Abstract
Background:
Obstructive sleep apnea (OSA) is associated with increased cardiac arrhythmia and sudden cardiac death. We recently developed a new method (neuECG) to non-invasively measure electrocardiogram and skin sympathetic nerve activity (SKNA).
Objectives:
To test the hypothesis that the SKNA measured during sleep study is higher in patients with OSA than without OSA.
Methods:
We prospectively recorded neuECG and polysomnography in 26 patients undergoing a sleep study. Sleep stages were scored into rapid eye movement (REM), non-REM (NREM) sleep stage 1, NREM2, NREM3. Average voltage of SKNA (aSKNA) and SKNA burst area were calculated for quantification. Apnea-hypopnea index (AHI) of >5/hr was used to diagnose OSA.
Results:
There was a positive correlation (r=0.549, p=0.018) between SKNA burst area and the arousal index in OSA but not in the control group. The average SKNA (aSKNA) during sleep was 0.61 ± 0.09 μV in OSA patients (N=18) and 0.53 ± 0.04 in control patients (N=8, p=0.025). The burst area was 3.26 [1.90-4.47] μV.s/min in OSA patients and 1.31 [0.67-1.94] μV.s/min in control (p=0.047). More apparent differences were found during NREM2, when the burst area in OSA (3.06 [1.46-5.52] μV.s/min) was much higher than that of the control (0.89 [0.79-1.65] μV.s/min, p=0.03).
Conclusions:
OSA patients have higher SKNA activity than control patients, with the most pronounced differences observed during NREM2. Arousal at the end of apnea episodes is associated with large SKNA bursts. The overlaps of aSKNA and SKNA burst area between groups suggest that not all OSA patients have increased sympathetic tone.
Keywords: Autonomic nervous system, Premature ventricular contractions, Skin sympathetic nerve activity, Sleep-disordered breathing
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by intermittent upper airway narrowing, which causes intermittent hypoxia and arousals. OSA is associated with increased cardiovascular morbidity and mortality, including hypertension, coronary artery disease, congestive heart failure, stroke and sudden cardiac death (SCD).1–4 One of the possible mechanisms associated with increased cardiovascular mortality and morbidity is that OSA increases sympathetic tone, as documented by plasma norepinephrine measurements and direct muscle sympathetic nerve activity (MSNA) recording using microneurography techniques.5–8 A major limitation of microneurography is the technical difficulty, which limited most of these studies to < 10 total patients. A second limitation of microneurography is a lack of voltage calibration, making it difficult to compare the SNA amplitudes between patients or disease conditions.9 A third limitation is that due to the difficulties in obtaining stable impalement over a prolonged period of time, there are no microneurography studies that documented the increased SNA at the onset of rare events such as spontaneous sustained cardiac arrhythmias. Large scale microneurography studies are also very difficult to perform. We recently developed a method (neuECG) for non-invasive and simultaneous skin SNA (SKNA) and electrocardiogram (ECG) recordings using standard patch electrodes.10, 11 We performed long-term recording to document that SKNA bursts are associated with the onset of both atrial and ventricular tachyarrhythmias.12, 13 Because SKNA is important in cardiac arrhythmogenesis, we hypothesize that patients with OSA have higher average SKNA (aSKNA) levels and more SKNA bursts during sleep than in patients without OSA. We further hypothesize that the magnitudes of aSKNA and SKNA burst areas overlap significantly between OSA and control patients, indicating that many OSA patients do not have consistently elevated sympathetic tone during sleep. The purpose of the present study was to perform neuECG recordings during sleep studies to test those hypotheses.
Methods
Participants
This research protocol was approved by the Institutional Review Board of the Indiana University School of Medicine. Patients with suspected sleep apnea referred to diagnostic polysomnography as part of their routine care were prospectively recruited for the study. Only one patient had prior CPAP therapy, but he voluntarily stopped the treatment 4-5 months before the sleep study. The remaining patients were CPAP naive. The studies were done at Indiana University Health Sleep Disorders Center between October 2016 and November 2018. Patients with central sleep apnea or gas exchange disorders were excluded.
