Summary
Background
Financial incentives promote utilization of HIV services and may support adherence to the sustained antiretroviral therapy (ART) necessary for viral suppression, but few studies have assessed a biomarker of adherence or evaluated optimal implementation. We sought to determine whether varying sized financial incentives for clinic attendance impact viral suppression among patients starting ART in Tanzania.
Methods
We conducted a three-arm parallel-group randomised controlled trial in Shinyanga region. At four health facilities, HIV-positive adult (≥18 years) ART initiates (≤30 days) were randomly allocated using a tablet-based application (1:1:1, stratified by site) to receive usual care (control group) or to additionally receive a cash incentive for monthly clinic attendance in one of two amounts: 10000 TZS (≈US $4.50) or 22500 TZS (≈US $10.00). Participants were masked to the existence of two incentive sizes. Incentives were provided for up to six months via mobile health technology (mHealth) that linked biometric attendance monitoring to automated mobile payments. We evaluated the primary outcome of retention in care with viral suppression (<1000 copies per mL) at six months using logistic regression. This completed trial was pre-registered (ClinicalTrials.gov NCT03351556).
Findings
From April 24, 2018 to December 14, 2018, we randomised 530 patients (184 control; 172 smaller incentive; 174 larger incentive). All participants were included in the primary intention-to-treat analysis. At six months, approximately 134 (73.0%) participants in the control group remained in care and achieved viral suppression, compared to 143 (82.9%) in the smaller incentive group [Risk Difference (RD)=9.8, 95% CI: 1.2–18.5] and 150 (86.1%) in the larger incentive group (RD=13.0, 95% CI: 4.5–21.5); we identified a positive trend between incentive size and viral suppression (P-trend=0.0032), although the incentive groups did not significantly differ (RD=3.2, 95% CI: −4.6–11.0). Adverse events included seven deaths (4%) in the control group and 11 deaths in the intervention groups (3%), none related to study participation.
Interpretation
Small financial incentives delivered using mHealth can improve retention in care and viral suppression among adults starting HIV treatment. While further research should investigate the durability of effects from short-term incentives, these findings strengthen the evidence for implementing financial incentives within standard HIV care.
Funding
National Institute of Mental Health at the US National Institutes of Health.
Introduction
Viral suppression of HIV through sustained antiretroviral therapy (ART) provides significant individual health benefits and also prevents transmission.1 Acting on this promise, global “treatment as prevention” efforts to end the HIV epidemic have markedly expanded access to ART.2 However, substantial gaps remain in achieving population-level viral suppression, which can only be accomplished through testing, linkage to and retention in care, and continued individual adherence. Within East and Southern Africa—the region most affected by HIV—only 58% of people living with HIV attained viral suppression in 2018.3 Attrition from care poses a key breakdown in the treatment cascade, especially in the first months after treatment initiation.4 To address these challenges, increasing attention has focused on social determinants of health that may undermine retention and adherence, including poverty, food insecurity, stigma, social exclusion, discrimination, and other vulnerabilities commonly experienced by HIV-positive individuals.5,6 As one strategy to mitigate social and structural barriers limiting engagement with care, particularly in resource-constrained settings, recent reviews and UNAIDS guidelines have recommended implementing financial incentives linked to clinic attendance as part of a comprehensive HIV response.6-8
Financial incentives show promise for increasing viral suppression through pathways grounded in both traditional and behavioral economic theories.9 First, incentives offset the price associated with clinic attendance, including transportation costs and opportunity costs such as time away from work, which impose substantial burdens for many individuals living with HIV. Depending on the size, incentives may also provide an income effect that partially alleviates poverty-related barriers to care. Insights from psychology suggest that incentives can counteract present bias, aiding individuals faced with day-to-day challenges of poverty to prioritize the future benefits of treatment.10 Furthermore, even small incentives can provide a motivational “nudge” by signaling the importance of the incentivised behaviour.11
Previous research based on process indicators, such as appointment attendance and medication dispensing, suggests that financial incentives linked to clinic attendance may improve ART adherence in low- and middle-income countries (LMICs).12-15 However, considerable gaps remain in understanding the viability of this strategy. Few studies of incentives have examined a biomarker for HIV viral suppression; of these, most focused on distinct sub-populations such as men who use drugs and pregnant women.15,16 Additionally, previous studies have rarely compared different incentive amounts, a key to understanding the mechanism for intervention impact and an important step toward identifying effective real-world implementation practices.11 Moreover, complicated incentive delivery mechanisms used in previous studies—often requiring research staff to manually monitor eligibility and distribute payments by hand—may prove difficult to administer at scale. Thus, by evaluating two values of automated mobile payments provided upon clinic attendance, compared to a control group who received no incentives, we sought to determine the effects of different sized financial incentives on viral suppression among adults starting HIV treatment in Tanzania.
Methods
Study design
We conducted a three-arm parallel-group randomised controlled trial at four HIV primary care facilities in Shinyanga region, Tanzania following procedures set forth in our study protocol. The Tanzania National Institute for Medical Research (NIMR/HQ/R.8c/Vol. IX/2677) and the Committee for Protection of Human Subjects at the University of California, Berkeley (#2017-12-10558) provided ethics approval for this study. Here we report the study design and results according to CONSORT 2010 guidelines.17
Participants
Study participants were recruited from the population of patients seeking care at the participating health facilities. Eligible individuals met the following inclusion criteria: 1) ≥18 years old; 2) living with HIV infection; and 3) initiated ART ≤30 days before. In coordination with facility staff, research assistants identified potential participants from facility records and then approached these patients upon attendance at the clinic to assess interest, confirm eligibility, and obtain written informed consent in Kiswahili.
Randomisation and masking
Participants were individually randomised in a 1:1:1 allocation ratio to receive usual HIV care provided by the health facilities (control) or to additionally receive a monthly cash transfer for up to six months, conditional on visit attendance, in one of two amounts: 10000 TZS (≈US $4.50) or 22500 TZS (≈US $10.00). After completing informed consent, participant registration in the study’s mobile health technology (mHealth) system, and a baseline interview to assess sociodemographic characteristics, research assistants conducted randomisation using the mHealth system’s custom application installed on tablet computers. The application sequentially allocated participants stratified within site using randomly permuted blocks of 30, which were generated by the application developers and concealed from research assistants and investigators. Neither participants nor research assistants were masked to intervention assignment, however participants were masked to the existence of two incentive sizes [at six months, only 6 of 457 (1.3%) participants surveyed indicated knowledge of both cash amounts under evaluation]. Clinic and laboratory staff (the latter of whom conducted viral load quantification at a separate facility) were not informed of intervention assignments.
