Abstract
Purpose
Type 2 diabetes (T2DM) is a leading cause of visual impairment. Its precursor, prediabetes (preDM), is growing in numbers every year. While it is well known that T2DM causes changes in retinal function early in the disease process, it is likely that some of these changes emerge during the preDM stage. This study evaluates retinal function measures in patients with preDM to determine if there are differences in colour vision, contrast sensitivity (CS), and multifocal electroretinogram (mfERG) measures present before T2DM is diagnosed.
Methods
The L’Anthony desaturated D-15 test, Mars Chart CS test, and mfERG were administered on the right eye of 43 participants; 15 controls (HbA1c 5.6%), 17 with preDM (HbA1c 5.7–6.4%), and 11 with T2DM (either physician diagnosed or with untreated HbA1c ≥ 6.5%). HbA1c values were measured at the time of the other tests. Colour vision confusion scores (CVCS) were calculated from the D-15 using the method developed by Torok. Multivariate regression (which controlled for age differences) was used to evaluate the relationship of HbA1c and functional measures. Kruskal-Wallis tests were also used to evaluate differences between groups with post-hoc analysis.
Results
CVCSs were significantly different between the three groups (p = 0.009). There was an association between higher CVCS and higher HbA1c values across all groups as well as specifically within the preDM group when controlling for age (R2 = 0.29, p = 0.01 and R2 = 0.39, p = 0.02 respectively). Multivariate regression of all of the functional tests together and HbA1c found only colour vision remained significant, indicating that the functional examination metrics may provide redundant data, with similar changes in prediabetes where colour vision may be the strongest indicator early in the process.
Conclusions
Patients with prediabetes have functional changes that can be measured in the retina before the diagnosis of diabetes, with the L’Anthony D-15 colour vision test providing the strongest association with glucose dysregulation in this population. This has important implications for follow up and screening for diabetes within optometric practices. Further studies are needed to follow these patients over time to see how and when these metrics change.
Keywords: mfERG, contrast sensitivity, colour vision, prediabetes, type 2 diabetes
Introduction
Diabetes mellitus is an important public health problem. In 2015, diabetes mellitus was estimated to affect 415 million individuals worldwide, with type 2 diabetes (T2DM) accounting for approximately 90% of that value.1 In spite of increasing knowledge surrounding the disease, global incidence and prevalence of T2DM is significantly on the rise, with estimates of 642 million individuals being affected by 2040.1 Chronic hyperglycemia associated with T2DM causes cellular alterations that leads to damage in the vascular and neurologic systems throughout the body, including the ocular system.1,2 Diabetic retinopathy (DR), a complication of T2DM, is one of the leading causes of moderate and severe visual impairment in the world. The number of individuals suffering with blindness from DR has increased by a factor of two from 1990 to 2015.3 In addition, there are millions of individuals with prediabetes.1 Prediabetes is a condition in which individuals have elevated blood glucose levels between normal values and the diagnostic criterion for T2DM, and it is associated with an increased risk of developing T2DM and related vascular and neurologic complications.1,4,5 However, there is very little data on prediabetes and visual function, likely because prediabetes is not always identified and the effect of prediabetes on vision is poorly understood.
It is well known that retinal functional changes occur in individuals with diabetes mellitus before vasculopathy as evidenced by colour vision assessment, contrast sensitivity testing and multifocal electroretinogram (mfERG) implicit times.2,6–11 Colour vision testing may serve as a clinically practical test to identify early changes that have occurred in retinal neurons as well as other areas in the visual system with diabetes.10,11 Previous studies have shown that colour vision defects in primarily blue-yellow but also red-green discrimination can develop in diabetes and can precede the detectable development of diabetic retinopathy with fundus examination.2,8,11,12 Contrast sensitivity, which examines a patient’s ability to discern objects from a background, is decreased in diabetic patients both with and without retinopathy due to changes in neuronal pathways in the inner retinal neurons (especially the ganglion cell layer), as well as in post-retinal areas, which implies that contrast sensitivity testing could be useful in detecting early diabetic changes in the eye.7,8,13 Lastly, the mfERG evaluates local electrophysiologic responses from different areas of the retina to localise retinal neural damage.14 Upon mfERG evaluation, individuals with short and long-term diagnosed diabetes have been shown to have an increase in implicit time in patients both with and without vascular retinopathy.8,9 In individuals without retinopathy, increased implicit time led to the individuals being more at risk for development of diabetic retinopathy compared to individuals with normal implicit times.6,8,9
However, it is not known if similar changes in neural function occur in prediabetes, which is one of the earliest stages of glucose dysregulation in the diabetic process. To our knowledge, they have not been included as a separate group in any functional eye study which included all of these tests. The purpose of this study was to evaluate changes in retinal function in patients with prediabetes. Knowing when changes begin in the eye in the course of the disease could be valuable for the development of biomarkers and future treatments.