Sleep study
All recordings were all taken after adequate adaptation and in a silenced room. Polysomnography was recorded using the Alice® 6 Diagnostics Sleep System (Philips-Respironics, Amsterdam, Netherlands), including signals of electrocardiography (ECG), electroencephalogram (EEG), electromyogram (EMG) and other physiological signals including oronasal thermal sensor, nasal pressure, thoracic and abdominal effort, and oxygen saturation (SaO2). The sleep stages were scored according to the American Academy of Sleep Medicine (AASM) manual.14 Sleep stages were scored in 30-s epochs and classified into non-rapid eye movement (NREM) sleep stage 1 (N1), NREM sleep stage 2 (N2), and NREM sleep stage 3 (N3) and rapid eye movement (R) sleep. Arousals were scored based on EEG shifts and arousal index was calculated as number of arousals per hour. Apnea was defined as a decrease in the oronasal thermal airflow signal by >90% for >10 s. Hypopnea was defined as reduction in nasal pressure airflow of at least 30%, compared with the baseline for at least 10 s, associated with a 4% decrease in oxygen saturation. The apnea-hypopnea index (AHI) was the number of apnea and hypopnea events per hr of sleep during the study. The oxygen desaturation index (ODI) was the average number of desaturation episodes per hr. Participants with an AHI ≥ 5.0/hr were categorized into the OSA group, and participants with an AHI < 5.0/hr were classified as controls.14, 15
SKNA recording and analysis
A modified portable ME6000 Biomonitor (Mega Electronics Ltd, Kuopio, Finland) was used for data acquisition.10 The signals were bandpass filtered 500-1000 Hz, rectified and integrated to display SKNA (Figures 1A, 1C and 1D). The recordings were analyzed using custom-written software and Labchart 8 (ADInstruments Ltd, Bella Vista, Australia).11 Burst area was also calculated for SKNA quantification (Figure 1B) as described in previous studies.11, 13 SKNA signals were integrated to generate integrated SKNA (iSKNA). Detailed description of SKNA recording are included in the Online Supplement.
Figure 1.
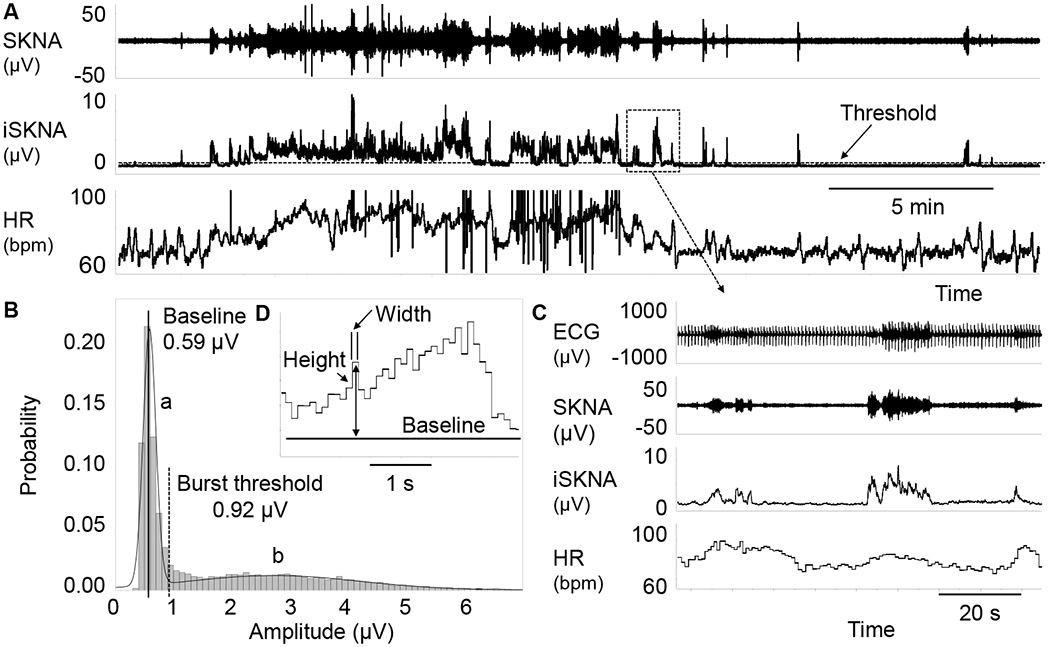
The methods for SKNA burst analysis. Panel A shows SKNA, iSKNA and HR. The broken line on iSKNA channel represents the threshold for burst detection. Plot B shows iSKNA values, with two normal distributions. The distribution on the left (a) represents the baseline signal and the distribution on the right (b) represents the burst activity. A vertical solid line indicates the mean while a broken line indicates 3X the standard deviation, which is taken as the threshold for burst detection. Panel C shows zoom-in of the box in Panel A, with positive association between HR and SKNA bursts. Graph D shows the method for burst area calculation. Each bin has a width of 0.1 s. The height of each bin is calculated as the absolute amplitude minus baseline. The area is calculated by multiplying width (s) with height (μV). The areas of all bins detected are added together as the burst area μV.s). HR, heart rate; iSKNA, integrated SKNA; SKNA, skin sympathetic nerve activity recorded by ECG patch electrodes.
Correlations between SKNA and ventricular arrhythmias
Three OSA patients had frequent PVCs (>1,000 PVCs/24h). We determined the Spearman’s correlations between aSKNA and the number of PVCs of every 5-min window during the entire recording.
Statistics
The data with normal distribution are presented as mean ± standard deviation. Otherwise they are presented as median [interquartile range]. Statistics were performed using SPSS 25 (SPSS Inc.). The demographics, sleep and respiratory characteristics, SKNA and HR between OSA group and controls were compared using unpaired t test (normal data), Mann-Whitney U test (skewed data) or Chi-squared test (categorical data). SKNA and HR during wakefulness and different sleep stages were compared using Wilcoxon Signed Rank test (skewed data) or paired t test (normal data). Correlations were analyzed by Spearman’s correlation. A P value ≤ 0.05 was considered statistically significant.