Procedures
All participants received usual clinical care as provided by the health facilities. As per national and global guidelines in place at these facilities, patients starting ART visit the clinic on a monthly basis for at least six months for clinical assessment and monitoring (weight, vital signs, screening for opportunistic infections) and medication dispensing, including antiretroviral drugs to treat HIV.18,19 In alignment with this standard care model, participants in the two intervention arms could receive cash transfers up to once per month during the six consecutive months following enrollment, conditional on attending a clinic visit. Participants could receive the first cash transfer at a visit ≥6 days after study enrollment (to accommodate typical clinic scheduling of the first appointment one to two weeks after starting ART) and thereafter at any visits ≥26 days apart—regardless of appointment timeliness—up to a maximum of six transfers (totaling a potential of US $27-$60 depending on arm and visit attendance).
The incentives were intended to motivate clinic attendance, with the amounts chosen in consultation with local and national stakeholders and designed to partially counter the costs of transportation, food, and lost wages for a day spent at the clinic. A previous randomized trial in the region piloted the larger of the two incentive amounts and determined it to be safe, acceptable, and effective (compared to a control group receiving usual care) based on appointment attendance and medication dispensing process indicators.12 This prior trial also evaluated the comparative effectiveness of cash incentive versus equivalently valued food baskets and found no statistically significant differences in retention or adherence, while cash was preferred by most study participants and logistically more feasible to distribute. The present study introduced the additional, smaller incentive amount to better understand the role of incentive size, including whether similar effectiveness could be achieved at a lower cost.
Attendance monitoring and cash transfer delivery was administered using the study’s tablet-based mHealth application, which linked biometric identification to an automated mobile payment system compatible with all mobile banking providers in Tanzania.20 Participant fingerprints and mobile banking account details were registered in the mHealth system during study enrollment. Subsequent clinic attendance was logged in the mHealth system upon a fingerprint scan administered by a pharmacist or research assistant at the pharmacy. After the system verified payment eligibility, participants in the intervention groups immediately received their assigned amount in their mobile banking account (either 10000 TZS or 22500 TZS, plus transfer fees), including a notification on their mobile phone. Participants who did not have access to a mobile banking account received cash in hand from a research assistant.
Viral load testing occurred approximately six months after ART initiation as part of routine care, consistent with WHO and Tanzanian guidelines for monitoring HIV infection (after which adherent patients with suppressed viral load can transition to less frequent clinic attendance).18,19 Laboratory staff conducted blood specimen collection at the first visit after 5.5 months on ART, although in some cases the specimen was collected earlier or later, including repeat collection following a failed test. Whole blood samples (4ml) were transported to the hospital laboratory within 6 hours, centrifuged to retrieve plasma, and stored at −20°C. Samples were transported biweekly to a laboratory in Dar es Salaam for testing. We used the Cobas AmpliPrep (CAP)/Cobas TaqMan (CTM) 96 HIV-1 assay (Roche Molecular Systems, Branchburg, NJ) and Cobas 4800 for HIV viral load quantification. Results were typically available within two days of sample arrival at the laboratory.
Study staff conducted interviews and abstracted medical records at baseline and approximately six months. All interviews were conducted in Kiswahili and assessed socio-demographic characteristics and other self-reported attributes. Medical record abstraction included body weight, height, CD4 cell count, WHO Clinical Stage, and other routinely collected data. Additionally, at each visit a research assistant or pharmacist entered data into the mHealth system including medication type and quantity dispensed and next appointment date.
As retention in care was a key outcome of interest, no study-related efforts were made to contact participants who stopped attending the clinic until the six-month follow-up period was complete (while any routine tracing initiated by the clinic continued as usual). After six months, research assistants attempted to trace missing participants following PEPFAR guidelines, using phone calls, engagement with community health workers who conduct routine tracing, and triangulation with other facilities.21 ‘Exhaustive’ tracing efforts as defined by PEPFAR (three attempts using at least two tracing methods) were completed for all participants who stopped attending the clinic before the end of follow-up. If information suggested that a participant had transferred to another health facility, the new facility was contacted to verify whether the transfer occurred. Medical records for confirmed transfers were obtained from the new facility, including visit attendance and viral load test results if available. If no transfer was confirmed, the medical records from the original clinic were considered the last engagement with facility-based HIV care.
Outcomes
The primary outcome is HIV viral suppression, defined as the proportion of patients retained in ART care and virally suppressed (<1000 copies per mL, the WHO threshold for virologic failure19) at six months after starting ART. This outcome definition is consistent with global “treatment as prevention” strategies including the UNAIDS Fast-Track targets, which aim for at least 95% of people living with HIV to know their status, 95% of these to be retained in care, and 95% of these to be virally suppressed by 2030.2 Following PEPFAR guidelines, patients considered not retained in ART care include those who died, disengaged from care or otherwise stopped ART, or had no evidence of facility-based care for ≥28 days after a missed appointment (i.e., the PEPFAR definition of lost to follow-up from facility-based care).21 Thus, as recommended by PEPFAR, participants who could not be found after exhaustive tracing efforts (described above) were classified as not retained in care. Only viral load results for specimens collected from 5 through 7 months on ART were included in the analysis. Patients retained in care but without a valid viral load result in this window were considered to have a missing outcome.
Pre-specified secondary outcomes include the component measures of the primary viral suppression outcome—the proportion retained in care at six months and the proportion virally suppressed among only those retained in care—and mean appointment attendance, defined for each participant as the proportion of scheduled visits attended on-time (±4 days) over the 6-month follow-up period.12 We also present the mean number of visits attended during this period.
Statistical analysis
The trial was pre-registered at ClinicalTrials.gov (NCT03351556) and was overseen by a data safety monitoring committee. As part of the study design, we calculated the sample size necessary to conduct a Cochran-Armitage linear trend test22 between incentive size and the proportion achieving HIV viral suppression at six months. Assuming that 63% of participants in the control group would remain in care and achieve viral suppression at six months (based on adherence data from a previous study at the same clinics12), we determined that 530 participants (150 per group given 15% loss to follow-up) would provide 80% power (two-sided α=0.05) to detect a trend if at least 70% of participants in the smaller incentive group and 78% in the larger incentive group achieved viral suppression.