Methods
Participants
Participants included were between 30 and 70 years of age and were recruited by word of mouth and fliers placed around the University of Houston (Houston, USA) and the University Eye Institute. Participants were classified into one of three groups based on HbA1c measurements at the time of the visit: control (HbA1c 5.6%), prediabetes (5.7–6.4%) and T2DM (previously physician diagnosed or with an untreated HbA1c ≥ 6.5%), which correspond with the ranges published by the American Diabetes Association.15 Participants who were pregnant females by self-report, had a positive history of eye surgery, had an active eye infection or clinically significant eye inflammation, had known eye disease other than diabetic eye disease, had Type 1 diabetes or were not safe to dilate were not included. Diabetes subjects with retinopathy or retinal oedema were not included in this analysis. Participants could not have known congenital colour anomalies. Written informed consent was obtained from all subjects before data collection began. This study was approved by and in compliance with regulations set by the University of Houston’s Institutional Review Board and complied with the Declaration of Helsinki. All subjects were consented prior to enrolment.
Preliminary Testing
All subjects underwent a screening slit lamp biomicroscopy examination using a Haag-Streit Diagnostics BQ 900© instrument (www.haag-streit.com). Gross examination of the cornea and lens was performed to check that no lens opacities, abnormalities, or cataracts of any type were present. Visual acuity was taken with the subject’s habitual correction at distance using an M&S Smart System® (www.mstech-eyes.com) to ensure the subject was 6/7.5 or better in each eye.
Colour Vision
The L’Anthony desaturated D-15 test (www.good-lite.com) was administered with all the room lights on. The lighting in the room was fluorescent “natural white lighting” with a colour rendering index (CRI) rating of 78 CRI and a temperature of 3500K. Lighting in the room was measured with a StellarNet Inc Black Comet spectrophotometer (www.stellarnet.us) using a cosine diffusor pointed upward from the location where the test was performed. It was found to have two major peaks with the largest at 544.5 nm (1.61 watts/m2) and the other at 611 nm (1.28 watts/m2). The overall illuminance on the surface where the test was conducted was 302 lux. The subjects wore their habitual correction using the common clinical technique for the test. The test was placed on a black tray and subjects performed the test with the right eye. Beginning with the cap labelled “0”, subjects were instructed, “out of the remaining caps, find the colour that looks most similar to the last one in the box.” The order of the caps was recorded on two-dimensional colour space according to the D-15 score sheet template to evaluate for evidence of protan, deutan and tritan colour deficiencies. Total error scores, colour vision confusion score and colour angle were calculated using computer programming designed by Torok16 from the methodology described by Vingrys and King-Smith.17 In addition, examiners recorded if the patient passed or failed according to the computer program and would pass or fail the colour test if administered clinically based on the following criteria: two crossovers of colour space, three or more single place errors or two or more other larger errors. The subject failed the test if any of these three criteria were met. It was also noted if the subject scored perfectly on the test or not, as this is another easy clinical criterion to employ. The direction of the defect was also noted.
Contrast Sensitivity
Contrast sensitivity was measured monocularly for the right eye using the Mars Letter Contrast Sensitivity Test (www.good-lite.com) at 60 cm under the same overhead lighting conditions as the colour vision testing described in detail above as well as an additional stand light, with the habitual correction in place. Subjects were instructed “to read the letters left to right across each line of the chart and guess even if the letters appear faint.” The test was complete when the subject missed two consecutive letters. The log contrast sensitivity score was given by the value at the lowest contrast letter identified just before two subsequently incorrectly identified letters, minus scoring correction for previously missed letters as is standard for the test.