Results
Participant characteristics
Eligible recordings were acquired in 26 patients (age 26 - 82 years, 7 males), including 18 with mild-to-severe OSA. As described in Table 1, sleep architecture was found to be different between the two groups with greater sleep fragmentation (sleep stage changes, 27.1 [19.7 – 38.7] events/hour vs 16.5 [13.0 – 21.0] events/hour, p=0.066) and a significant decrease in stage R sleep (11.2% [3.3%-15.1%] vs 17.9% [13.1%-22.2%], p=0.026) in OSA patients. Those with OSA seemed to have shorter stage N3 and longer stage N1 compared with control group. All control subjects had good representation of all stages of sleep. In comparison, stage N3 was not found in seven OSA patients, and stage R was absent in three OSA patients. As expected, the arousal index, AHI and ODI were significantly higher in OSA patients. No significant differences in age, gender, body mass index, sleep efficiency and average SpO2 were found between the OSA and control group. Two patients in OSA group and two patients in control group were taking beta-blockers.
Table 1.
Demographics, sleep and respiratory characteristics of the subjects
| Variables | Control (n=8) | OSA (n=18) |
|---|---|---|
| Age (years) | 41 ± 11 | 50 ± 17 |
| Gender (male/female) | 1/7 | 6/12 |
| BMI (kg/m2) | 32.4 ± 5.1 | 37.5 ± 12.1 |
| Sleep efficiency (%) | 81.5 ± 11.8 | 74.8 ± 14.9 |
| Sleep stage changes | 16.5 (13.0 – 21.0) | 27.1 (19.7 – 38.7) § |
| Sleep architecture | ||
| -NREM1 (%) | 7.7 (4.6 – 11.1) | 15.2 (8.8 – 19.9) |
| -NREM2 (%) | 61.1 (53.0 – 63.2) | 62.3 (45.9 – 74.8) |
| -NREM3 (%) | 10.1 (8.8 – 16.6) | 6.2 (0 – 13.3) |
| -REM (%) | 17.9 (13.1 – 22.2) | 11.2 (3.3 – 15.1) * |
| Arousal index (events /hour) | 10.1 (8.6 – 13.7) | 18.8 (12.0 – 29.9) * |
| AHI (events/hour) | 0.9 (0.7 – 1.6) | 21.5 (10.7 – 39.3) *** |
| ODI (events/hour) | 0.9 (0.7 – 1.4) | 22.6 (9.8 – 38.3) *** |
| Average SpO2 (%) | 96 ± 1 | 94 ± 3 |
Continuous variables with normal distribution are expressed as mean ± SD and compared with ANOVA. Continuous variables with skewed distribution are expressed as median (interquartile range) and compared with Mann Whitney U test. Categorical variable was expressed as fractions and compared with Chi-squared test. The duration of each sleep stage is expressed as percentage of total sleep time.
p<0.05
p<0.001
p=0.066 vs control group.
AHI, apnea/hypopnea index; BMI, body mass index; NREM1, NREM2 and NREM3, non-REM sleep stage 1, stage 2 and stage 3; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; REM, rapid eye movement sleep; SpO2, pulse oxygen saturation.
Sympathetic nerve activity in patients with OSA
As shown in Figure 2, the overall SKNA was significantly lower during sleep than during wakefulness in both control and OSA groups. In control group, the aSKNA and burst area during sleep was 0.53 ± 0.04 μV and 1.31 [0.67-1.94] μV.s/min, respectively, significantly lower than those during wakefulness ( aSKNA, 0.89 ± 0.17 μV, p<0.001; Burst area, 18.9 [13.3-27.75] μV.s/min, p=0.012). Similar results were found in the OSA group, for which the aSKNA during sleep and wakefulness was 0.61 ± 0.09 μV and 0.96 ± 0.36 μV, respectively (p<0.001). The burst area during sleep and wakefulness was 3.26 [1.90-4.47] μV.s/min and 12.38 [5.14-31.35] μV.s/min, respectively (p<0.001). In addition, HR was significantly lower during sleep than during wakefulness in both control (62 ± 9 vs 68 ± 9 bpm, p=0.006) and OSA (73 ± 10 vs 77 ± 9 bpm, p=0.002) groups. Compared with controls, patients with OSA had a significantly higher aSKNA during sleep (0.61 ± 0.09 vs 0.53 ± 0.04 μV, p=0.025; Burst area, 3.26 [1.90-4.47] vs 1.31 [0.67-1.94] μV.s/min, p=0.047). When looking at specific stages of sleep, SKNA during Stage N2 sleep was significantly higher in patients with OSA compared with controls (aSKNA, 0.62 ± 0.10 vs 0.53 ± 0.04 μV, p=0.017; Burst area, 3.06 [1.46-5.52] vs 0.89 [0.79-1.65] μV.s/min, p=0.030). The HR during both wakefulness (77 ± 9 vs 68 ± 9 bpm, p=0.037) and sleep (73 ± 10 vs 62 ± 9 bpm, p=0.011) in patients with OSA was significantly higher than those in control subjects. However, no difference was noted in SKNA during wakefulness between OSA patients and controls. In spite of significant statistical significance, large overlaps were noted in aSKNA, SKNA burst area and HR between OSA and control patients, indicating that only a minority of OSA patients have consistently elevated SKNA during sleep.