Following our pre-specified analysis plan, we first tested for a dose-response relationship between incentive size and 6-month viral suppression using a logistic regression model (corresponding to the Cochran-Armitage trend test), logit(p) = a + b*x, where p is the binomial proportion virally suppressed and x is the ordinal incentive amount (coded as 0, 10000, or 22500 TZS), against the null hypotheses of no trend (H0: b = 0 vs. Ha: b ≠ 0) at α=0.05. Next, we calculated pairwise differences between groups for all outcomes by modeling intervention arm as a three-level categorical variable, using logistic regression for binary outcomes and linear regression for appointment attendance. No adjustment was made for multiple comparisons. We controlled only for enrollment site in the primary analyses, to account for stratified randomisation.
In pre-specified secondary analyses, we (1) additionally adjusted for prognostic factors including age, sex, and baseline WHO clinical stage and (2) assessed effect heterogeneity using Wald tests for interaction. To conserve statistical power, heterogeneity analyses compared the pooled incentive arms to the control. Pre-specified baseline variables examined for effect heterogeneity included sex, age, wealth index (relative within the study population; constructed using polychoric principal components analysis on the reported number of common household assets, similar to Demographic and Health Surveys23), and time since HIV diagnosis at ART initiation.
The primary intention-to-treat analysis included all randomised participants. As per our prespecified analysis plan, we used multiple imputation24 to estimate viral suppression status for a small minority who remained in care at six months but lacked a valid viral load result (i.e., missing values for the primary outcome, details described in Results). Note as a sensitivity analysis, we also report complete-case estimates for viral suppression outcomes excluding participants who remained in care but lacked a viral load result. We implemented multiple imputation with 20 iterations separately for each study arm using a logistic model, including the same pre-specified prognostic factors as in the secondary adjusted analysis (clinic, age, sex, and baseline WHO clinical stage). Results were combined according to Rubin’s rules24 using the “mi estimate” command in Stata.25 All statistical analyses used Stata 14 (College Station, TX).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From April 24, 2018 to December 14, 2018, we recruited and randomised 530 participants; follow-up continued through June 20, 2019 and the primary analysis included all participants (Figure 1). Adverse events included seven deaths (4%) in the control group and 11 deaths in the intervention groups (3%), none related to study participation. Participants were majority female with a median age of 35 years (Table 1). Nearly a quarter had no formal education and two in five had not worked in the past week, while over half were characterized as WHO Clinical Stage 1 (asymptomatic). All measured baseline characteristics were balanced between randomisation arms.
Figure 1. Trial profile.
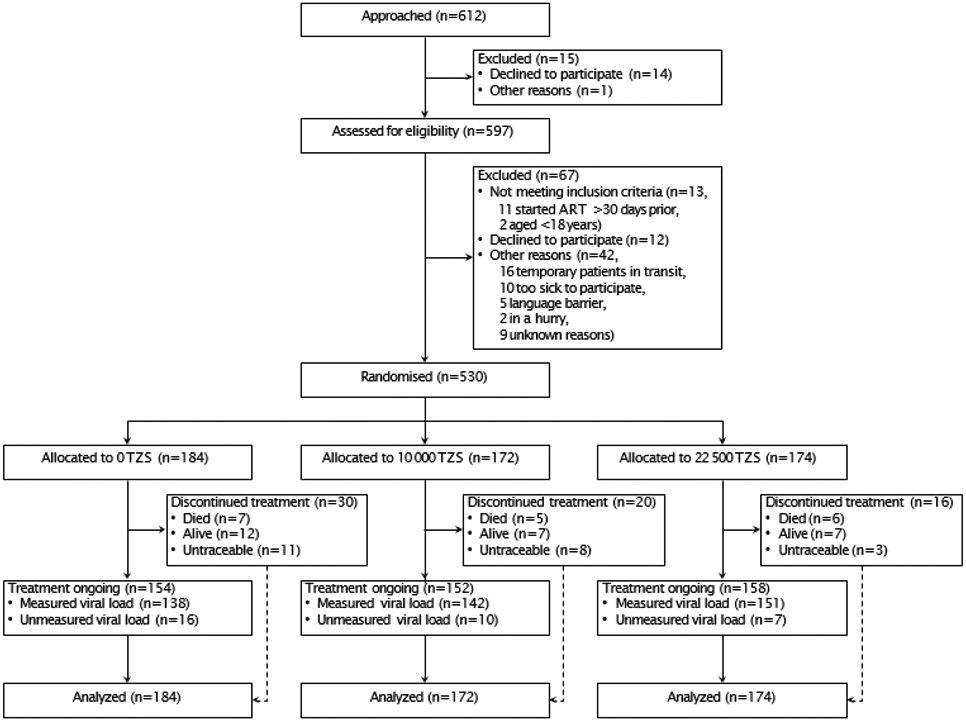
Table 1.
Baseline characteristics of the intention-to-treat population, HIV treatment initiates in Tanzania, 2018-2019.
