Abstract
Vascular disease plays an important role in all kinds of cognitive impairment and dementia. Diabetes increases the risk of vascular disease and dementia. However, it is not clear how existing vascular disease in the brain accelerates the development of small vessel disease and promotes cognitive dysfunction in diabetes. We used microemboli (ME) injection model in the current study to test the hypothesis that cerebrovascular dysfunction in diabetes facilitates entrapment of ME leading to inflammation and cognitive decline. We investigated cognitive function, axonal/white matter (WM) changes, neurovascular coupling, and microglial activation in control and diabetic male and female Wistar rats subjected to sham or low/high dose ME injection. Diabetic male animals had cognitive deficits, WM demyelination and greater microglial activation than the control animals even at baseline. Functional hyperemia gradually declined in diabetic male animals after ME injection. Both low and high ME injection worsened WM damage and increased microglial activation in diabetic male and female animals. Low ME did not cause cognitive decline in controls, while promoting learning/memory deficits in diabetic female rats and no further decline in diabetic male animals. High ME led to cognitive decline in control male rats and exacerbated the deficits in diabetic cohort. These results suggest that the existing cerebrovascular dysfunction in diabetes may facilitate ME-mediated demyelination leading to cognitive decline. It is important to integrate comorbidities/sex as a biological variable into experimental models for the development of preventive or therapeutic targets.
Keywords: microemboli, vascular cognitive impairment, diabetes, vascular dysfunction, microglial activation
1. Introduction
Vascular contributions to cognitive impairment and dementia (VCID) spectrum disorders are the second leading cause of dementia after Alzheimer’s Disease (Alber et al., 2019; Chornenkyy, Wang, Wei, & Nelson, 2019; Toth, Tarantini, Csiszar, & Ungvari, 2017; Trigiani & Hamel, 2017) and affect millions worldwide causing loss of creativity, productivity and quality of life. VCID includes all patients with an intellectual decline associated with cerebrovascular dysfunction (Corriveau et al., 2016). It is largely attributed to a heterogeneous group of diseases including large vessel disease with single or multiple strokes and small vessel disease (SVD) with lacunar infarcts and progressive damage to the deep white matter (Madigan, Wilcock, & Hainsworth, 2016; Rincon & Wright, 2013). It is clear that in all forms of cognitive impairment/dementia cases, vascular disease plays an important role and as such the American Heart Association issued a recent statement highlighting the importance of cardio/cerebrovascular health for brain health (Gorelick et al., 2017; Iadecola, 2017). While it has been long known that there is central neuropathy and diabetic individuals develop cognitive decline, with a prevalence as high as 40% in poorly controlled and long-standing diabetes, cognitive impairment remains to be one of the less understood and less studied complications of diabetes (Biessels & Despa, 2018; Mauricio, Alonso, & Gratacos, 2020). This is in part due to the wide spectrum of deficits observed and the varying impact of diabetes duration and severity on the symptoms of cognitive impairment. While VCID in diabetes is certainly multifactorial, SVD in the brain plays an important role in its pathogenesis. Diabetes is a well-known risk factor for both the micro- and macrovascular diseases, however, it is less known how existing vascular disease in the brain accelerates the development of SVD and VCID in diabetes.
Diabetes also increases the risk of cardiovascular disease including atherosclerosis and carotid stenosis, which are associated with increased level of microemboli (ME) (Dempsey et al., 2018; Silva, Miranda, Liu, Tse, & Roever, 2019). ME can be detected frequently in the cerebral circulation (Vukovic-Cvetkovic, 2012). Given that penetrating parenchymal arterioles, which branch out from pial arteries, are terminal arterioles without any collaterals and uniquely supply the brain tissue around them (Nishimura, Schaffer, Friedman, Lyden, & Kleinfeld, 2007), the brain would be predicted to be highly susceptible to ME. However, clinical studies using transcranial Doppler suggest that silent emboli are present even in asymptomatic individuals (Purkayastha & Sorond, 2012). It has been suggested that brain has the ability to “wash out” ME (Caplan & Hennerici, 1998; Lam, Yoo, Hiner, Liu, & Grutzendler, 2010), but smaller size of ME can lodge in the parenchymal arterioles and brain may have a selective vulnerability to ME in different zones (Bergui et al., 2015; Zhu et al., 2012). Given that pial and penetrating arteries are very important for the regulation of cerebral blood flow (CBF) and perfusion, how ME that entrapped in the brain microvasculature affect brain function is not well understood. ME have been shown to lead to increased microinfarcts and cognitive impairment in human (Goldberg, Auriel, Russell, & Korczyn, 2012; Rapp, Pan, et al., 2008; Venkat et al., 2017). Interestingly not all ME are occlusive to cause microinfarcts (Zhu et al., 2012) and even non-occlusive ME have been shown to trigger cortical spreading depression in mice (Nozari et al., 2010). Previous study showed that injection of ME composed of cholesterol crystals can disrupt the blood brain barrier (BBB) and induce microglial activation in a rat model (Rapp, Hollenbeck, & Pan, 2008). It has also showed that the same ME model can cause microinfarction and a temporary cognitive decline in young and aged mice (Wang et al., 2017; Wang et al., 2012) and rats (Goldberg et al., 2012; Rapp, Pan, et al., 2008; Venkat et al., 2017). However, these past studies have been conducted in otherwise healthy animals without comorbidities such as diabetes that is commonly found in patients with VCID. Based on these grounds, we used the ME injection animal model to test the hypothesis that one mechanism by which cerebrovascular dysfunction causes cognitive impairment is by facilitating the entrapment of ME in dysfunctional small vessels resulting in inflammation.