Figure 2.
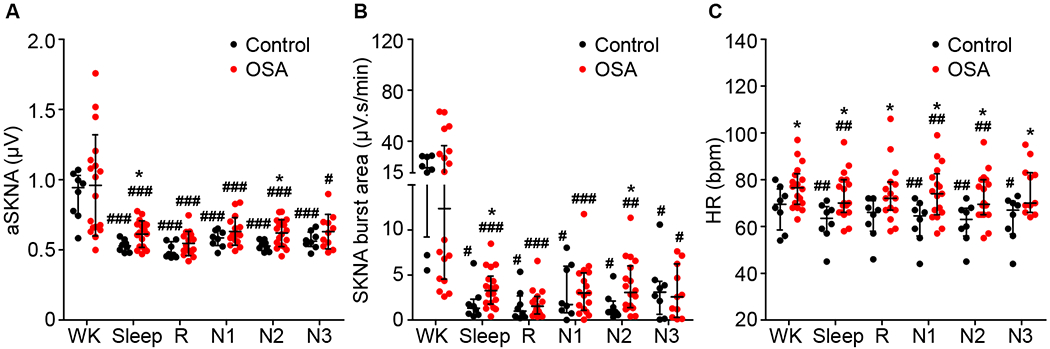
SKNA and HR during wakefulness and different sleep stages. Plots A, B and C compare aSKNA, SKNA burst area and HR, respectively, between OSA patients and controls during wakefulness and different sleep stages. aSKNA and HR were expressed as scatter plots with mean (horizontal lines) and standard deviation (whiskers) and SKNA burst areas were expressed as scatter plots with median (horizontal lines) and interquartile range (whiskers). *p<0.05 vs control group; #p<0.05, ##p<0.01, ###p<0.001 vs Wake. aSKNA, averaged SKNA; HR, heart rate; N1, N2 and N3, non-REM sleep stage 1, stage 2 and stage 3; OSA, obstructive sleep apnea; R, rapid eye movement sleep (REM); SKNA, skin sympathetic nerve activity recorded by ECG patch electrodes; W, wake.
We used RR intervals to determine the spectral heart rate variability (HRV) during different sleep stages. The results show nonREM sleep was associated with significantly lower low frequency/high frequency ratio than wakefulness and REM sleep within control and OSA groups, but there were no differences between groups. The details are shown in the Online Supplement Figures 1 and 2.
Correlation between SKNA, arousals and respiratory events
The sleep disordered breathing events (e.g. apneas, hypopneas, desaturations) and the related arousals were correlated with sympathetic activation. Figure 3 shows a typical example of obstructive apneas accompanied with desaturations and arousals. After the onset of sleep apnea, a gradual increase in SKNA and desaturation in pulse oximetry was observed, followed by arousal and simultaneous transient recovery of breathing. Following the onset of apnea, a gradual increase in SKNA was observed preceding arousals, with simultaneous transient recovery of ventilation. It should be noted that each arousal event was accompanied by SKNA burst activities, which was subsequently followed by an increase of HR. Figure 4 further illustrates a typical example of the association between arousals and SKNA bursts. The first apnea episode was not followed by arousal (changes in electroencephalogram), and this episode was not accompanied by evident SKNA bursts. In comparison, the subsequent two apnea episodes were associated with arousals and distinct SKNA bursts.
Figure 3.