| Cash Incentive Amount |
||||
|---|---|---|---|---|
| Total N=530 |
0 TZS (Control) N=184 |
10,000 TZS N=172 |
22,500 TZS N=174 |
|
| Sex | ||||
| Male | 200 (37.7%) | 68 (37.0%) | 63 (36.6%) | 69 (39.7%) |
| Female | 330 (62.3%) | 116 (63.0%) | 109 (63.4%) | 105 (60.3%) |
| Age (years) | 34.7 (28.4-42.2) | 34.6 (28.3-42.8) | 36.0 (29.4-40.8) | 33.6 (28.1-42.0) |
| 18-24 years | 67 (12.6%) | 25 (13.6%) | 19 (11.0%) | 23 (13.2%) |
| 25-34 years | 204 (38.5%) | 68 (37.0%) | 62 (36.0%) | 74 (42.5%) |
| ≥35 years | 259 (48.9%) | 91 (49.5%) | 91 (52.9%) | 77 (44.3%) |
| Language | ||||
| Swahili | 244 (46.0%) | 75 (40.8%) | 88 (51.2%) | 81 (46.6%) |
| Sukuma | 264 (49.8%) | 99 (53.8%) | 78 (45.3%) | 87 (50.0%) |
| Other | 22 (4.2%) | 10 (5.4%) | 6 (3.5%) | 6 (3.4%) |
| Education level | ||||
| No formal education completed | 115 (21.7%) | 35 (19.0%) | 43 (25.0%) | 37 (21.3%) |
| Some primary | 84 (15.8%) | 31 (16.8%) | 27 (15.7%) | 26 (14.9%) |
| Primary completed | 331 (62.5%) | 118 (64.1%) | 102 (59.3%) | 111 (63.8%) |
| Occupation | ||||
| Farming | 114 (21.5%) | 41 (22.3%) | 30 (17.4%) | 43 (24.7%) |
| Business | 115 (21.7%) | 42 (22.8%) | 37 (21.5%) | 36 (20.7%) |
| Other | 249 (47.0%) | 81 (44.0%) | 85 (49.4%) | 83 (47.7%) |
| Unemployed | 52 (9.8%) | 20 (10.9%) | 20 (11.6%) | 12 (6.9%) |
| Worked in the past week | 309 (58.3%) | 107 (58.2%) | 93 (54.1%) | 109 (62.6%) |
| Married or with partner | 288 (54.3%) | 100 (54.3%) | 93 (54.1%) | 95 (54.6%) |
| Head or joint head of household | 401 (75.7%) | 141 (76.6%) | 127 (73.8%) | 133 (76.4%) |
| Household size | ||||
| 1-3 members | 232 (43.8%) | 79 (42.9%) | 73 (42.4%) | 80 (46.0%) |
| 4-13 members | 298 (56.2%) | 105 (57.1%) | 99 (57.6%) | 94 (54.0%) |
| Wealth index | ||||
| Low | 177 (33.4%) | 58 (31.5%) | 57 (33.1%) | 62 (35.6%) |
| Middle | 177 (33.4%) | 68 (37.0%) | 55 (32.0%) | 54 (31.0%) |
| High | 176 (33.2%) | 58 (31.5%) | 60 (34.9%) | 58 (33.3%) |
| Transit cost for clinic visit (TZS) | 1000 (0-2000) | 1000 (0-2000) | 1000 (0-2000) | 1000 (0-2000) |
| Facility | ||||
| Referral hospital | 42 (7.9%) | 14 (7.6%) | 14 (8.1%) | 14 (8.0%) |
| Hospital | 326 (61.5%) | 114 (62.0%) | 106 (61.6%) | 106 (60.9%) |
| Health center | 79 (14.9%) | 27 (14.7%) | 25 (14.5%) | 27 (15.5%) |
| Dispensary | 83 (15.7%) | 29 (15.8%) | 27 (15.7%) | 27 (15.5%) |
| Time since ART start (days) | 10.6 (7.0) | 10.3 (6.9) | 10.0 (7.2) | 11.4 (6.7) |
| Delay starting ART after diagnosis | ||||
| 0-1 days | 169 (31.9%) | 55 (29.9%) | 58 (33.7%) | 56 (32.2%) |
| 2-7 days | 204 (38.5%) | 77 (41.8%) | 61 (35.5%) | 66 (37.9%) |
| >1 week | 157 (29.6%) | 52 (28.3%) | 53 (30.8%) | 52 (29.9%) |
| Weight (kg) | 58.3 (11.0) | 58.0 (11.3) | 58.7 (10.6) | 58.3 (11.2) |
| WHO Clinical Stage | ||||
| Stage 1 | 281 (53.0%) | 98 (53.3%) | 91 (52.9%) | 92 (52.9%) |
| Stage 2 | 187 (35.3%) | 62 (33.7%) | 63 (36.6%) | 62 (35.6%) |
| Stage 3 | 59 (11.1%) | 23 (12.5%) | 17 (9.9%) | 19 (10.9%) |
| Stage 4 | 3 (0.6%) | 1 (0.5%) | 1 (0.6%) | 1 (0.6%) |
| CD4+ count (cells per μl) | 369 (209-539) | 352 (174-601) | 394 (173-552) | 414 (246-532) |
| Pregnant | 18 (3.4%) | 4 (2.2%) | 7 (4.1%) | 7 (4.0%) |
Data are n (%), median (IQR) or mean (SD). TZS=Tanzanian Shillings. ART=antiretroviral therapy. WHO=World Health Organization. All variables are fully observed except for CD4+ cell count (n=229).
During the six-month intervention period, participants received a total of 1631 payments upon visit attendance, mostly delivered through mobile banking (77.5%). Almost all participants in the incentive arms received at least one payment ([163 (95%) and 168 (97%) within the smaller and larger incentive arms, respectively]. On average, participants in the smaller incentive arm each received 4.6 total payments (SD=1.7) with 73% delivered through mobile banking, while participants in the larger incentive arm each received 4.8 (SD=1.6) total payments with 78% through mobile banking; these differences were not statistically significant.
At six months, overall 464 (87.6%) participants remained in care, including 28 confirmed transfers to another facility (Figure 1). Of 66 (12.5%) participants not in care, 18 had died, 26 were confirmed alive but not in care, and 22 could not be located after exhaustive tracing attempts (classified as not in care according to PEPFAR guidelines). A total of 33 participants [6.2%; 16 (8.7%) control, 10 (5.8%) smaller incentive, and 7 (4.0%) larger incentive; P=0.181] who remained in care lacked a valid viral load result because either a blood specimen was not drawn on time (n=22), the test failed (n=8), or the result was not returned from the laboratory (n=3); viral suppression was multiply imputed for these participants.
Following our pre-specified analysis plan, we first identified a positive dose-response relationship between incentive size and six-month viral suppression (OR=1.10 per 2500 TZS, 95% CI: 1.03–1.17, P-trend=0.0032). In pairwise comparisons (Table 2 and Figure 2), relative to the control arm, a substantially greater proportion of participants remained in care and achieved viral suppression in both the smaller [82.9% vs. 73.0%, Risk Difference (RD)=9.8, 95% CI: 1.2–18.5] and larger incentive arms (86.1% vs. 73.0%, RD=13.0, 95% CI: 4.5–21.5). However, there was no statistically significant difference between incentive arms (RD=3.2, 95% CI: −4.6–11.0).
Table 2.
