full recording) and the false alarm rate was 4% (corresponding to one false alarm total).
Figure 6.
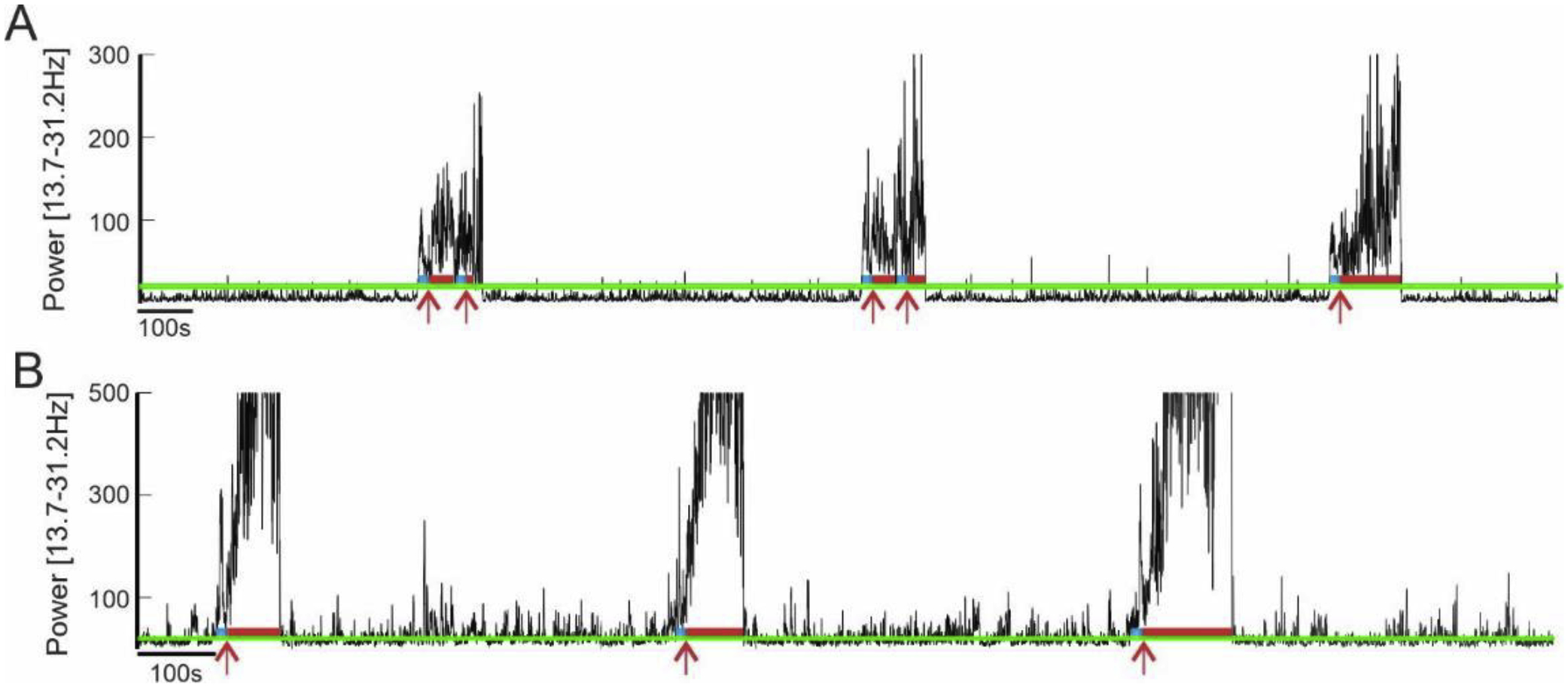
Alarm triggering based on the power in the [13.7–31.2Hz] band for Monkey A (in A) and Monkey C (in B). The green line corresponds to the power threshold (20db). The blue lines show that the power is above the threshold for 10s, and the red line correspond to the alarm being ON. The red arrows indicate when the email was sent.
4. Discussion
There are several telemetries currently available for NHP, but at the time of our purchase, none of these systems had the functional and technical specification that we needed for our research experiment (rechargeable battery and real-time online analysis). Consequently, we decided to adapt a head-mounted telemeter originally conceived for rodents. This report describes the adaptation and validation of the RodentPACK telemeter for NHP utilization. We designed an enclosure that protects the connector and telemeter for more than a year while being of a suitable size for the animal’s head. This enclosure allowed us to record good quality EEG data for more up to year on the same animal (experiments are still in progress in Monkey B and C).