2. Results
2.1. Low dose (LD) ME caused parenchymal injury and WM demyelination but no cognitive decline in male diabetic animals
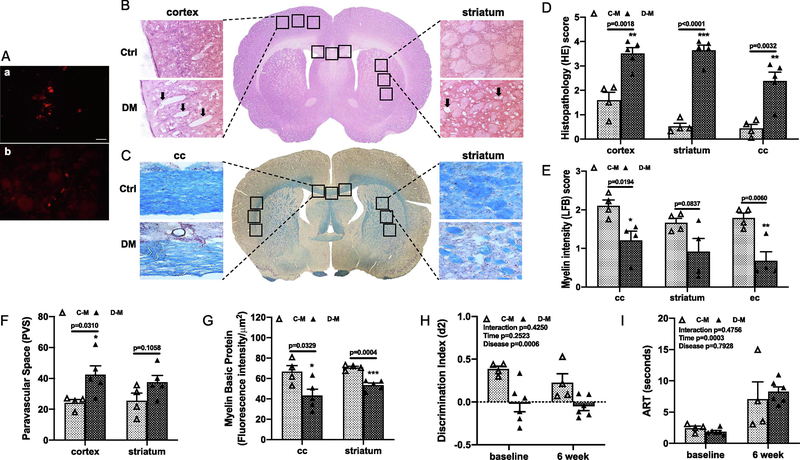
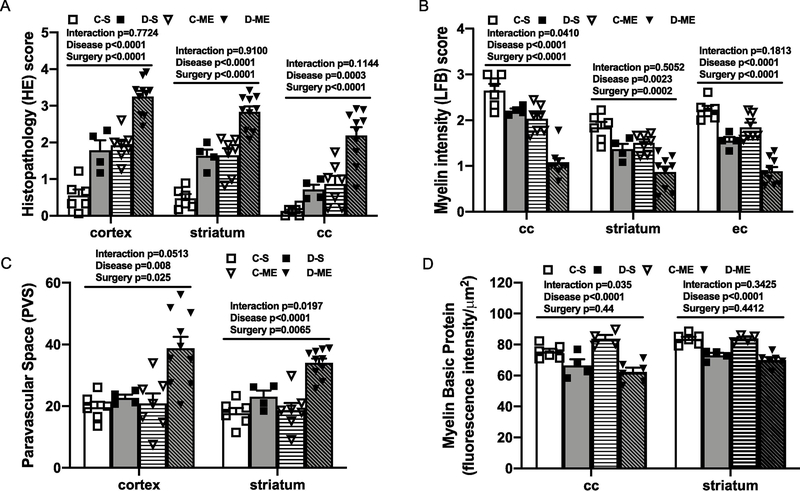
First, to confirm that ME reached and stayed in the brain, we labeled the crystals with fluorescent dye and sacrificed two rats at 3 days after injection. As shown in Fig. 1A, labeled ME are visible in brain sections. We next evaluated brain histology in control and diabetic male animals after 8 weeks. LD ME injection caused inflammatory cell infiltration and parenchymal injury in control animals but vacuolization, inflammatory cells, loss of tissue elements, axonal damage, and white matter (WM) rarefaction were evident only in diabetic animals (Fig. 1B and C, Suppl. Fig. 2). Pathology scores based on hematoxylin and eosin (HE) and Luxol fast blue (LFB) staining showed that diabetic male animals had worse damage and demyelination as well as increased perivascular space (PVS) index than the control group (Fig. 1D to F, Suppl. Fig. 2). To further confirm the demyelination, the expression of myelin basic protein (MBP) protein was measured by the fluorescence intensity, which was significantly lower at corpus callosum and striatum in diabetic animals compared to the controls (Fig. 1G).
Fig. 1,
LD ME prompted histopathological damage in the brain of male diabetic animals. Fluorescence dye labeled ME were detected in petri dish (a in A) and in brain sections (b in A) at 3 days after injection (bar = 50 μm). The histopathological damage in the brain sections were analyzed with HE (B) and LFB (C) staining. Diabetic male animals with LD ME had more neuronal injury and vacuolation (arrow) in the cortex indicated by the higher HE score (D), lower LFB score (E), higher PVS index (F), and lower MBP intensity (G) in multiple brain areas indicating a greater degree of cerebral damage and demyelination compared to control animals. Diabetic male rats also had significantly lower discrimination index (d2) at baseline and 6 weeks after LD ME (H). However, the LD ME did not exacerbate cognitive deficits (H) and sensorimotor function in diabetic male rats (I). Ctrl: control, DM: diabetes, C-M: control male, D-M: diabetic male, cc: corpus callosum, ec: external capsule.
At baseline, diabetic male rats had a lower d2 score compared with the control animals indicating baseline cognitive deficits (Fig. 1H). LD ME did not cause a decline in working memory in either groups by week 6, but diabetic rats maintained the baseline cognitive deficits (Fig. 1 H). The fine sensorimotor function tested by adhesive removal test (ART) showed there was no difference between control and diabetic animals at either time point, but it took the animals longer time to remove the adhesive dot at week 6 after ME injection (Fig. 1I).
2.2. LD ME caused parenchymal injury, WM demyelination and cognitive decline in female diabetic animals
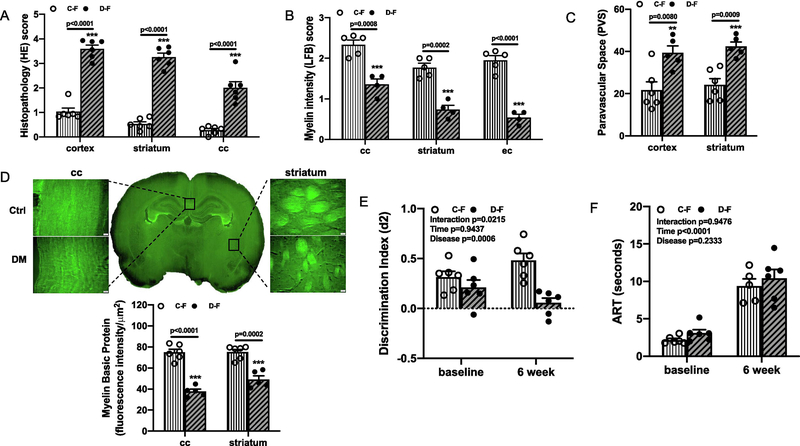
Similar analyses were performed in a cohort of female animals. Compared to control animals, the diabetic female animals had significant parenchymal damage, loss of tissue elements, higher volume of vacuolization, and WM degeneration as indicated by the higher HE score, lower LFB score, higher PVS index, and less MBP intensity in multiple areas (Fig. 2A–D).