Summary of the effects of financial incentives on HIV viral suppression and retention on antiretroviral therapy, Tanzania, 2018-2019.
| Outcome at six months | N | Group estimate (SE)* |
Between-group difference (95% CI), p-value* |
||||
|---|---|---|---|---|---|---|---|
| 0 TZS | 10,000 TZS | 22,500 TZS | 10,000 vs. 0 TZS | 22,500 vs. 0 TZS | 22,500 vs. 10,000 | ||
| Retained in care and virally suppressed† | 530 | 73.0% (0.034) | 82.9% (0.029) | 86.1% (0.026) | 9.8 (1.2, 18.5), 0.026 | 13.0 (4.5, 21.5), 0.0027 | 3.2 (−4.6, 11.0), 0.42 |
| Retained in care | 530 | 83.7% (0.027) | 88.4% (0.024) | 90.8% (0.022) | 4.7 (−2.5, 11.8), 0.20 | 7.1 (0.3, 13.9), 0.041 | 2.4 (−4.0, 8.8), 0.46 |
| Virally suppressed‡ | 464 | 87.1% (0.030) | 93.8% (0.021) | 94.9% (0.018) | 6.7 (−0.2, 13.6), 0.057 | 7.8 (0.9, 14.7), 0.027 | 1.1 (−4.4, 6.6), 0.70 |
| Appointment attendance (%)§ | 530 | 80.0% (0.017) | 87.4% (0.017) | 90.5% (0.017) | 7.4 (2.7, 12.1), 0.0020 | 10.5 (5.9, 15.2), <0.0001 | 3.1 (−1.7, 7.9), 0.20 |
| Total number of visits attended | 530 | 4.30 (0.129) | 4.83 (0.133) | 5.03 (0.133) | 0.54 (0.17, 0.90), 0.0041 | 0.73 (0.37, 0.11), 0.0001 | 0.19 (−0.18, 0.56), 0.30 |
Data are estimates from generalized linear models adjusted for the clinic where randomisation occurred. TZS=Tanzanian Shillings.
Viral suppression status was multiply imputed for 33 (6.2% of 530 overall) participants, who remained in care but were missing a valid viral load result.
Primary outcome; the composite proportion of patients who remained in care at six months and had a viral load <1000 copies per mL.
Among those retained in care (n=464 overall).
The mean patient’s proportion of scheduled appointments over six months that were attended within 4 days of the scheduled date.
Figure 2. Retention in care and HIV viral suppression (<1000 copies per mL) at 6 months by intervention group.
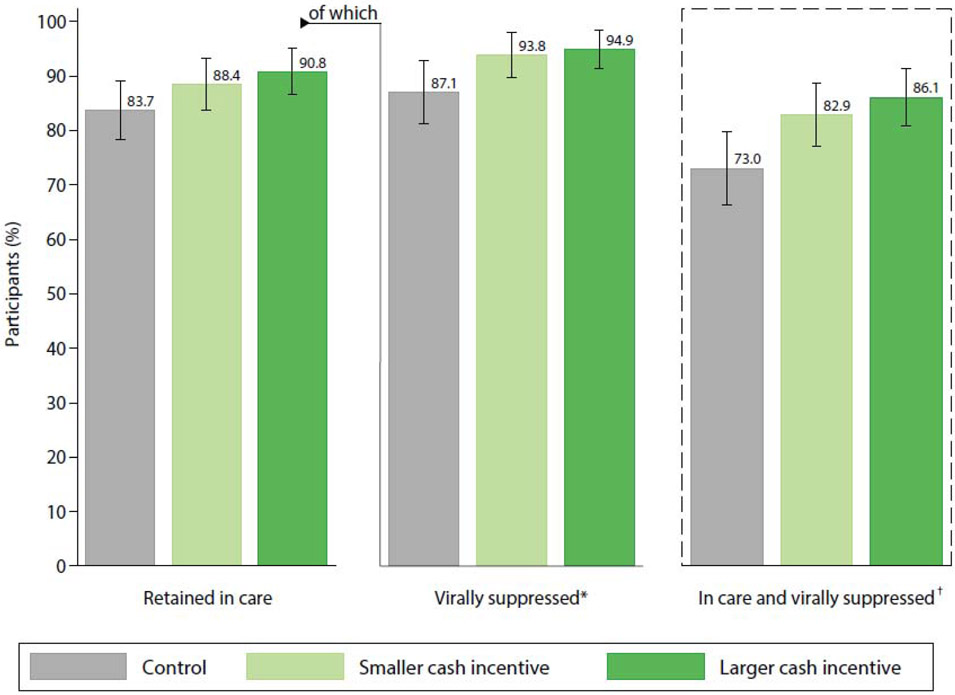
Data are predictive marginal probabilities and 95% confidence intervals estimated from logistic regression models adjusted for clinic.
*Of those retained in care at 6 months (N=464 out of total sample N=530).
†Primary outcome; the composite proportion of patients who remained in care at six months and had a viral load <1000 copies per mL.
Similar patterns were observed for the secondary component outcomes of retention in care alone and viral suppression among those retained in care. Compared to the control arm, the larger incentive achieved significantly higher 6-month retention in care (90.8% vs. 83.7%, RD=7.1, 95% CI: 0.3–13.9) and viral suppression among those retained in care (94.9% vs. 87.1%, RD=7.8, 95% CI: 0.9–14.7); lesser improvements in these measures with the smaller incentive were not statistically significant (retention in care: 88.4% vs. 83.7%, RD=4.7, 95% CI: −2.5–11.8; viral suppression given retention in care: 93.8% vs. 87.1%, RD=6.7, 95% CI: −0.2–13.6). The mean proportion of appointments attended on-time substantially exceeded the control arm (80.0%) for both the smaller (87.4%, RD=7.4, 95% CI: 2.7–12.1) and larger incentive arms (90.5%, RD=10.5, 95% CI: 5.9–15.2). There were no statistically significant differences between incentive arms for any measures.
In secondary analyses, results were similar when additionally adjusting for prognostic factors (appendix pp. 1) and in the complete-case sensitivity analysis (appendix pp. 2). In heterogeneity analyses (Figure 3, appendix pp. 3), disaggregating by relative wealth showed variation in the effectiveness of incentives (combined incentive arms compared to the control): In the lowest wealth tertile, incentives elevated the proportion retained in care and virally suppressed from 68.2% (control) to 87.1% (RD=18.9, 95% CI: 5.1–32.7), while in the highest wealth tertile incentives had no detectable effect as both groups achieved relatively high viral suppression (83.1% control vs. 83.6% cash, RD=0. 5, 95% CI: −11.9–12.9; P-interaction=0.068). Incentive effects on viral suppression did not substantially vary by sex, age, or timeliness of ART start after HIV-positive diagnosis (P-interaction0.20 for all analyses).