4.1. The design, material, and manufacturing
The major design criteria of the enclosure was to optimize robustness while minimizing the encumbrance on the animal head. This was particularly relevant in our case, since all of our animals were also equipped with a standard recording chamber (on the contralateral side) and a head holder on the back of the head (Crist Instruments, Hagerstown, MD). While the Crist recording chamber was too small to accommodate the RodentPACK, their designs inspired us to include the textured threading around the bottom and to cut the base at variable angles.
We were able to maintain the smallest form factor of the enclosure, such that the final design is only 2 mm larger than the MRI compatible Crist recording chamber. The small size of the enclosure permits the usage of the telemetry system for other primates, including Macaca fascicularis A typical adult Macaca fascicularis has a 55.1 mm posterior cranial breadth, which gives ample space to fit the 33.5 mm wide enclosure (Bolly, Indah et al. 2019). In addition, our experiments required us to maintain the MRI compatibility of the animals’ acrylic cap. Although the brass nuts on the lid of the enclosure were melted directly into the plastic, we tapped screw threads onto the base. The tapped threads, however, may crack the plastic overtime due to daily wear and tear. As a result, we highly recommend adding the brass nuts to the base if MRI compatibility is not an issue. Note that while the current telemeter itself is not MRI compatible, the circular Omnetic connector permanently implanted during the surgery can be made with MRI compatible elements.
To minimize the burden for the animal, we designed lids with two heights. The tall lid was used when the RodentPACK telemeter was connected, while the short lid aims to only protect the connector. The overall height could be reduced by moving the holes of the screws closer to the edges of the base and lid. However, we do not recommend doing this, since inserting and removing the screws induce pressure that can lead to cracks overtime. Moreover, there needs to be sufficient material around the hole to grip the brass nuts.
The type of material was another important concern to ensure sturdiness of the chamber while allowing for robust signal transmission from the telemeter. Since metal would interfere the communication between the telemeter and the receiver, we needed to choose a strong plastic that can withstand frequent impact. Moreover, another factor to consider when using 3D printing technology of plastics is the manufacturing. For our first animal (Monkey A), we began by using fused deposition modeling (FDM), which is a common plastic printing method where thermoplastic filaments are layered and melted together. A common filament of FDM is Acrylonitrile Butadiene Styrene (ABS), which is known to have excellent strength. However, we found that ABS was too brittle and would crack over time. These cracks were most likely to form between the edge of the lid or base and the holes for the screws, which is why we only recorded 132 days on Monkey A. We then chose to use Selective Laser Sintering (SLS), which fuses small particles of polymer powder to make the 3D print. This method is great for small parts with thin walls and does not require printing supports. We used Nylon PA11, a bioplastic polyamide powder derived from castor oil. As a material, PA11 is not as strong as ABS. However, PA11 has a greater elongation at break and is more elastic: proving it to be a better material for frequent impacts. Although the surface finish is grainy, we slightly increased the outer surface area to account for post-processing sanding. Moreover, because Nylon PA11 is made from renewable raw materials derived from vegetable oil, it has a lower environmental impact than PA12. While this was not in our priority when choosing the material, we still considered this as an added benefit. Other materials, such as ULTEM and PEEK, are also excellent in strength and impact resistance. However, these materials are much more expensive than PA11, and manufacturers using this material are rare.
4.2. Advantages of the RodentPACK compared to other telemetry devices
DSI telemeters (Data Sciences International, St. Paul, Minnesota) are a popular choice in the NHP research community (Durmuller, Guillaume et al. 2007, Authier, Paquette et al. 2009, Bassett, Troncy et al. 2014). These internally implantable systems have an integrated non-rechargeable battery that can last up to 120 days and can stream data online. In contrast, the RodentPACK system uses batteries that can last up to 150 hours of continuous recording and can be easily replaced when needed. It is interesting to note that rechargeable, implantable batteries have recently become incorporated in neurostimulation devices designed for human use (Restor Rechargable neurostimulator, Medtronic, Minneapolis, MN). These neurostimulators can be recharged using an antenna positioned on the skin surface. The recharging procedure can be completed within a few hours and can last for a couple of days depending on usage. However, while this technology has been used for several years in human, it has yet to be develop commercially for animals.
The Neurologger (NewBehavior, TSE system, MO, USA) is an unique alternative that has been previously used in NHP (Hoffmann, Coolen et al. 2012). This head-mounted system checks the rechargeable batteries criteria and the use of a standard connector in order to be compatible with other electrophysiological recording system. However, it does not allow for data streaming, which is a critical feature for online seizure monitoring. Using the RodentPACK real-time data analysis could drastically prevent or change the prognosis of generalized seizures. It is a sustainable and economical alternative to monitoring the animals by night shifts. Furthermore, remote monitoring can decrease the stress level of the target animal and other animals in the housing area. Since physical inspection is only necessary if there is a high suspicion that intervention procedures may be necessary, monitoring the animals visually through a surveillance camera and physiologically with RodentPACK can provide uninterrupted sleep to the animals.