Fig. 2,
LD ME caused tissue damage and cognitive impairment in female diabetic animals. Brain sections from female groups were also analyzed with HE and LFB staining as in male animals. Compared to the control animals, diabetic female rats had significantly higher HE score (A), lower LFB score (B), increased PVS index (C), and lower MBP intensity (D) in multiple brain areas as well. Interestingly, cognitive function was similar at baseline between control and diabetes female groups, but LD ME caused deficits at week 6 only in diabetic female animals (E, interaction p=0.0215) while sensorimotor function was similar at both time points (F). Ctrl: control, DM: diabetes, C-F: control female, D-F: diabetes female, cc: corpus callosum, ec: external capsule.
In contrast to the male groups, there was no difference in baseline d2 score of control and diabetic female animals. However, there was a significant interaction [F (1,20) =6.2, p=0.0215] such that cognitive decline was greater in the diabetic but not control group by week 6 after LD ME injection (Fig. 2E). The sensorimotor function was the same as seen in the male groups. There was no difference between control and diabetic female animals at baseline or after LD ME injection, but the time for adhesive removal was increased at week 6 in both groups (Fig. 2F).
2.3. LD ME led to diverse microglial morphology in both male and female diabetic animals
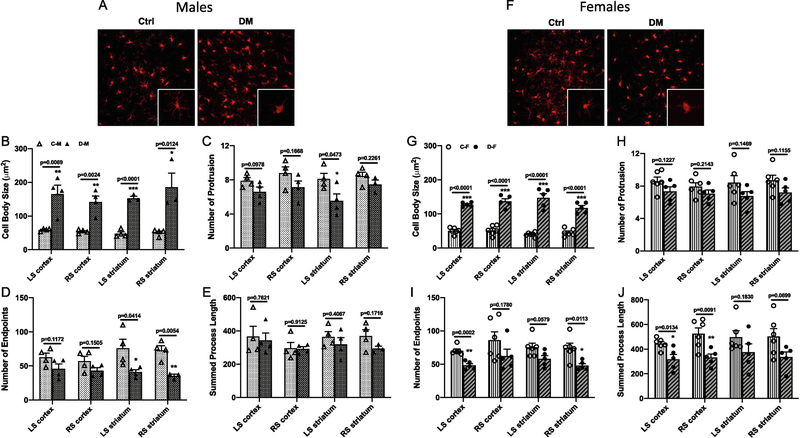
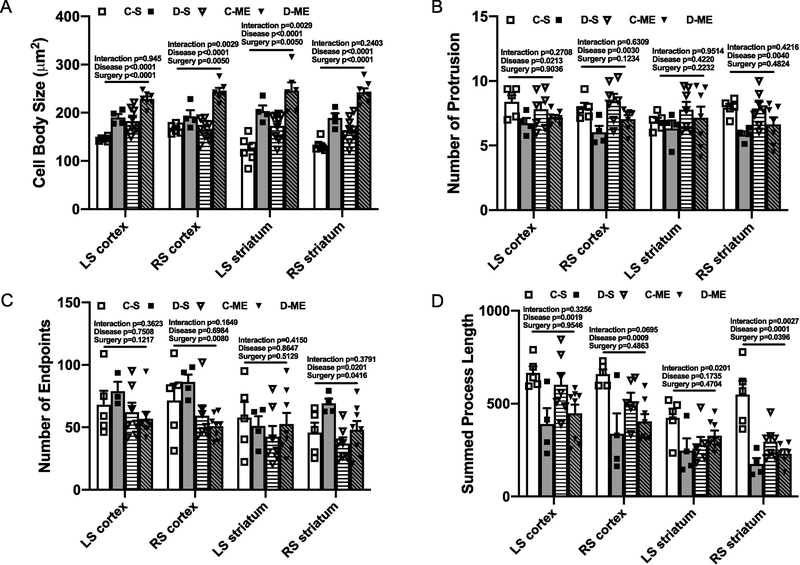
Because inflammation plays an essential role in cognitive impairment and diabetes, we next examined microglial morphology in the cortical and striatal brain regions in both hemispheres. In male animals, cell body swelling was significantly greater in all areas in the diabetic group (Fig. 3B). Number of protrusions was found to be different between groups only in left striatum, while number of endpoints was significantly lower in the striatum of diabetic rats and summed process length had no difference between groups (Fig. 3C to E).
Fig. 3,
Microglia were activated in both male and female diabetic animals with LD ME. Expression of Iba-1 was evaluated with immunohistochemistry staining and representative images from cortex of male (A) and female (F) animals are given in panels. In males, diabetes with LD ME had significantly increased cell body swelling (B), decreased number of protrusions (C) and endpoints of processes (D), but had no change for the process length (E). In females, LD ME significantly increased the cell body swelling (G), decreased the number of endpoints of processes (I) and the total process length (J) in diabetic animals, but had no effect on the number of protrusions (H). C-M: control male, D-M: diabetes male, C-F: control female, D-F: diabetes female, LS: left hemisphere, RS: right hemisphere.
In female animals, cell body swelling was significantly higher in all brain areas in the diabetic group (Fig. 3G). While there was no difference in number or protrusions between the groups, number of endpoints and process length were lower in diabetic animals (Fig. 3H to J), collectively suggesting amplified activation of microglia in both male and female diabetic rats.