Figure 3. Effects of incentives (combined groups) on viral suppression within subgroups according to baseline characteristics.
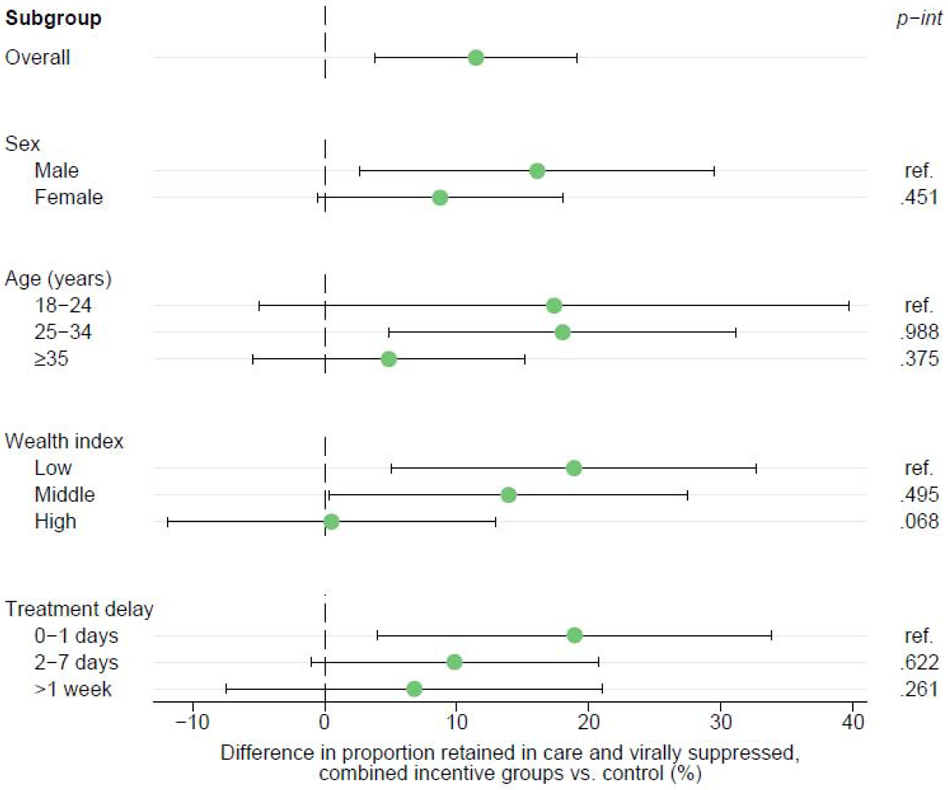
Data are risk differences with 95% confidence intervals estimated from generalized linear models adjusted for clinic, comparing the combined financial incentive arms to the control arm who received no incentives. P-values for each interaction are shown.
Discussion
In this randomised trial of 530 HIV-positive adults starting ART in Shinyanga, Tanzania, we found that small financial incentives improved the proportion of patients who remained in care and achieved viral suppression at six months by as much as 13 percentage points (with the larger of two incentive amounts tested), an 18% relative improvement over usual care. We identified a positive trend between incentive amount and viral suppression, while further demonstrating effectiveness of both tested amounts compared to the control. Incentives also bolstered retention in care alone, viral suppression among those retained in care, and appointment attendance. While direct comparison of incentive amounts showed no statistically significant differences for any outcome—which suggests possible diminishing returns from increasing incentive amount—our study has limited power for this determination (as it was not designed for pairwise comparisons between arms). These findings contribute to a critical gap in understanding the effects of financial incentives on a biomarker of HIV treatment adherence. To our knowledge, this is the first randomized trial in a LMIC to evaluate the impact of financial incentives for treatment-seeking behaviour on HIV viral suppression among a general population.
A recent review of conditional economic incentives for HIV treatment and prevention in LMICs found burgeoning evidence for positive effects on prevention outcomes and process indicators of treatment retention and adherence, but identified only three randomized trials that measured impacts on viral suppression.9 The first of these studies, of 433 HIV-positive pregnant women in the Democratic Republic of the Congo, found that cash incentives delivered upon attending scheduled clinic visits and accepting proposed services (valued at US $5 for the first visit and escalating by $1 at each consecutive visit, up to a maximum total of $45 over six visits) resulted in higher retention in care at six weeks postpartum compared to a control group who received no incentives.14 This trial evaluated viral suppression as a secondary outcome among the subset of participants who remained in care, finding no effect [66.1% intervention vs. 69.7% control virally suppressed among 174 (80.6%) and 158 (72.8%) respectively retained].16 These findings translate to approximately 53.3% of the full intervention group retained in care and virally suppressed versus 50.7% of the control group, a risk difference of 2.6 percentage points. This smaller effect than in the current study may relate to differences in populations, study procedures, or treatment context.
The second study, of 120 HIV-positive men who use drugs in India, found that quarterly delivery of non-monetary vouchers redeemable for food or household goods (valued at US $4 for ART initiation, $4 for monthly visit attendance, and $8 for viral suppression at 6 or 12 months) increased linkage and retention in care compared to a control group who could receive similarly sized lottery prizes at quarterly visits, but did not improve viral suppression or CD4 cell count.15 The authors outlined limitations including a small sample size and potentially restricted generalizability to other populations, and speculated that delayed payment for monthly visit attendance until the end of three-month intervals may have diminished the potential salience of incentives for motivating daily adherence behaviors.
Lastly, a recent trial of adult patients on ART in Uganda incentivised the achievement of viral suppression at four timepoints, from 6 to 48 weeks, with escalating incentives (increasing from US $4 to $12.5) delivered after home-based viral load measurement; they too found no effect on viral suppression.26 The authors noted possible explanations including high baseline viral suppression and frequent provision of viral load testing and counselling to all participants. Like the India-based study, they also suggested that incentives might have been too infrequent and not linked to interim behaviours on the pathway to viral suppression, including medication adherence. In summary, until now, no studies have investigated whether incentives delivered at the time of clinic attendance to a general adult population can improve viral suppression.