Pupil Dilation and mfERG
The pupil was dilated with 1% tropicamide and 2.5% phenylephrine to at least 6 mm and the left eye was patched. A ground electrode was placed on the subject’s left ear after exfoliation. After the right eye was anaesthetised with 0.5% proparacaine, a Burian-Allen lens (www.hansenlab.com) filled with Celluvisc lubricant eye gel (www.refreshbrand.com) was placed on the cornea to record signals. VERIS 6.4.3 by Electrodiagnostic Imaging (EDI) software (www.veris-edi.com) was used to record the mfERG. The stimulus was displayed on a screen and consisted of an array of 103 hexagons where the luminance of each hexagon alternated in a 4-minute pseudo-random binary m-sequence near 100% contrast between white (200 cd/m2) and black (<5 cd/m2) at 75 Hz. A central fixation cross was placed in the middle of the stimulus and room illumination approximately matched that of the stimulus screen. The overall recording time was 4 minutes and was divided into eight 30-second segments. Implicit times at P1 and mfERG amplitude measured from trough of N1 to peak of P1 were measured for the entire eye averaged together and for the fovea. The data processing used one iteration of artifact removal and 10% averaging from neighbours from the first order kernel.
Fundus examination
After the mfERG was completed, a fundus examination including a photograph and optical coherence tomography was performed for both eyes. A Heidelberg Spectralis OCT (Heidelberg Engineering, www.business-lounge.heidelbergengineering.com), software version 6.8, was used with an acquisition rate of 40,000 A scans per second. A 7-line volumetric scan over a 30 × 5° area and a 21-line volumetric scan over a 20×20° area over the macula were generated to check for diabetic macular oedema and to assure that the retina was healthy. Using a Topcon TRC-NW400 non-mydriatic retinal camera (www.topconmedical.com), fundus photographs were taken on both the undilated left eye and dilated right eye over the central 45°. Photographs were assessed for signs of diabetic retinopathy. Patients with any retinopathy or signs of diabetic oedema were excluded from analysis.
Blood Collection and Biochemical Testing
Fingerstick blood was collected for analysis. After the tip of the third or fourth finger was swabbed with an alcohol pad, a 21-gauge 2.5 mm-penetration lancet was used to stick the finger. HbA1C levels were quantified via a Siemens DCA HbA1c Analyzer (Siemens Medical Solutions USA, www.siemens-healthineers.com) and were used to define control (≤ 5.6%), prediabetes (5.7–6.4%) and diabetes (≥ 6.5%) groups.
Statistical Analysis
Kruskal-Wallis tests were used to evaluate differences between groups. If the overall differences were significant, Mann-Whitney U testing was performed post-hoc to detect which groups were different from each other, and Bonferroni corrections were used to account for the three comparisons between groups (changing the significant p-value to 0.02). Multivariate regression (which controlled for age differences between groups) and Pearson correlations were used to evaluate the relationship of HbA1c and the functional measures.
Results
Participants
Forty-three participants were enrolled in the study: 15 control subjects, 17 with prediabetes, and 11 with T2DM. Subject characteristics are shown in Table 1. The majority of the participants were female. The control group, prediabetes group and T2DM group were clinically similar in age (middle aged adults). However, the control group was statistically younger than the prediabetes and T2DM groups (p = 0.01), thus regression calculations controlled for age in the model. There was no difference in acuity between the three groups. Subjects in the T2DM group had no retinopathy in either eye and HbA1c levels were overall well-controlled in this group (HbA1c ranging from 6.5–9.1%). In the T2DM group based on self-report, seven patients were on a diabetic medication alone, one patient was on insulin alone, one patient was on insulin with an additional diabetic medication, and two were not taking any medications.
Table 1.
Primary demographic information and functional retinal outcomes in the study population. Values given in counts and median [IQR].