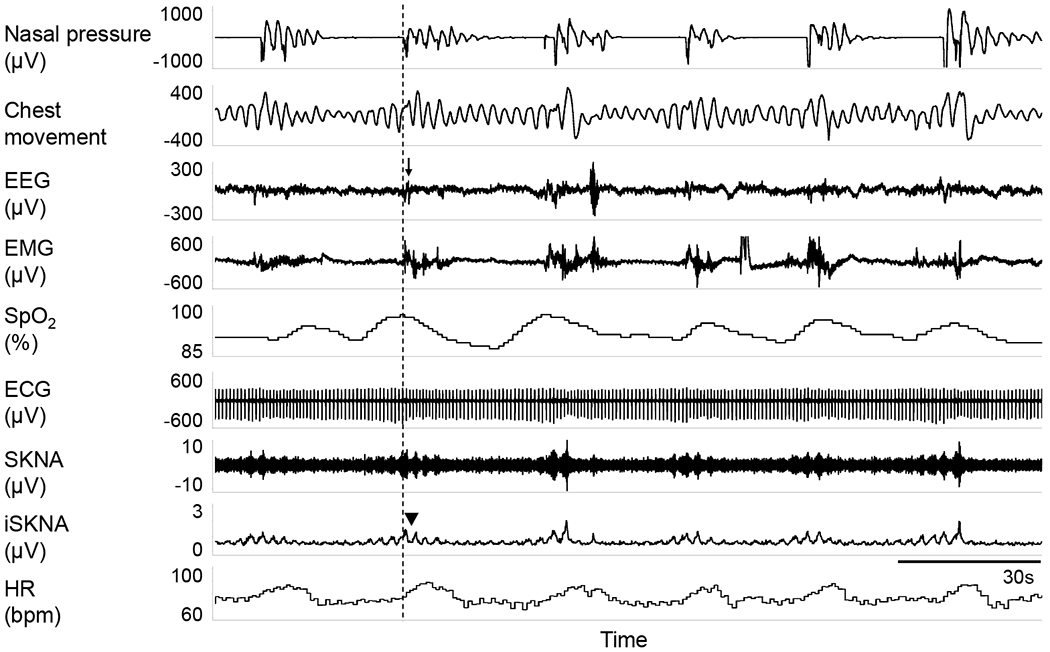
Typical episodes of obstructive apnea accompanied with desaturation, arousals and sympathetic surges. This recording segment is from non-REM sleep in a patient with severe OSA. Black arrow shows arousals characterized by abrupt changes in both EEG and EMG, accompanied with SKNA burst activities (black triangle). EEG, electroencephalogram; EMG, electromyogram; SpO2, pulse oxygen saturation.
Figure 4.
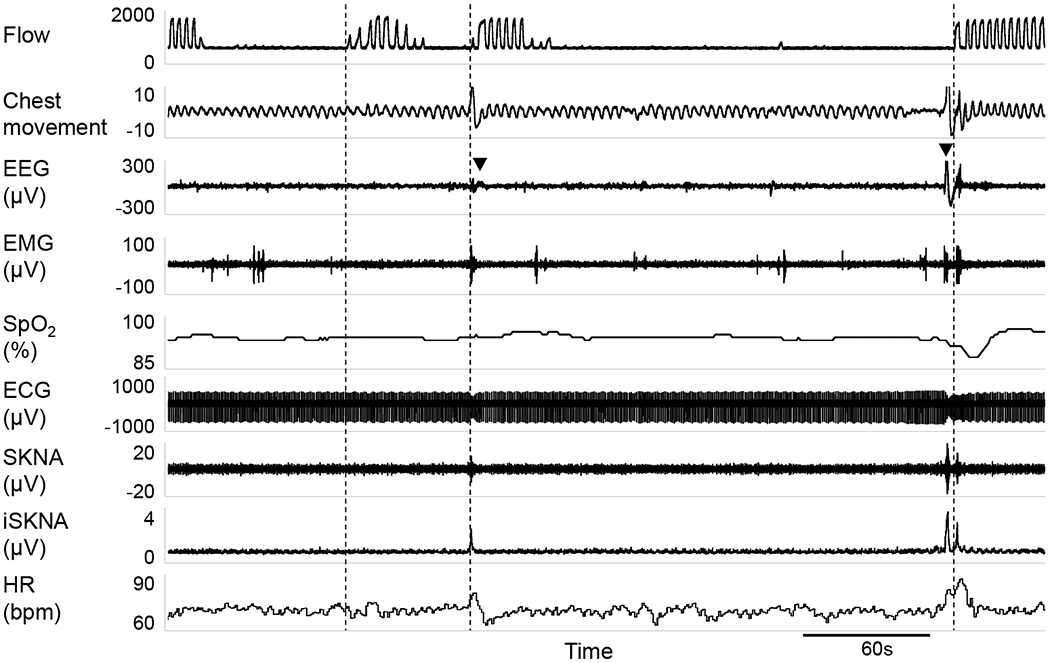
Association between arousal and SKNA bursts. There are 3 apnea episodes in this recording. Vertical dotted line indicates the end of each apnea episode. The first episode was not associated with either arousal or changes in SKNA. The terminations of the next 2 apnea episodes were marked by both arousals (black triangles indicate EEG changes) and large SKNA bursts.
The arousal index, AHI, and ODI during different sleep stages in OSA and control group are shown in Figure 5. In addition to higher AHI, OSA patients also had significantly higher ODI during stage N1, N2 and R compared with control group. Arousals, apneas/hypopneas and desaturations were rarely seen during stage N3 in either group. Arousal index during the entire sleep period and stage N2 sleep were significantly higher in OSA patients than in control subjects, which might be associated with a significant SKNA increase during sleep and stage N2 in OSA patients (Figure 2, Panel A and B).
Figure 5.