2.4. High dose (HD) ME worsened cognitive deficits in diabetic male rats
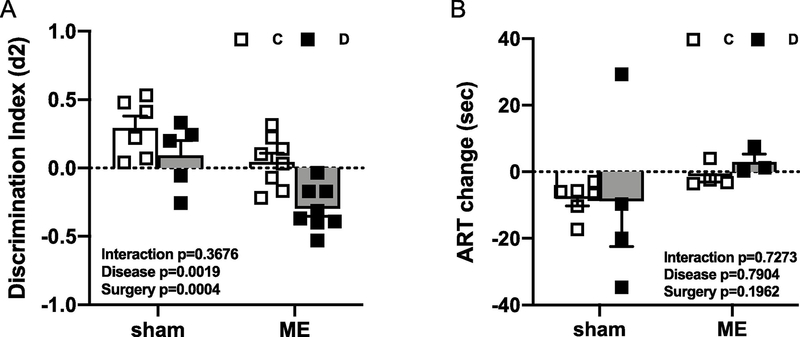
In Experiment 1, since we did not see a decline in cognitive function despite parenchymal injury by week 6 after LD ME in male animals, we next tested the impact of a higher dose of ME over a longer (16 weeks) period of time. We also included sham groups to evaluate the effect of intervention. By week 12 after HD ME, both control and diabetic animals had lower d2 scores than sham groups, and diabetic male animals had a significantly lower d2 score as compared to controls [Fig. 4A, Disease (control vs. diabetes) p=0.0019, Surgery (sham vs. ME) p=0.0004]. Even diabetic sham animals had a lower d2 scores than the control group. On the other hand, the HD ME had no effect on the sensorimotor function in both control and diabetic animals as measured by ART (Fig. 4B).
Fig. 4,
HD ME exacerbated cognitive impairment in diabetic male animals. Sham diabetic male animals had lower discrimination index (d2) than the control group, and HD ME further worsened it (A) without affecting the sensorimotor function (B) at 12 weeks after ME injection.
2.5. HD ME mediated parenchymal damage and WM demyelination in diabetes
To investigate whether the higher dose and longer period of ME worsens tissue damage, we next analyzed the histology of the brain sections. Compared to the control group, parenchymal injury and WM demyelination were evident in all brain areas even in the diabetic sham group, which was exacerbated by HD ME (Fig. 5A to D). The corpus callosum had the most significant WM demyelination with HD ME as there was an interaction in both LFB score and MBP intensity in this area [Fig. 5B, F (1,22) =4.712, p=0.0410 in LFB score, and Fig. 5D, F (1,16) =5.302, p=0.0351 in MBP intensity], and there was an interaction trend for the HE score in this area as well [Fig. 5A, F (1,23) =2.692, p=0.1144]. For PVS index, there was an interaction that diabetic animals with HD ME had worst PVS index at multiple brain areas [Fig. 5C, F (1,23) =4.229, p=0.0513 in cortex, F (1,23) =6.286, p=0.0197 in striatum].
Fig. 5,
HD ME induced tissue damage in control and exacerbated the existing injury in diabetic male animals. Sham diabetic animals had higher HE score (A), lower LFB score (B) and MBP intensity (D) than the controls. This was further intensified with HD ME, which caused even higher HE score (A) and lower LFB score (B) in both control and diabetic groups. HD ME caused significantly higher PVS index (C) in diabetic animals, with no effect on MBP intensity (D) in both control and diabetic animals. C-S: control sham, D-S: diabetic sham, C-ME: control with HD ME, D-ME: diabetes with HD ME, cc: corpus callosum, ec: external capsule.
2.6. HD ME induced microglial activation in male diabetic animals
Since previous study has showed a positive correlation between inflammation and the number of injected emboli (Rapp, Pan, et al., 2008), and we have found diverse changes in microglial morphology in the LD ME study, we next evaluated the microglial morphology in the HD ME groups. Diabetes increased cell body swelling, decreased the number of protrusions and branch length in all brain areas even in sham groups (Fig. 6A to D), indicating the increases in activated microglia. Furthermore, HD ME induced further increased cell body swelling in both control and diabetic groups, but decreased branch length only in the control animals and decreased number of endpoints only in diabetic animals (Fig. 6A to D).
Fig. 6,
HD ME exacerbated the increased inflammation in diabetic male animals. In sham animals, diabetes was associated with an increased cell body swelling (A), decreased number of protrusions (B) and total process length (C) of the microglia in all brain areas. HD ME further aggravated the increase of cell body swelling in both control and diabetes, while decreasing the process length in control animals (D). C-S: control sham, D-S: diabetic sham, C-ME: control with HD ME, D-ME: diabetes with HD ME, LS: left hemisphere, RS: right hemisphere.
2.7. HD ME reduced neurovascular coupling in diabetic male animals
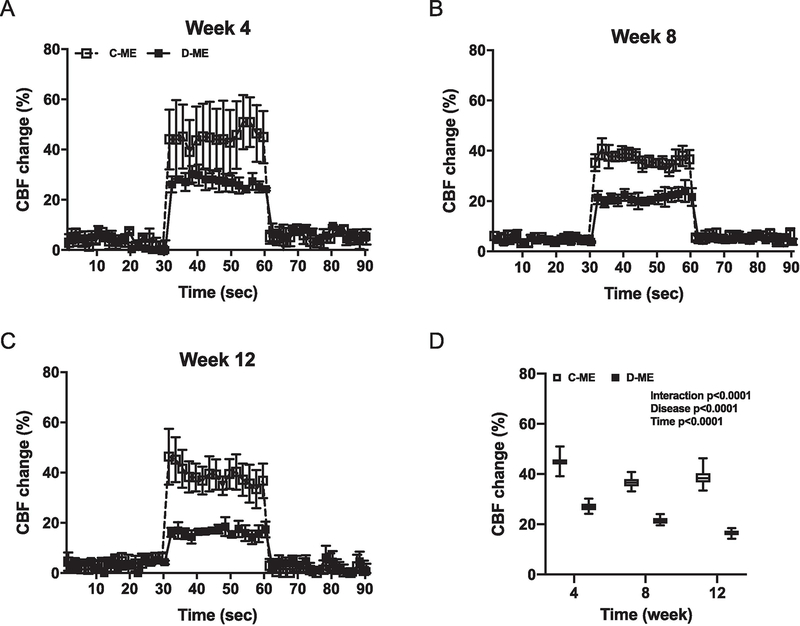
The brain relies on a constant blood supply for proper function. To determine whether ME in the cerebral circulation affect the vascular responses to firing neurons, we assessed functional hyperemia induced by whisker stimulation as a measure of neurovascular coupling (Li et al., 2013). The percent increase in relative CBF change in response to stimulation was lower and progressively declined in diabetes (Fig. 7A to D).