The present trial advances knowledge gained from previous studies in several important ways. This study was designed to evaluate a relatively simple implementation model feasible to administer in real-life clinical settings, including an automated mHealth system to regularly deliver monthly incentives through mobile banking. Incentives were provided on the ‘soft’ condition of clinic attendance, regardless of timeliness, to further simplify implementation and to avoid excluding disadvantaged patients facing the greatest obstacles to keeping appointments.27 Payments were delivered in the pharmacy at the time of medication refill and thus directly tied to a component of adherence. This may partly explain the difference in effects on viral suppression between the present study and prior trials despite relatively similar incentive sizes across studies. Behavioral economic theory supports the notion that timing and salience matter for the success of health incentives.11 In the present study, the effectiveness of both incentive amounts and the small non-significant difference between them—despite the larger value more than doubling the smaller one—suggests that these behavioral economic factors may have exerted an influence above and beyond the size of the incentive.
Additionally, the present study specifically targeted patients initiating ART, as numerous studies show that risk of attrition from care peaks in the first six months of treatment.4 The rationale for this focus additionally considered the heightened clinical and economic vulnerability of patients at this time28 and the potential to influence early habit formation for durable effects.29 Our findings suggest that financial incentives offered to adult ART initiates were indeed effective at least in the short term. While more work is needed to understand long term effects, preliminary data from the follow-up of a previous trial suggests that incentives for ART initiates do no harm and may have benefits even after withdrawn.30 The present study also showed that poorer individuals may benefit most from incentives, however attempting to target incentives based on wealth could complicate implementation and thereby hinder effectiveness, and again risk excluding individuals who stand to benefit—particularly given widespread poverty and challenges measuring wealth.
This study had important limitations. First, the trial was not powered to determine pairwise differences between groups (although these secondary comparisons were prespecified); it was primarily designed to evaluate the more efficient test for trend between incentive size and viral suppression. Therefore, given the imprecision in these pairwise measures of effect, our finding of no statistically significant difference between the incentive groups should be interpreted with caution. Moreover, the magnitude of effect may be viewed as only one of many important considerations when determining the optimal incentive size in a given setting, including availability of resources, stakeholder preferences, and the local economic context. We also note that observed viral suppression surpassed expectations used in power calculations (based on an older study using a process-based adherence indicator), however the proportion virally suppressed among those in care in the control group matches current national data and significant improvements were still achieved.3
Additional limitations are countered by important strengths. While we lacked viral load results for some participants who remained in care at six months, we followed standard multiple imputation procedures for these missing outcomes in order to include all participants in the primary analysis. This strategy sought to preserve the benefits of randomisation and improve statistical power. Because these participants were few (6.2%), did not vary from others in terms of observable baseline characteristics apart from a small difference in mean age (which was included in the imputation model), and a complete-case sensitivity analysis yielded similar results, we anticipate limited resultant bias.
Separately, a small number of participants could not be located to confirm their status after exhaustive tracing and were thereby classified as not retained in care. While this protocol followed gold-standard PEPFAR indicator guidelines, misclassification may have occurred if patients had an undocumented facility transfer and achieved viral suppression. The proportion of untraceable participants among those not retained in care did not significantly vary by study arm, suggesting that potential misclassification may be non-differential by arm and would therefore bias results towards the null. As a final strength, findings from this study are supported by those from our previous trial of financial incentives for ART initiates in the same region, which found positive effects on medication possession ratio, an indicator of adherence.12
In conclusion, this study provides an important contribution to understanding the potential of financial incentives to achieve viral suppression in LMICs. While further research should investigate the durability of effects from short-term incentives such as those provided in this study, these findings strengthen the evidence for implementing small financial incentives within standard HIV care as part of a comprehensive strategy for epidemic control.
Supplementary Material
Panel: Research in context.
Evidence before this study: Treatment for HIV with antiretroviral therapy (ART) can promote individual health and eliminate transmission, however these benefits rely on retention in care and sustained adherence to achieve viral suppression. Financial incentives offer a social protection strategy for overcoming individual barriers to engagement in HIV care. One recent review examined the impacts of financial incentives on HIV prevention and treatment in low- and middle-income countries. We searched PubMed on March 11, 2020 for any additional studies, using the search terms "incentives" OR "cash" AND "HIV" AND "suppression". We identified three studies investigating effects of financial incentives on viral suppression, in India, the Democratic Republic of Congo, and Uganda, all of which were randomised controlled trials; none found any impact on viral suppression. Two of these studies were conducted among special populations (drug users and pregnant women) and evaluated viral suppression as a secondary outcome. The third study conditioned incentives on achieving viral suppression, rather than engagement in care, and was conducted among a population with high baseline viral suppression. No previous study with viral suppression as the primary outcome evaluated incentives tied to clinic attendance, which is often required on a monthly basis and poses a significant obstacle to achieving viral suppression, especially in the first months of treatment when substantial attrition from care occurs. The considerable variability in target population and incentive size, conditions, and method for compensation used in previous studies leaves uncertainties regarding both effectiveness and implementation best practices.
Added value of this study: To our knowledge, this is the first study primarily designed to evaluate the impact of financial incentives for clinic attendance on viral suppression in a low- or middle-income country. This study also provides useful evidence to inform optimal implementation strategies, including considerations of incentive size and administration method. Using mobile health technology that linked biometric attendance monitoring to automated mobile payments upon monthly clinic visits, we compared usual care to two incentive values among patients starting treatment. We found that financial incentives bolstered the probability of remaining in care and achieving viral suppression at six months. Both incentive sizes were more effective than usual care, while there was a trend toward greater viral suppression with increasing incentive size.
Implications of all the available evidence: While further research should evaluate the durability of effects from short-term incentives, this study shows potential for a scalable financial incentive program to increase the use of HIV services and achieve viral suppression, a critical goal for ending the HIV epidemic.
Acknowledgements
The authors are grateful to the local research team, clinic staff, and study participants. This study was supported by a grant from the National Institute of Mental Health at the US National Institutes of Health (R01MH112432). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health.
SOURCES OF SUPPORT: Funding for the study is provided by the National Institute of Mental Health (McCoy R01MH112432). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors have no conflicts of interest to declare.