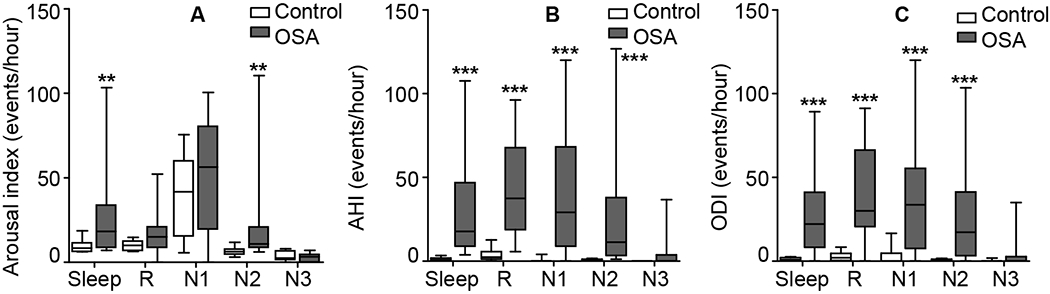
Comparison of arousal index, AHI and ODI during different sleep stages. The arousal index (A), AHI (B) and ODI (C) during different sleep stages were compared between OSA patients and controls. Data are expressed as median (horizontal lines), interquartile range (box) and range minimum to maximum (whiskers). *p<0.05, **p<0.01, ***p<0.001 vs control group. AHI, apnea/hypopnea index; N1, N2 and N3, non-REM sleep stage 1, stage 2 and stage 3; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; R, rapid eye movement sleep (REM).
The correlation between overall SKNA burst area and arousal index, AHI or ODI was evaluated among the 18 patients in the OSA group (Figure 6). SKNA burst area was positively correlated with the arousal index (r=0.549, p=0.018). However, we found no significant correlation between SKNA burst area and AHI or ODI. Similarly, the overall aSKNA was also significantly correlated with arousal index (r=0.484, p=0.042) while not correlated with AHI or ODI. For the 8 patients in the control group, no significant correlation between SKNA and arousal index was found (SKNA burst area, r=0.000, p=1.000; aSKNA, r=0.395, p=0.333).
Figure 6.
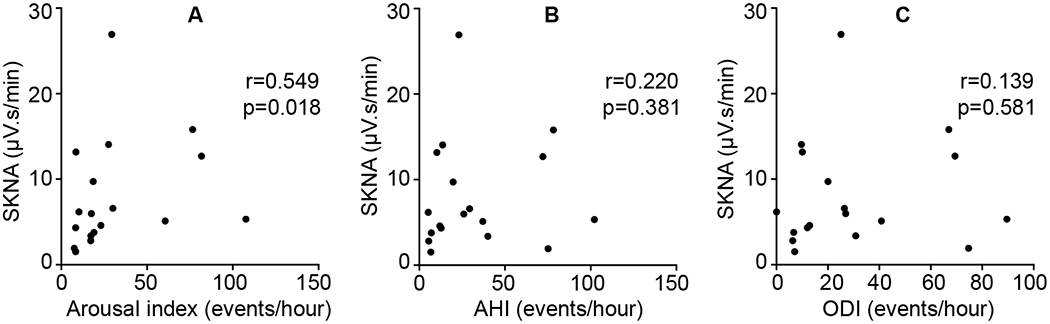
Correlations between SKNA and arousal index, AHI or ODI. The correlation between overall SKNA burst area and arousal index (A), AHI (B) or ODI (C) among the 18 patients in the OSA group was evaluated by Spearman’s correlation. AHI, apnea/hypopnea index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SKNA, skin sympathetic nerve activity recorded by ECG patch electrodes.
Correlation between SKNA and PVC
Frequent PVCs (>1000 PVCs/24h) were observed in 3 OSA patients (2 females and 1 male, age 58 ± 7, PVC frequency 4759 ± 1351 / 24h) and none of the controls. Online supplement Figure 3 shows the correlation between aSKNA and PVC count or HR in all 3 patients. One patient had a weak but significant (r=0.297, p=0.010) positive correlation. A second patient had weak and insignificant positive correlation (r=0.203, p=0.058). A third patient had a significant negative correlation (r=−0.551, p<0.001). At the same time, HR was positively correlated with aSKNA in all the 3 patients (Patient 1, r=0.286, p=0.013; Patient 2, r=0.3, p=0.005; Patient 3, r=0.727, p<0.001). Online Supplement Figure 4 shows typical examples. For PVCs with positive correlation with SKNA (Panel A), PVC clusters were always accompanied by SKNA bursts. In comparison, for PVCs that negatively correlated with aSKNA (Panel B), PVCs were not associated with SKNA bursts.