| Group | Control (HbA1c ≤ 5.6%) | Prediabetes (HbA1c 5.7–6.4%) | Diabetes (HbA1c ≥ 6.5%) | p-values |
|---|---|---|---|---|
| n | 15 | 17 | 11 | |
|
Gender Male : Female |
4 : 11 | 4 : 13 | 3 : 8 | |
| Age (years) | 41 [35–52] | 53 [43–61] | 56 [53–61] | 0.01 |
| HbA1C (%) | 5.3 [5.0–5.3] | 5.8 [5.7–6.0] | 7.0 [6.7–7.5] | 0.00 |
| Visual acuity (logMAR) | 0.0 [−0.04–0.02] | 0.0 [−0.08–0.04] | −0.04 [−0.08–0.04] | 0.8871 |
| Color Vision Confusion Score | 1.12 [1.09–1.73] | 1.85 [1.23–2.14] | 2.03 [1.28–2.63] | 0.009 |
| Color Vision Total Error Score | 6.8 [6.7–9.7] | 10.2 [7.4–13.2] | 11.1 [7.5–14.0] | 0.0113 |
| Color Vision Fail % | ||||
| Clinical designation | 26.7% | 70.6% | 72.7% | |
| Computer designation | 13.3% | 64.7% | 72.7% | |
| Color Vision % of Patients with No Errors | 20% | 12% | 0% | |
| Color Vision Angle | 63.5 [61.3–68.2] | 66.3 [60.5–75.4] | 66.8 [61.5–73.7] | |
| Contrast Sensitivity (logCS) | 1.80 [1.71–1.80] | 1.72 [1.68–1.8] | 1.56 [1.44–1.76] | 0.026 |
| mfERG Whole Eye IT (ms) | 28.5 [27.8–29.2] | 28.3 [27.8–28.9] | 29.3 [28.4–30.2] | 0.31 |
| mfERG Whole Eye Amplitude (nV/deg2) | 24.7 [21.0–36.3] | 24.7 [21.8–30.3] | 26.1 [23.0–28.0] | 0.87 |
| mfERG Foveal IT (ms) | 29.7 [28.9–30.7] | 29.4 [28.3–30.6] | 29.8 [29.2–30.7] | 0.32 |
| mfERG Foveal Amplitude (nV/deg2) | 82.4 [70.6–99.2] | 73.3 [64.6–100.4] | 88.8 [62.5–95.6] | 0.67 |
Colour vision
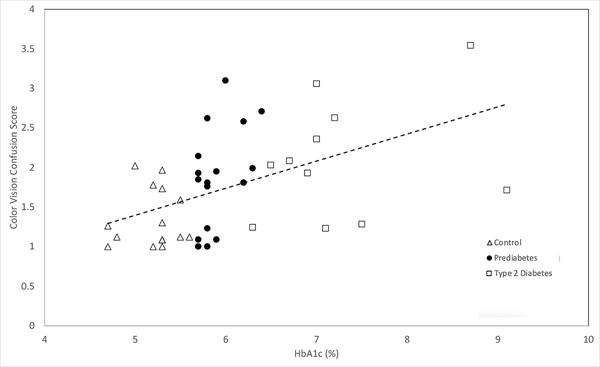
Colour vision confusion score was significantly related to HbA1c across all groups (and after controlling for age) (adjusted R2 = 0.29, p = 0.01) as well as specifically within the prediabetes group alone (adjusted R2 = 0.39, p = 0.02, Figure 1).
Figure 1.
HbA1c vs. Color Vision Confusion Score in controls, prediabetes, and type 2 diabetes study groups. (R2=0.29, p<0.01). Data calculations controlled for age differences in groups.
Colour vision confusion score was also different between the groups (Table 1) and post hoc analysis found significant differences between the control and the T2DM group (p = 0.003), but no significant differences between the prediabetes and T2DM groups (p = 0.33). The difference between the control and prediabetes groups (p = 0.029) approached significance but did not quite meet the threshold after correction for multiple comparisons. Total error scores for the D-15 test showed a similar result (Table 1).
In addition, when evaluating clinical results as would be used in an eye examination, the percentage of patients that would fail the colour vision test increased between the prediabetes and control patients but this same jump in percentage did not occur between prediabetes and diabetes subjects. There were only two patients in the study who had red/green direction crosses; all other defects noted were tritan in direction.
Relationship of other functional tests, colour vision, and HbA1c
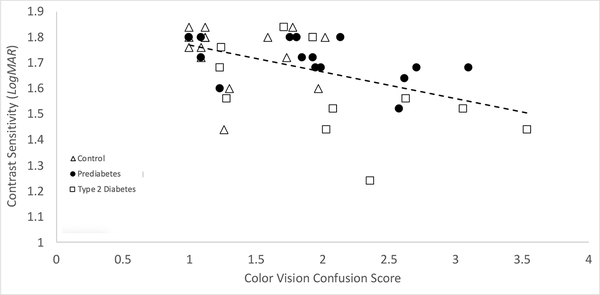
There was a negative correlation between colour vision confusion scores and log contrast sensitivity across groups (R2 = 0.28, p = 0.014, Figure 2). The contrast sensitivity was different between the three groups (Table 1) and post hoc analysis showed the control and prediabetes participants were not significantly different (p = 0.22) and neither were the prediabetes and T2DM groups (p = 0.032) but the T2DM group had worse contrast sensitivity than the control group (p = 0.017).