Finally, RodentPACK has the capability to use the same telemeter for multiple animals, each equipped with an enclosure and connector, by designating a configuration for each animal. Yet, this can only be done if one does not have to monitor the same animal continuously. In our situation, we primarily wanted to ensure the safety and wellness of our animals after seizure induction sessions. Although we did not need to monitor more than one animal at a given time, the basic system can handle up to four telemeters.
4.3. Limitation and further optimization
To the best of our knowledge, at the time of this experiment, there was no better system that would allow long term recording, flexibility in the data acquisition system, cost effectiveness, and had an alarm system for seizures detection.
Despite these advantages, the system has some restrictions. The numbers of bio-potential channel are currently limited to four, but the 12pin connector could accommodate up to six channels in bipolar configuration. Though, the two channels that are not being recorded by the RodentPACK can be connected and recorded by a secondary electrophysiological system. Furthermore, there are many factors that can play a role in signal drop, albeit small. For example, high monkey activity, such as jumping or shaking of the cage, can produce signal drops. Human factors, such as shifting the animal cages deeper into the room, can cause more signal drops that may not be overtly noticeable at the start of the recording. In addition, while the batteries can last up to 150 hours, the system does not keep track of the battery charge. This makes it necessary to track battery usage in order to avoid losing power during remote monitoring. For that, we highly recommend to buy a battery tester and to replace the battery when the half charge is past. Finally, since metal material can interfere with data transmission, the telemeter’s enclosure must be manufactured in plastic, and one needs to consider the quantity of metal between the telemeter and the receiver. However, Nylon PA11 has proved to be pretty resistant and despite the housing of our animal being in metal, we did not experience a great interference in signal (Figure 4B and 4D).
Currently, the software alarm system can send emails, but it would be an improvement if it can prompt an automatic phone call or text message. Lastly, it is important to recognize that in order to accurately set a threshold, one need to analyze the frequency component of the seizures specifically in each animal. In our experiments we have been able to use the same threshold for Monkey A and C, however further analysis would be necessary to generate a standardize threshold for seizures detection (Gotman 1990, Paul 2018). It is also important to keep in mind that telemeter will in general continue to decrease in size and companies will develop systems with even more enhance features.
NHP represent an important model in pharmacology and neurosciences in general. EEG monitoring is a common procedure that could inform on cortical changes, sleep disorder, and seizures. Ensuring the wellness and safety of animals is a priority in NHP laboratories, and this can best be achieved with an online, alarm EEG system. Here, we report the design of a 3D printed enclosure to house a head-mounted rodent telemeter (RodentPACK). We showed that this enclosure is capable to withstand and protect the telemeter against NHP’s strength for more than a year. Furthermore, we validate the system for long-term recording, sleep scoring and seizures detection with alarm monitoring.
Supplementary Material
Highlights.
Our new 3D printed design allow for long time telemetry recording on monkey.
EEG monitoring can be performed for several months.
This method refines the current sleep and seizure monitoring methods for NHP.
With minimum of adaptions, rodent telemeter are suitable for NHP experiments.
Acknowledgements:
Supported by a grant from the NIH Office of Research Infrastructure Programs OD P51-OD011132 to the Yerkes National Primate Research Center and a NIH/NINDS grant 1UG3NS100559-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- Authier S, Paquette D, Gauvin D, Sammut V, Fournier S, Chaurand F and Troncy E (2009). “Video-electroencephalography in conscious non human primate using radiotelemetry and computerized analysis: refinement of a safety pharmacology model.” J Pharmacol Toxicol Methods 60(1): 88–93. [DOI] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F and Ghorayeb I (2009). “Sleep disorders in Parkinson’s disease: The contribution of the MPTP non-human primate model.” Experimental Neurology 219(2): 574–582. [DOI] [PubMed] [Google Scholar]