Fig. 7,
HD ME caused neurovascular uncoupling and gradually impaired the functional hyperemia in diabetic male animals. Functional hyperemia was assessed as the CBF change with whisker stimulation at each time point (A-C). The percentage increase of CBF has consistently decreased in DM group, but not Ctrl group, with ME injection (D). C-ME: control with HD ME, D-ME: diabetes with HD ME.
3. Discussion
It is increasingly recognized that prolonged hypoperfusion resulting from cerebrovascular dysfunction mediates BBB disruption, microinfarction and WM degeneration leading to dementias including Alzheimer’s Disease and VCID (Sweeney, Kisler, Montagne, Toga, & Zlokovic, 2018; Sweeney, Montagne, et al., 2019; Sweeney, Zhao, Montagne, Nelson, & Zlokovic, 2019). It is postulated that one possible mechanism by which vascular dysfunction leads to cognitive decline is hypoxia-mediated neuroinflammation. Thus, most studies investigating the mechanisms leading to cognitive decline model experimental VCID by restricting blood flow to the brain which bypasses early changes that can be detected and intervened effectively. Our group and others have previously shown that diabetes mediates vascular dysfunction and CBF is decreased in experimental models of diabetes (Hardigan, Hernandez, Ward, Hoda, & Ergul, 2017; Nie, Zhu, Zhang, Leng, & Wang, 2019; Shah et al., 2011). We have also reported impaired neurovascular coupling in diabetes (Kelly-Cobbs et al., 2012). Given that 1) regulation of blood flow is critical for proper brain function, 2) diabetes mediates early cerebrovascular dysfunction and a hypercoagulable state, and 3) ME are quite common in the cerebral circulation and can penetrate into the brain parenchymal arterioles, we postulated that entrapment of ME in dysfunctional vessel walls leads to the development of SVD ultimately resulting in VCID in a sex independent manner in diabetic but not control animals. Our findings show that: 1) diabetes is associated with vascular dysfunction that results in cognitive deficits which are further amplified by ME-mediated injury over time; 2) lower behavior scores are associated with greater tissue damage and demyelination in diabetic animals; and 3) diabetic rats also show a progressive decline in their ability to increase CBF upon neuronal stimulation, suggesting worsening of neurovascular uncoupling.
Global hypoperfusion models including bilateral carotid artery occlusion in rats (Chung, Iwasaki, Mishima, Egashira, & Fujiwara, 2002) and bilateral carotid artery stenosis with wire coils in mice (Nishio et al., 2010; Shibata, Ohtani, Ihara, & Tomimoto, 2004) have been frequently used in VCID research. Gradual perfusion reduction is produced by placing ameroid microconstrictor cuffs filled with casein around the carotid artery in rats and mice (Hattori et al., 2015; Kitamura et al., 2012). These models mimic the low perfusion pressure as seen in the deep white matter areas of human brain (Nonaka et al., 2003). However, the pathogenesis of white matter injury in these hypoperfusion models is believed to be different from human SVD. The majority of white matter degeneration and lacunes in human brains are thought to be a direct result of local changes in small vessels walls (Pantoni, 2010), rather than due to embolic events or episodes of global hypoperfusion. Some studies have used cholesterol crystals as ME to develop a “multiple microinfarcts” model (Venkat et al., 2017; Wang et al., 2017; Wang et al., 2012). Microinfarcts are presenting in routine neuropathological specimens and are generally attributed to SVD. Clinical studies have shown that VCID patients are associated with ME in the cerebral circulation (Dempsey et al., 2018; Laza et al., 2013). In preclinical studies, multiple microinfarcts and cognitive deficits were found after variable sizes of cholesterol crystals injected in male healthy control mice and rats (40–70 μm for mice (Wang et al., 2017; Wang et al., 2012) and 60–100 μm for rats (Rapp, Pan, et al., 2008; Venkat et al., 2017)). It was also found that repeated injection of ME in a rat model only caused minor neuronal injury but no infarction (Rapp, Pan, et al., 2008). In the current study, we used the smaller size cholesterol crystals and observed cognitive decline in both male and female diabetic animals. To our knowledge, this is the first study to explore the development of cognitive decline in both male and female diabetic animals with ME injection model.
Inflammation in the central nervous system has been implicated in the pathogenesis of cognitive impairment. It has been suggested that brain inflammation initially occurs as a protective response, the microglia phagocytes amyloid fibrils but then their activity declines. Later, a second phase of increased microglial activation occurs which is neurotoxic as tau tangles accumulate, and this phenotype drives disease progression (Fan, Brooks, Okello, & Edison, 2017). Diabetes is also a chronic inflammatory state. ME as a secondary insult may exacerbate the inflammation in the brain and deteriorate the cognitive function (Rapp, Pan, et al., 2008; Wang et al., 2012). As resident immune cells in the central nervous system, microglial cells are the primary initiators of the inflammatory response in acute and chronic disorders. While accumulating evidence suggest that microglial cells display diverse regional and functional heterogeneity, traditionally these cells are characterized by a ramified shape with small cell body and extensive branching off the soma in the surveillance (homeostatic) mode and a phagocytic phenotype with enlarged soma and retraction of processes in the activated (reactive) mode (Lauro & Limatola, 2020; Stratoulias, Venero, Tremblay, & Joseph, 2019). Previously we have found increased microglia reactivity in the hippocampus of male diabetic rats, which was associated with decreased cognitive function after ischemic cerebral injury (Ward et al., 2018). Recent clinical study also reported that mild cognitive impairment with low baseline but rising amyloid load had correlated levels of microglial activation which then later decline when the amyloid load approaches Alzheimer’s Disease level (Ismail et al., 2020). In this current study, we assessed microglial activity by measuring several indices of microglia morphology using Iba-1-stained brain sections. There is an increased microglial activity in both male and female diabetic animals in LD ME groups. In HD ME groups, we found that diabetes alone increased the microglial activity in sham animals and HD ME further activated microglial cells in both control and diabetic male animals. These results suggest that diabetes amplifies the inflammatory response and contributes to the cognitive impairment.