Figure 2.
Color Vision Confusion Score vs. Contrast Sensitivity in controls, prediabetes, and type 2 diabetes study groups. (R2= 0.28, p<0.014). Data calculations controlled for age differences in groups.
There was also a weak correlation between increased colour vision confusion scores and increased whole eye mfERG implicit time across all groups (R2 = 0.09, p = 0.05). There were no differences between the three groups for the mfERG values (Table 1).
Backwards multivariate regression of all of the functional tests together and HbA1c found only colour vision remained significant, indicating that the functional examination metrics may provide redundant data, with similar changes in prediabetes where colour vision may be the strongest indicator early in the process.
Discussion
In this study we found that prediabetes has functional changes that can be measured in the retina before the diagnosis of diabetes. This has important implications for follow up and screening of diabetes within optometric practices. Approximately 5–10% of prediabetic patients convert to T2DM each year. Thus, prediabetes increases the risk for development of diabetic changes in a significant portion of the population.18 There have been numerous studies that have looked to understand functional neuronal changes that occur in early T2DM, including before vasculopathy has developed.6–13 However, none of these to our knowledge looked at prediabetes as a separate category defined by HbA1c levels at the time of testing.
Similar to other studies, this investigation found that colour vision testing is sensitive to changes caused by diabetes before the presentation of clinical retinopathy.10–13 However, we were also able to show that alterations in colour vision as evidenced by the desaturated D-15 test are associated with HbA1c, and can occur as early as the prediabetic stage. Colour vision testing in particular appears to have the potential to differentiate healthy patients from those with glucose dysregulation as early as the prediabetes stage.
There have been many proposed mechanisms as to how acquired colour vision defects occur in diabetes. One study performed in donor retinas found significant reduction in the percentage of S-cones within the retina, suggesting this to be the cause of tritan-like deficits in patients with diabetic retinopathy.19 This coincides with our study, as the subjects used here almost all demonstrated defects that were tritan in nature. Another proposed mechanism, which seems plausible given our findings in individuals with prediabetes, revolves around the inner retinal circulation. As a primarily vascular disease, damage to the inner retinal microcirculation could cause alterations in metabolism and fluid changes in the retina that ultimately leads to death and damage of the ganglion cells as well as cells in the inner nuclear layer..20,21 This has been evidenced in other studies. Research performed in a prediabetic rat model has shown thinning in the retinal nerve fibre layer occurring before vasculopathy,5 while another study revealed macular thinning in individuals with prediabetes as diagnosed by fasting blood glucose.22 There is also the possibility that the changes we measured in this study are coming from higher visual centres. Additional testing will be needed to learn if this is indeed the case.
Other functional tests such as mfERG and contrast sensitivity have been shown to be altered significantly in individuals with T2DM before the development of retinopathy.6–8,10 However, contrast sensitivity and mfERG testing showed no significant differences between controls and those with prediabetes in this study. While these tests appear to be redundant and more correlated to each other than to HbA1c, they may still have a place in screening for ocular changes caused by diabetes. More studies are needed with a larger sample and wider ranges of HbA1c to truly understand the relationship between these metrics and screening for changes. Other methods for measuring contrast sensitivity may also need to be evaluated.
One of the limitations of all studies of prediabetes is that we do not know how long subjects have had the condition. Other investigations have shown that retinal changes in individuals are correlated with the duration of diabetes, so examining subjects from the non-diabetic to prediabetic to diabetic state could give us more insight into the timing and nature of these changes.23 Interestingly, the majority of our prediabetes group found out they had HbA1c status consistent with prediabetes during the study and had no knowledge of it before presenting to participate.
In addition, while cataracts or any other visible lens changes were an exclusion criterion here, we do not have any objective measure of potential subtle changes in the lens that would not have been visible by slit lamp examination. Lens changes could impact contrast and colour testing. Clinically our groups were very similar by standard subjective testing, including visual acuity; however the control group was 7–10 years younger than both the prediabetes and T2DM groups. Berninger et al. reported no correlation between age and central colour vision thresholds between individuals aged 6 to 71 years after correcting for spectral sensitivity.24 While our age range was not as large, we did not measure differences in spectral sensitivity in this study. Roy et. al reported no difference in the L’Anthony D-15 test in individuals between the ages of 40 and 55 in normal observers with no lens changes.25 However, Roy et al. did note colour changes in older subjects. While most of our subjects were in the same age range as Roy’s study, we did include subjects in their 60s. Based on our results and these other studies, we do not believe the lens is responsible for the changes measured, but plan to include objective lens assessments in future studies.