Contributor Information
Carolyn A. Fahey, Department of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA
Prosper F. Njau, Ministry of Health, Community Development, Gender, Elderly and Children, Dodoma, Tanzania; Health for a Prosperous Nation, Dar es Salaam, Tanzania
Emmanuel Katabaro, Health for a Prosperous Nation, Dar es Salaam, Tanzania.
Rashid S. Mfaume, Ministry of Health, Community Development, Gender, Elderly and Children, Dodoma, Tanzania
Nzovu Ulenga, Management and Development for Health, Dar es Salaam, Tanzania.
Natalino Mwenda, Rasello, Dar es Salaam, Tanzania.
Patrick T. Bradshaw, Department of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA
William H. Dow, Department of Health Policy and Management, School of Public Health, University of California, Berkeley, California, USA
Nancy S. Padian, Department of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA
Nicholas P. Jewell, Department of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA
Sandra I. McCoy, Department of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA
References
- 1.Cohen MS, Smith MK, Muessig KE, Hallett TB, Powers KA, Kashuba AD. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013;382(9903):1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Understanding Fast-Track: accelerating action to end the AIDS epidemic by 2030. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2015. https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf (accessed Feb 26 2020). [Google Scholar]
- 3.UNAIDS. UNAIDS Data 2019. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2019. https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed Oct 27 2019). [Google Scholar]
- 4.Fox MP, MPA SR. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ Glob Health. 2016;l(4):e000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. Social protection: a Fast-Track commitment to end AIDS — Guidance for policy-makers, and people living with, at risk of or affected by HIV. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2018. https://www.unaids.org/sites/default/files/media_asset/jc2922_social-protection-fast-track-commitment-end-aids_en.pdf (accessed Nov 13 2019). [Google Scholar]
- 7.Heise L, Lutz B, Ranganathan M, Watts C. Cash transfers for HIV prevention: considering their potential. J Int AIDS Soc. 2013;16(1):18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassett IV, Wilson D, Taaffe J, Freedberg KA. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS. 2015;10(6):451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galárraga O, Sosa-Rubí SG. Conditional economic incentives to improve HIV prevention and treatment in low-income and middle-income countries. Lancet HIV. 2019;6(10):e705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haushofer J, Fehr E. On the psychology of poverty. Science. 2014;344(6186):862–7. [DOI] [PubMed] [Google Scholar]
- 11.Dow WH, White JS. Incentivizing use of health care. New York: United Nations, 2013. [Google Scholar]
- 12.McCoy SI, Njau PF, Fahey C, Kapologwe N, Kadiyala S, Jewell NP, et al. Cash vs. food assistance to improve adherence to antiretroviral therapy among HIV-infected adults in Tanzania. AIDS. 2017;31(6):815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linnemayr S, Stecher C, Mukasa B. Behavioral economic incentives to improve adherence to antiretroviral medication. AIDS. 2017;31(5):719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yotebieng M, Thirumurthy H, Moracco KE, Kawende B, Chalachala JL, Wenzi LK, et al. Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV. 2016;3(2):e85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon SS, Srikrishnan AK, Vasudevan CK, Anand S, Kumar MS, Balakrishnan P, et al. Voucher incentives improve linkage to and retention in care among HIV-infected drug users in Chennai, India. Clin Infect Dis. 2014;59(4):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yotebieng M, Thirumurthy H, Moracco KE, Edmonds A, Tabala M, Kawende B, et al. Conditional cash transfers to increase retention in PMTCT care, antiretroviral adherence, and postpartum virological suppression: a randomized controlled trial. J Acquir Immune Defic Syndr. 2016;72(Suppl 2):S124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 Statement. JAA4A. 2019;321(16):1610–20. [DOI] [PubMed] [Google Scholar]
- 18.National AIDS Control Programme. National guidelines for the management of HIV and AIDS: sixth edition. Dar es Salaam: United Republic of Tanzania Ministry of Health, Community Development, Gender, Elderly, and Children, 2017. [Google Scholar]
- 19.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: second edition. Geneva: World Health Organization (WHO), 2016. [PubMed] [Google Scholar]
- 20.Fahey CA, Mwenda N, Bhattarai P, Mneney J, Katabaro E, Njau P, et al. Feasibility and acceptability of a biometric mHealth system for monitoring retention in HIV services and delivering financial incentives to adults initiating antiretroviral therapy in Tanzania. Mexico City: 10th IAS Conference on HIV Science, 2019. http://programme.ias2019.org/Abstract/Abstract/915 (accessed Mar 6 2020). [Google Scholar]
- 21.PEPFAR. Monitoring, Evaluation, and Reporting Indicator Reference Guide: MER 2.0 (Version 2.3). Washington, DC: PEPFAR, 2018. [Google Scholar]
- 22.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1995;11(3):375–86. [Google Scholar]
- 23.Rutstein SO, Johnson K. The DHS wealth index DHS Comparative Reports No. 6. Calverton, MD: ORC Macro, 2004. [Google Scholar]
- 24.Rubin DR. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc., 1987. [Google Scholar]
- 25.Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw. 2011;45(1):1–20. [Google Scholar]
- 26.Thirumurthy H, Ndyabakira A, Marson K, Emperador D, Kamya M, Havlir D, et al. Financial incentives for achieving and maintaining viral suppression among HIV-positive adults in Uganda: a randomised controlled trial. Lancet HIV. 2019;6(3):e155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia M, Moore CMT. The Cash Dividend: The Rise of Cash Transfer Programs in Sub-Saharan Africa. Washington, DC: The World Bank, 2012. [Google Scholar]
- 28.Whetten K, Reif S, Whetten R, Murphy-McMillan LK. Trauma, mental health, distrust, and stigma among HIV-positive persons: implications for effective care. Psychosom Med. 2008;70(5):531–8. [DOI] [PubMed] [Google Scholar]
- 29.Charness G, Gneezy U. Incentives to exercise. Econometrica. 2009;77(3):909–31. [Google Scholar]
- 30.Fahey CA, Kelly N, Mnyippembe A, Hassan K, Msasa J, Mfaume R, et al. Mortality after receipt of short-term incentives for HIV care among adults initiating ART in Tanzania: follow-up of a randomized trial. Mexico City: 10th IAS Conference on HIV Science, 2019. http://programme.ias2019.org/Abstract/Abstract/4785 (accessed Mar 6 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