It has been demonstrated that the WM damage is the most common marker of SVD in the brain and has been frequently found on brain MRIs of patients with cognitive impairment (Wardlaw et al., 2013). The WM hyperintensities appear mostly in the periventricular area and have a modest association with cognitive dysfunction seen in these patients (van den Berg, Geerlings, Biessels, Nederkoorn, & Kloppenborg, 2018). In preclinical studies with cerebral hypoperfusion models, the WM lesions are predominantly located at corpus callosum and striatum in rodents and at deep white matters in baboon (Washida, Hattori, & Ihara, 2019). In the current study, we demonstrated that diabetes alone induces significant WM damage and that ME further exacerbates this in adult Wistar rats. The characteristic histopathological changes were seen in both male and female animals. Myelinated axons are essential for the fiber communication to maintain the normal working memories. The worsened WM damage in diabetic rats with ME may hasten the development of cognitive impairment. WM damage is also associated with increased perivascular space. Prominent perivascular space has been considered as a pathological marker for SVD and may related to glymphatic dysfunction (Iadecola, 2017; Wang et al., 2017; Wardlaw et al., 2020). In the current study, we observed increased PVS index in both male and female diabetic rats with ME. Together with the increased vacuolization, demyelination, and dilated PVS, ME worsened the SVD in both male and female diabetic animals.
Previous studies in our laboratory have showed that cerebral vasculature develops variable regeneration patterns after ischemic injury in diabetic male and female animals depending on the stroke and diabetes models (Li et al., 2019; Ward et al., 2018). We also found that both male and female diabetic animals develop cognitive impairment at baseline and early after cerebral ischemic injury (Li et al., 2019; Ward et al., 2018). In the current study, we explored the cognitive function change before and after ME injection in both male and female animals. We found that while LD ME had no effect on cognitive impairment in males, it worsened the cognitive function of diabetic female animals 6 weeks after the insult. Tissue damage was greater in female animals. On the other hand, HD ME significantly decreased the cognitive function in both control and diabetic male groups. These results suggest that diabetes affects the cognitive function differently in male and female animals and that the development of cognitive impairment may differ depending on the severity and duration of the comorbidities. Given that women with diabetes are particularly vulnerable to cerebrovascular disease (Madsen et al., 2018), and are more likely to enter nursing home after stroke because of greater cognitive impairment (Bushnell et al., 2014; Gall, Tran, Martin, Blizzard, & Srikanth, 2012), there is a need to further explore whether improvement of endothelial function is an effective strategy for prevention and treatment of cognitive decline in females.
There are several limitations in our study. First, this study was focused on the development of an ME model to study cognitive impairment in diabetic animals of both sexes, therefore, no mechanistic experiments were included. Second, we only studied male animals in the HD ME experiments. Further studies with sham female animals are needed to determine the contribution of diabetes and inflammation to the development of neurovascular dysfunction and cognitive impairment in both sexes. Additionally, this study did not include ovariectomized female animals to investigate the effects of sex hormones. Previous studies have demonstrated that cognitive impairment is associated with neurophysiological changes in postpartum and postmenopausal period but our findings suggest that in the presence of diabetes even young animals develop cognitive decline. Third, the experiments were done in relatively young animals. Cognitive impairment is considered an aging disease, and although diabetes does increase the risk of vascular disease in the aging population, little is known about neurovascular changes in aged animals. Lastly, we studied the microglial activation based on morphology but did not differentiate pro-inflammatory status which causes demyelination, or immune-regulatory status which may result in remyelination depending on the region of the brain and stage of the disease (Lee, Hamanaka, Lo, & Arai, 2019).
Revisiting our hypothesis, our findings support the concept that early cerebrovascular dysfunction in diabetes facilitates and enhances ME-mediated neurodegeneration and demyelination leading to cognitive decline. Hence improvement of vascular function may prevent and/or retard the onset cognitive decline in comorbid disease states. Our findings highlight the importance of integrating comorbid disease models into experimental paradigms for the development of preventive and therapeutic targets.
4. Experimental Procedures
4.1. Animals
Animal studies were conducted at the Augusta University in Augusta, GA before the investigators relocated to the Medical University of South Carolina in Charleston, SC. Rats were housed in the animal care facility at Augusta University, which is approved by the American Association for Accreditation of Laboratory Animal Care. All experiments were conducted in accordance with the National Institute of Health (NIH) guidelines for the care and use of laboratory animals in research. Furthermore, all protocols were approved by the institutional animal care and use committee.
Diabetes was induced in male and female Wistar rats (Envigo RMS, Inc., Indianapolis, IN) using a high-fat diet/low-dose streptozotocin (HFD/STZ) combination (Ward et al., 2018). The rats were received at 4 weeks of age and immediately started and maintained on a 45% kcal fat diet for the remainder of the study (D12451, Research Diets Inc., New Brunswick, NJ). A single dose STZ injection (35 mg/kg; Cayman Chemical, Ann Arbor, MI) was administered intraperitoneally at 6 weeks of age. If blood glucose was not above 150 mg/dL at 5 days post-injection, a second small dose (20 mg/kg) was administered. In combination with HFD, this low dose STZ injection results in elevated blood glucose in at least 90% of the animals. Control rats were received at 10–11 weeks of age and maintained on regular chow with 4% kcal fat. Body weight and blood glucose were measured once a week until euthanasia.