Further, although the colour confusion and total error scores increase from controls through prediabetes to diabetes, it may still be difficult to differentiate these results from each other in a clinical setting based on score or angle alone. The computer program by Torok16 which uses the methods of Vingrys and King-Smith17 indicates results similar to the clinical results independently evaluated by the authors. Specifically, it indicated that many of our subjects had “pathological colour discrimination, probably diffuse colour discrimination error.” Longitudinal studies are needed to fully understand the underlying nature of the colour discrimination errors seen in this investigation.
Lighting conditions used for all subjects in the study were similar to those found in typical clinical practice settings, but differed from the ideal lighting for a L’Anthony D-15. We noted that bulbs with a bimodal colour distribution may not be as useful for colour testing as a true light source. The lighting differences in our study may have led to altered perception of the test caps. However, it is also important to understand how testing would vary in typical clinical settings, and all subjects here were tested in the same lighting conditions. More work evaluating different lighting would be helpful.
Lastly, we acknowledge that this is a cross sectional study of a small sample of patients with diabetes and prediabetes. The goal was to pilot and explore the most promising screening factors in prediabetes for future studies. A larger sample may show stronger correlations with blood glucose control and other functional measures which were either not investigated or shown to be significant here.
In the future, we aim to incorporate different lighting and colour vision tests such as the anomaloscope, Hardy-Rand-Ritter or Rabin Cone Contrast Test in order to see if these tests produce similar results to the L’Anthony D-15, as well as to see if one test is more sensitive than another. We chose the L’Anthony D-15 in this study for its clinical ease of use; however, we acknowledge that it is variable even within the normal population.26 We also want to follow individuals with prediabetes over time, to see if classical lifestyle interventions are able to reverse the changes that we have observed in colour vision.
In conclusion, while functional retinal testing has shown changes that occur in early type 2 diabetes before vasculopathy has developed, there are also functional changes in patients with prediabetes. Colour vision testing appears to be a candidate to differentiate those with glucose dysregulation as early as the prediabetes stage. These colour vision errors are positively correlated with increased HbA1c in patients across a range of prediabetes and diabetes. This study adds to the body of evidence that incorporating colour vision testing into diabetes screening protocols may be a helpful tool to detect changes early in these patients. This is important because changes in colour vision could serve as potential biomarkers that may aid in earlier diagnosis of diabetic eye disease, and could also provide a measure that could potentially serve as the foundation for earlier treatments.
Acknowledgements
We would like to acknowledge Allison Jussel and Rachel Wang for assistance in data collection, Dr. Minal Bhadane for assistance in recruiting of patients, Dr. Ted Zderic and the UH TORC staff for assistance in data processing, Amy Wozniak for general statistical advice, Dr. Daniel Coates for assistance with the spectrophotometer, and Dr. Laura Frishman for her help in this manuscript. This work was supported by the Fight for Sight Summer Student Fellowship, the NIH T35EY007088 Summer Research Grant, and University of Houston startup funds to WH and KR.