- Bassett L, Troncy E, Pouliot M, Paquette D, Ascah A and Authier S (2014). “Telemetry video-electroencephalography (EEG) in rats, dogs and non-human primates: methods in follow-up safety pharmacology seizure liability assessments.” J Pharmacol Toxicol Methods 70(3): 230–240. [DOI] [PubMed] [Google Scholar]
- Bolly H, Indah AR, Faried A, Noverina R, Zafrullah A and Wirakusumah F (2019). “Cranial Characteristics, Maxillofacial, and Skull Base Structure of Non-Human Primate (Adult Macaca fascicularis): A Preliminary Study for Cranial Craniotomy Model.” MKP 51(2). [Google Scholar]
- Devergnas A, Piallat B, Prabhu S, Torres N, Benabid AL, David O and Chabardes S (2012). “The subcortical hidden side of focal motor seizures: evidence from micro-recordings and local field potentials.” Brain 135: 2263–2276. [DOI] [PubMed] [Google Scholar]
- Dupin M, Garcia S, Boulanger-Bertolus J, Buonviso N and Mouly AM (2019). “New Insights from 22-kHz Ultrasonic Vocalizations to Characterize Fear Responses: Relationship with Respiration and Brain Oscillatory Dynamics.” eNeuro 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmuller N, Guillaume P, Lacroix P, Porsolt RD and Moser P (2007). “The use of the dog electroencephalogram (EEG) in safety pharmacology to evaluate proconvulsant risk.” J Pharmacol Toxicol Methods 56(2): 234–238. [DOI] [PubMed] [Google Scholar]
- Froget G, Peyon G, Bigot K, Napoleoni JG and Esneault E (2017). “Assessment of Seizure Activity in the Telemetered Dog: A New Procedure Using the rodentPack System.” Journal of Pharmacological and Toxicological Methods 88: 222–222. [Google Scholar]
- Gainza-Lein M, Fernandez IS, Ulate-Campos A, Loddenkemper T and Ostendorf AP (2019). “Timing in the treatment of status epilepticus: From basics to the clinic.” Seizure 68: 22–30. [DOI] [PubMed] [Google Scholar]
- Garber J, Barbee R, Bielitzki J, Clayton L, Donovan J, Hendriksen C, Kohn D, Lipman N, Locke P, Melcher J, Quimby F, Turner P, Wood G and W. H (2011). “Guide for the Care and Use of Laboratory Animals (8th ed.).” Washington, DC: National Academies Press. [Google Scholar]
- Goonawardena AV, Morairty SR, Orellana GA, Willoughby AR, Wallace TL and Kilduff TS (2019). “Electrophysiological characterization of sleep/wake, activity and the response to caffeine in adult cynomolgus macaques.” Neurobiol Sleep Circadian Rhythms 6: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Plano A, Robinson L, Platt B, Hampson RE and Riedel G (2011). “A Pilot Study into the Effects of the CB1 Cannabinoid Receptor Agonist WIN55,212–2 or the Antagonist/Inverse Agonist AM251 on Sleep in Rats.” Sleep Disord 2011: 178469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J (1990). “Automatic seizure detection: improvements and evaluation.” Electroencephalogr Clin Neurophysiol 76(4): 317–324. [DOI] [PubMed] [Google Scholar]
- Gurbanova AA, Aker RG, Sirvanci S, Demiralp T and Onat FY (2008). “Intra-amygdaloid injection of kainic acid in rats with genetic absence epilepsy: The relationship of typical absence epilepsy and temporal lobe epilepsy.” Journal of Neuroscience 28(31): 7828–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Coolen A, Schlumbohm C, Meerlo P and Fuchs E (2012). “Remote long-term registrations of sleep-wake rhythms, core body temperature and activity in marmoset monkeys.” Behav Brain Res 235(2): 113–123. [DOI] [PubMed] [Google Scholar]
- Lundt A, Wormuth C, Siwek ME, Muller R, Ehninger D, Henseler C, Broich K, Papazoglou A and Weiergraber M (2016). “EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research.” Neural Plast 2016: 8213878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Y (2018). “Various epileptic seizure detection techniques using biomedical signals: a review.” Brain Inform 5(2): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu, Chabardes S, Sherdil A, Devergnas A, Michallat S, Bhattacharjee M, Mathieu H, David O and Piallat B (2015). “Effect of subthalamic nucleus stimulation on penicillin induced focal motor seizures in primate.” Brain Stimul 8(2): 177–184. [DOI] [PubMed] [Google Scholar]
- Prabhu, Piallat B, Devergnas A, Blauwblomme T, Sherdil A, Chivoret N, David O and Chabardes S (2014). “Characteristics of a primate model of focal motor cortical seizures suitable for preclinical testing of therapies like DBS.” World Journal of Neuroscience 04: 47–57. [Google Scholar]
- Rachalski A, Authier S, Bassett L, Pouliot M, Tremblay G and Mongrain V (2014). “Sleep electroencephalographic characteristics of the Cynomolgus monkey measured by telemetry.” J Sleep Res 23(6): 619–627. [DOI] [PubMed] [Google Scholar]
- Saleem K and Logothetis N (2017). A combined MRI and Histology Atlas of the Rhesus Monkey Brain.
- Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR and Iber C (2007). “The visual scoring of sleep in adults.” J Clin Sleep Med 3(2): 121–131. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