4.2. Preparation and injection of ME
Cholesterol crystals were used as ME and prepared as described previously (Wang et al., 2012). Briefly, USP-grade free cholesterol crystals (Sigma) were filtered through a 70 μm and then a 40 μm cell strainer (352340; BD Biosciences). Crystals in size of 40–70 μm were collected and counted with a hemocytometer. Fluorescence labeled ME were prepared as described previously (Lam et al., 2010). 100 mg of cholesterol crystals were melted at 150 °C and Texas Red-X succimidyl ester were added to label them fluorescently. The solution was crystallized at room temperature, sonicated in saline, filtered through cell strainer as mentioned above and resuspended.
All rats were randomly assigned to ME or saline (sham) injection groups. Animals were anesthetized with isoflurane (5% for induction and 2% for maintenance) during the surgical process. The right common carotid artery (CCA), the internal carotid artery (ICA), and the external carotid artery (ECA) were carefully isolated. The ECA was permanently ligated and cauterized. A PTFE 160 catheter was inserted into the incision on the ECA stump and forwarded into the intracranial portion of ICA. Total 200 μl of saline with crystal suspension or saline alone was injected over a period of 5 min. After injection, the catheter was removed, the ECA stump was closed with silk suture, and the skin incision was closed with surgical clips. The animals were closely monitored for 24 h after surgery.
Experiment 1: LD ME injection
Control and diabetic male and female rats were subjected to ME injection with 15 crystal/μl saline and followed for 8 weeks (Suppl. Fig. 1, n=6 in each group). 2 rats in control male group and 1 in diabetic male group died within 3 days of ME injection and were not included in the results. Novel object recognition (NOR) and ART were performed at baseline and week 6 after surgery to evaluate cognitive and sensorimotor deficits, respectively, as described below. All female rats underwent surgery during the diestrus phase after careful monitoring of the estrus cycle by vaginal swab.
Experiment 2: HD ME injection
Control and diabetic male rats were subjected to sham (saline only) or ME injection with 30 crystals/μl saline and followed for 16 weeks (Suppl. Fig. 1, n=6 for each of the control sham and diabetic sham, 8 for each of the control ME and diabetic ME). 1 rat in each of the diabetic sham and control ME groups died, and were not included in the final analyses. Final animal numbers are n=6 of each for control sham, 5 for diabetic sham, 7 for control ME, and 8 for diabetic ME. NOR and ART were conducted at baseline and at week 12 after surgery. Functional hyperemia was assessed over time at weeks 4, 8 and 12.
4.3. Evaluation of sensorimotor and cognitive functions
Sensorimotor and cognitive tests were recorded and scored in a blinded fashion. Animals were handled and trained in the room where tests were to be carried out. Sensorimotor function was examined through the ART as previously described (Li et al., 2017). Briefly, animals were trained to remove an adhesive paper dot for 5 days before baseline recording. Contact and removal latency was reported at baseline and at each time point after ME injection. For each day, three trials were averaged with the maximum removal latency at 180 s per trial.
Cognitive function was assessed through NOR test as previously described (Prakash, Johnson, Fagan, & Ergul, 2013; Ward et al., 2018). Animals were habituated to the test apparatus for 4 days, 10 min per time per day before baseline testing. On the day of testing, the animal had three phases: acclimation, familiarization, and novel. The acclimation phase allowed the animal to habituate to the empty box for 5 min each day. During the familiarization phase, or A/A session, animals were allowed to explore two identical objects for 5 min. Rats were placed back into their home cages for a 15 min delay followed by a 5 min novel phase (A/B session) in which the familiar object from the A/A session was paired with a novel object. Objects were placed equidistant from the walls, with 20 cm between the two objects. Rats were placed in the center of the box for each session. Between each phase, objects and the testing apparatus were cleaned with 30% ethanol. The time spent exploring each object was recorded, and the time spent with novel object (N), familiar object (F), recognition index (RI = N / [N + F]), and discrimination index 2 (d2 = [N-F] / (N+F]) was calculated for both A/A and A/B sessions. Only animals that explored objects for a minimum of 30 s within the 5 min trial were included in the analysis.
4.4. Functional hyperemia
Functional hyperemia was assessed at multiple time points after the ME injection by measuring the change in CBF in the somatosensory cortex upon whisker stimulation as described before (Kelly-Cobbs et al., 2013). Animals were anesthetized with ketamine-xylazine (100 and 10 mg/kg) injection, and trimmed contralateral whiskers were gently stroked at a frequency of 10 Hz with an applicator attached to a vortex. The PIM3 laser Doppler scanning system (LDS, Perimed, Ardmore, PA) was programmed to scan an area covering somatosensory cortex, which is supplied by the middle cerebral artery (MCA), without tissue contact. CBF changes were expressed as percent increase relative to resting levels.
4.5. Histopathology assessment
After sacrifice, brains were isolated and preserved in 4% paraformaldehyde/PBS for 2 days before transfer to a 30% sucrose/PBS solution. Coronal sections (20 μm thickness) stained with HE were used to assess the histopathology (cortex, striatum and corpus callosum) and PVS in the cortex and striatum. A four-point scale as previously described (Mangalam et al., 2013) was used: 0, no pathology; 1, no tissue destruction but only minimal inflammation; 2, early tissue destruction (loss of architecture) and moderate inflammation; 3, definite tissue destruction (demyelination, parenchymal damage, cell death, neuronal vacuolation); and 4, necrosis (complete loss of all tissue elements with associated cellular debris).
The PVS was quantified as previously reported (Ampawong et al., 2011). Briefly, all visible vessels on each stained section were selected with software Fiji. The outline of the PVS was drawn manually to determine its inner and outer limits. The length of the vessel was also measured. The PVS index was calculated by subtraction of the two surfaces and then divided by the vessel length to correct the errors caused by the vessel size.