Discussion
neuECG is a well-validated method for simultaneous non-invasive recording of ECG and SKNA.10, 11 Using these methods, we were able to temporally match the SKNA with sleep stages and wakefulness. We found that on average, OSA increases aSKNA during sleep and arousal, but not during wakefulness. Consistent with previous microneurography studies, the most significant increase of SNA during sleep occurred in sleep stage N2.8 These findings suggest that neuECG is a useful non-invasive tool to estimate sympathetic tone in patients undergoing sleep studies. However, large overlaps of aSKNA and SKNA bursts between OSA and control patients suggest that only a minority of patients with OSA had elevated sympathetic tone during sleep, a finding with potentially significant clinical implications.
Increased sympathetic tone in OSA patients
Sleep disorders like OSA may be accompanied by impaired autonomic regulation and increased sympathetic tone, which probably plays a role in increasing the risk of cardiovascular diseases. This increased sympathetic tone might be due to various stimuli such as hypoxia, arousal, and sleep fragmentation. In OSA patients, the apneic episodes usually cause low oxygen saturations and increased carbon dioxide levels, and are terminated by arousals from sleep as shown in our example. Hypoxia and hypercapnia have a synergistic effect on sympathetic activation.16 Several mechanisms have been proposed for this sympathetic overactivation. Hypoxia during apneas increased sympathetic firing through the carotid chemoreflex.17 Two weeks of chronic intermittent hypoxia simulating OSA compromised baroreflex control of sympathetic outflow contributing to sympathetic overactivity with resultant increase in MSNA and daytime blood pressure.18 In a chronic intermittent hypoxia model during wakefulness, chronic intermittent hypoxia increased the hypoxic chemosensitivity in humans,19 which might play a role in the excess sympathetic activation in OSA. The loss of sympatho-inhibitory input from the pulmonary stretch receptors during apnea may contribute to the increase of sympathetic nerve activity.18 Apnea-induced sympathetic responses can be eliminated by cardiac afferent denervation in canine models.20
Arousals from sleep may be another key factor for the sympathetic overactivity in patients with OSA. It has been reported that acoustic arousals, per se, can evoke sympathetic nerve bursts.21 Arousal index was shown to be the single strongest predictor of MSNA during wakefulness,22 which is consistent with the findings in the present study. In addition, sleep fragmentation is also a contributing factor to the increased wakefulness sympathetic tone in OSA patients.22 The improvement of the arousal index and related abnormal sleep architecture might be important for the recovery of the elevated sympathetic tone in OSA patients.
The BMI is higher in OSA group than control group. The increased BMI could contribute to the heightened sympathetic tone in this study.23
SKNA is increased during sleep and especially stage NREM2
It has been reported that MSNA is increased during both wakefulness and sleep in OSA patients.8 On the other hand, spectral HRV is different during selected sleep stages but not during awake between patients with myocardial infarction and matched controls.24 In this study we showed SKNA is increased only during sleep. The MSNA is synchronous to blood pressure and respiration while SKNA is sensitive to emotional stimulation and body temperature.25 These two types of SNA do not always increase or decrease in parallel to each other. For example, MSNA is suppressed, while skin SNA measured with microneurography techniques (SSNA) is increased, during white coat hypertension.26 On the other hand, SKNA is increased during stage N2 sleep, consistent with that found by MSNA recordings.8 Interestingly, arousal index in OSA patients was significantly increased only during stage N2 when compared with controls, while the AHI and ODI were increased during stage N1, N2 and REM sleep. Considering the close relationship between SKNA and arousals, the increased SKNA during stage N2 might be related to frequent arousals.
Involvement of sympathetic hyperactivity in cardiac arrhythmias for OSA patients
It is widely accepted that increased sympathetic tone will facilitate the occurrence of ventricular arrhythmias. However, He et al27 previously reported that the frequencies of PVCs could either increase or decrease with heart rate, suggesting a variable relationship between SNA and PVC frequencies. Consistent with that hypothesis, we found a variable relationship between aSKNA and PVCs. The mechanism of the negative correlation between SNA and PVC in some cases is unknown, but overdrive suppression might be a possible mechanism.
The significance of SKNA recording in OSA patients
Because SKNA bursts are known to be proarrhythmic in both atrial and ventricles,12, 13, 28 identification of increased SKNA bursts during sleep could help arrhythmia risk stratification among patients with OSA. The early noninvasive detection of patients with a high sympathetic tone may help identify OSA patients who will benefit from treatment.29 However, we discovered that a majority of OSA patients actually had aSKNA and SKNA burst area in the range of controls. Continuous positive airway pressure (CPAP) is a widely accepted treatment for patients with OSA and CSA, which can help to attenuate the apneas/hypopneas, desaturations and arousals in these patients. However, based on the evidence from randomized clinical trials, CPAP failed to improve survival or prevent major cardiovascular events in OSA patients.30 In another study using heart rate variability as indices for autonomic tone, CPAP therapy only increased a parasympathetic parameter during wakefulness.6 It is possible that poor adherence to CPAP underlies these negative findings. However, based on the results of our study, most patients with OSA actually did not have abnormally high SKNA. If the primary therapeutic benefit of CPAP is to reduce the detrimental effects of persistently elevated SNA, then a large number of OSA patients with normal SNA during sleep would not have improved cardiovascular outcomes with CPAP therapy. It is still possible that if the clinical trials enroll only patients with heightened SNA, then the results might show a significant beneficial effect of CPAP.