Footnotes
Disclosure
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
References
- 1.Chatterjee S, Kamlesh K, Davies MJ. Type 2 diabetes. The Lancet. 2017;389:2239–2251. [DOI] [PubMed] [Google Scholar]
- 2.Harrison W, Yevseyenkov V. Early interventions to prevent retinal vasculopathy in diabetes: a review. Clinical Optometry. 2015;7:71–80. [Google Scholar]
- 3.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithewaite T, Cincinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. The Lancet. 2017;5:1221–1234. [DOI] [PubMed] [Google Scholar]
- 4.Onofrey B, Skorin L, Holdeman N. Ocular therapeutics handbook. 3rd ed. Philadelphia: Wolters Kluwer; 2015:398–410 [Google Scholar]
- 5.Alves MRP, Boia R, Campos EJ, Martins J, Nunes S, Maderia MH, et al. Subtle thinning of retinal layers without overt vascular and inflammatory alterations in a rat model of prediabetes. Mol Vis. 2018;24:353–366. [PMC free article] [PubMed] [Google Scholar]
- 6.Bronson-Castain K, Bearse M, Neuville J, Jonasdottir S, King-Hooper B, Barez S, et al. Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina. 2012;32:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misra S, Saxena S, Kishore P, Bhasker SK, Misra A, Meyer CH. Association of contrast sensitivity with LogMAR visual acuity and glycosylated hemoglobin in non-insulin dependent diabetes mellitus. J Ocul Biol Dis Infor. 2010;3:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Survey of Ophthalmology. 2018;63:601–608. [DOI] [PubMed] [Google Scholar]
- 9.Tyrberg M, Lindbald U, Melander A, Lovestam-Adrian M, Ponjavic B, Andreasson S. Electrophysiology studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc Ophthalmol. 2011;123:193–198. [DOI] [PubMed] [Google Scholar]
- 10.Wolff BE, Bearse MA Jr, Schneck ME, Dhamdhere K, Harrison WW, Barez S, et al. Color vision and neuroretinal function in diabetes. Doc Ophthalmol. 2015;130:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piro A, Tagarelli A, Lagonia P, Nicoletti G, Quattrone A. Color vision study to assess the impaired retina-brain cortex pathway in type 2 diabetes: a pilot study in Calabria (Southern Italy). Neurological Sciences. 2019;40:1939–1942. [DOI] [PubMed] [Google Scholar]
- 12.Feitosa-Santana C, Paramei GV, Nishi M, Gualtieri M, Costa MF, Ventura DF. Color vision impairment in type 2 diabetes assessed by the D-15d test and the Cambridge Colour Test. Ophthalmic and Physiological Optics. 2010;30:717–723. [DOI] [PubMed] [Google Scholar]
- 13.Gella K, Raman R, Pal SS, Ganesan S, Sharma T. Contrast sensitivity and its determinants in people with diabetes: SN-DREAMS-II, Report No 6. Eye (Lond). 2017;31:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons J, et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Documenta Ophthalmologica. 2012;124:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standard of Medical Care in Diabetes—2020. Diabetes Care. 2020;43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 16.Torok B Farnsworth D-15 and Lanthony D-15 Color Vision Test Scoring. 2013.
- 17.Vingrys AJ and King-Smith PE. A quantitative scoring technique for panel tests of color vision. Invest Ophthalmol Vis Sci.1988;29:50–63. [PubMed] [Google Scholar]
- 18.Tabak AG, Herder C, Wolfgang R, Brunner EJ, Kivimaki M. Prediabetes: A high-risk state for developing diabetes. The Lancet. 2012;379:2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho NC, Poulsen GL, Ver Hoeve JN, Nork TM. Selective loss of S-cones in diabetic retinopathy. Arch Ophthalmol. 2000;118:1393–1400. [DOI] [PubMed] [Google Scholar]
- 20.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Survey of Ophthalmol. 2002;47:S253–S262. [DOI] [PubMed] [Google Scholar]
- 21.Gella K, Raman R, Kulothungan V, Pal SS, Ganesan S, Sharma T. Impairment of colour vision in diabetes with no retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SNDREAMS-II, Report 3). PLoS One. 2015;10: e0129391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Clerck EEB, Schouten JSAG, Berendschot TTJM, Goezinne F, Dagnelie PC, Schaper NC, et al. Macular thinning in prediabetes or type 2 diabetes without diabetic retinopathy: the Maastricht Study. Acta Ophthalmol. 2017; 96:174–182. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Diabetic Retinopathy Position Statement. Diabetes Care. 2002;25:590–593. [Google Scholar]
- 24.Berninger T, Drobner B, Hogg C, Ruolph G, Arden CB, Kampik A. Color vision in relation to age: a study of normal values. Klin Monatsbl Augenheilkd. 1999;215:37–42. [DOI] [PubMed] [Google Scholar]
- 25.Roy MS, Podgor MJ, Collier B, Gunkel RD. Color vision and age in a normal North American population. Graefe’s Arch Clin Exp Ophthalmol. 1991;229:139–144. [DOI] [PubMed] [Google Scholar]
- 26.Good GW, Schepler A, Nichols JJ. The reliability of the Lanthony Desaturated D-15 Test. Optometry and Vision Science. 2005;82:1054–1059. [DOI] [PubMed] [Google Scholar]