Limitations
Due to the technical difficulty in performing microneurography in OSA patients during sleep, we did not have simultaneous MSNA recording in these patients. The SKNA does not contain information on parasympathetic nerve activity. Whether or not OSA increases parasympathetic nerve activity remains unknown.
Conclusions
SKNA, heart rate and arousals are elevated in obstructive apnea patients during sleep. neuECG recordings may be a simple non-invasive tool to measure increased sympathetic tone and might help to evaluate the cardiovascular risk and therapeutic options in OSA patients.
Supplementary Material
Acknowledgements:
We thank Ane Fiems, RRT, RPSGT for technical assistance.
Funding
This study was supported by NIH Grants R42DA043391, TR002208-01, R01 HL139829, a Charles Fisch Research Award endowed by Dr Suzanne B. Knoebel, a Medtronic-Zipes Endowment of the Indiana University and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts
Indiana University was awarded U.S. patent 10478623 for inventing the method of neuECG recordings.
References
- 1.Ni YM, Rusinaru C, Reinier K, et al. Unexpected shift in circadian and septadian variation of sudden cardiac arrest: the Oregon Sudden Unexpected Death Study. Heart Rhythm 2019;16:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeidan-Shwiri T, Aronson D, Atalla K, et al. Circadian pattern of life-threatening ventricular arrhythmia in patients with sleep-disordered breathing and implantable cardioverter-defibrillators. Heart Rhythm 2011;8:657–662. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 5.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 1993;103:1763–1768. [DOI] [PubMed] [Google Scholar]
- 6.Grau N, Bazan V, Kallouchi M, et al. Long-term Impact of Continuous Positive Airway Pressure Therapy on Arrhythmia and Heart Rate Variability in Patients With Sleep Apnea. Arch Bronconeumol 2016;52:17–23. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303–307. [DOI] [PubMed] [Google Scholar]
- 8.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart EC, Head GA, Carter JR, et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 2017;312:H1031–H1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm 2017;14:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusayama T, Wong J, Liu X, et al. Simultaneous non-invasive recording of electrocardiogram and skin sympathetic nerve activity (neuECG). Nat Protoc 2020;15:1853–1877. [DOI] [PubMed] [Google Scholar]
- 12.Uradu A, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm 2017;14:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusayama T, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985) 1989;67:2101–2106. [DOI] [PubMed] [Google Scholar]
- 17.Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol (1985) 1993;74:2969–2975. [DOI] [PubMed] [Google Scholar]
- 18.Tamisier R, Pepin JL, Remy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 2011;37:119–128. [DOI] [PubMed] [Google Scholar]
- 19.Pialoux V, Hanly PJ, Foster GE, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med 2009;180:1002–1009. [DOI] [PubMed] [Google Scholar]
- 20.Tavares L, Rodriguez-Manero M, Kreidieh B, et al. Cardiac Afferent Denervation Abolishes Ganglionated Plexi and Sympathetic Responses to Apnea: Implications for Atrial Fibrillation. Circ Arrhythm Electrophysiol 2019;12:e006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol (1985) 1996;80:1627–1636. [DOI] [PubMed] [Google Scholar]
- 22.Taylor KS, Murai H, Millar PJ, et al. Arousal From Sleep and Sympathetic Excitation During Wakefulness. Hypertension 2016;68:1467–1474. [DOI] [PubMed] [Google Scholar]
- 23.Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension 2000;36:531–537. [DOI] [PubMed] [Google Scholar]
- 24.Vanoli E, Adamson PB, Ba L, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation 1995;91:1918–1922. [DOI] [PubMed] [Google Scholar]
- 25.Gilbey MP. Sympathetic rhythms and nervous integration. Clin Exp Pharmacol Physiol 2007;34:356–361. [DOI] [PubMed] [Google Scholar]
- 26.Grassi G, Seravalle G, Buzzi S, et al. Muscle and skin sympathetic nerve traffic during physician and nurse blood pressure measurement. J Hypertens 2013;31:1131–1135. [DOI] [PubMed] [Google Scholar]
- 27.He W, Lu Z, Bao M, et al. Autonomic involvement in idiopathic premature ventricular contractions. Clin Res Cardiol 2013;102:361–370. [DOI] [PubMed] [Google Scholar]
- 28.Kabir RA, Doytchinova A, Liu X, et al. Crescendo Skin Sympathetic Nerve Activity and Ventricular Arrhythmia. J Am Coll Cardiol 2017;70:3201–3202. [DOI] [PubMed] [Google Scholar]
- 29.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 2019;15:301–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva Paulitsch F, Zhang L. Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: a meta-analysis of randomized trials. Sleep Med 2019;54:28–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