LFB, a demyelination marker, staining was performed to detect the severity of WM damage (Khan et al., 2018). The lesion was graded into four levels on the basis of LFB staining as: 0, no stain; 1, low stain; 2, moderate stain; and 3, high stain in cortex, striatum and external capsule with higher score indicating less damage.
For all measurements, 3 sections from each brain were used and 3 ROI in each brain section were scored. Results are given as the average of 9 readings.
4.6. Immunohistochemistry
To visualize the activated microglia and macrophages, the expression of ionized calcium-binding adapter molecule 1 (Iba-1) was assessed in the brain sections by incubating with anti-Iba-1 antibody (1:500, Wako) overnight at 4°C followed by incubation with appropriate fluorescence labeled secondary antibody (1:1000, Vector) for 1 h at room temperature. Sections were imaged on a Zeiss Axiovert 200 microscope (Carl Zeiss Micro-Imaging) in the cortex and striatum area using a 20x objective. Identical threshold parameters were used across all sections. These images were then converted to binary and skeletonized using Fiji software, and analyzed as we previously published (Ward et al., 2018). Cell body area, number of protrusions, summed process end points, and summed process length of the microglia were reported.
MBP was evaluated in brain sections with the primary antibody (anti-MBP, 1:400, Abcam) overnight at 4°C and then incubated with the secondary antibody (goat anti-rabbit IgG, 1:1000; ThermoFisher). Fluorescence pictures were captured with Olympus IX73 fluorescence microscope with Olympus DP74 camera. Image acquisition settings were kept constant across the groups. Fluorescence intensity in the region of interest was analyzed using Fiji ImageJ software and mean integrated intensity was reported.
4.7. Data Analysis
The results were analyzed by GraphPad Prism 8 and expressed as mean ± SEM. For Experiment 1, endpoints measured in control and diabetic animals were compared with two tailed t-test, respectively (Fig 1C–F, Fig 2A–D, and Fig 3B–E and G–J). Behavior tests were analyzed by a two-way ANOVA for (control vs. diabetes) and (baseline vs. 6 week) comparisons (Fig 1G–H and Fig 2E–F). For Experiment 2, these indices were analyzed by a two-way ANOVA for (control vs. diabetes) and (sham vs. ME) comparisons (Figs 4–6). The effect of ME on neurovascular coupling in control and diabetic rats was compared with repeated measures of (Fig 7D). Statistical significance was determined at p<0.05 and indicated by symbols or ANOVA tables where appropriate.
Supplementary Material
Table 1,
The animal basic information in the study.
| Experiment 1: LD ME (15 crystals/μL) | ||||
| Groups | C-M (n=4) | D-M (n=5) | C-F (n=6) | D-F (n=6) |
| Body weight (g) | 379.1 ± 4.3 | 431.2 ± 29.9 | 253.3 ± 3.6* | 316.8 ± 23.7* |
| Blood glucose (mg/dL) | 81.8 ± 3.4 | 335.2 ± 22.2*** | 80.7 ± 3.3 | 338.2 ± 15.5*** |
| Experiment 2: HD ME (30 crystals/μL) | ||||
| Groups | C-S (n=6) | D-S (n=5) | C-ME (n=7) | D-ME (n=8) |
| Body weight (g) | 622.2 ± 15.1 | 554.9 ± 47.1 | 588.4 ± 22.8 | 421.2 ± 32.8** |
| Blood glucose (mg/dL) | 82.0 ± 4.9 | 311.5 ± 7.0*** | 72.8 ± 2.2 | 337.8 ± 37.6*** |
p<0.001 vs. C-M or D-M, respectively
p<0.01 vs. D-S or C-ME, respectively
p<0.0001 vs. C, respectively.
Highlights.
Diabetic animals had worse cerebral histopathological damage with microemboli.
Microemboli-mediated injury amplifies cognitive impairment in diabetic animals.
Microemboli increased microglial activation in diabetic male and female animals.
Neurovascular coupling is progressively declined in diabetic animal with microemboli.
Acknowledgements
This study was supported by Veterans Affairs (VA) Merit Review (BX000347), VA Senior Research Career Scientist Award (IK6 BX004471), National Institute of Health (NIH) RF1 NS083559 and R01 NS104573 (multi-PI, Susan C. Fagan as co-PI) to Adviye Ergul; and Diabetic Complications Research Consortium DiaComp awards 17AU3831/18AU3903 (DK076169/115255) to Weiguo Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, et al. (2019). White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y), 5, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampawong S, Combes V, Hunt NH, Radford J, Chan-Ling T, Pongponratn E, et al. (2011). Quantitation of brain edema and localisation of aquaporin 4 expression in relation to susceptibility to experimental cerebral malaria. Int J Clin Exp Pathol, 4(6), 566–574. [PMC free article] [PubMed] [Google Scholar]
- Bergui M, Castagno D, D’Agata F, Cicerale A, Anselmino M, Maria Ferrio F, et al. (2015). Selective Vulnerability of Cortical Border Zone to Microembolic Infarct. Stroke, 46(7), 1864–1869. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, & Despa F (2018). Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol, 14(10), 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, et al. (2014). Sex differences in quality of life after ischemic stroke. Neurology, 82(11), 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LR, & Hennerici M (1998). Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol, 55(11), 1475–1482. [DOI] [PubMed] [Google Scholar]
- Chornenkyy Y, Wang WX, Wei A, & Nelson PT (2019). Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol, 29(1), 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Iwasaki K, Mishima K, Egashira N, & Fujiwara M (2002). Repeated cerebral ischemia induced hippocampal cell death and impairments of spatial cognition in the rat. Life Sci, 72(4–5), 609–619. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. (2016). The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol, 36(2), 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RJ, Varghese T, Jackson DC, Wang X, Meshram NH, Mitchell CC, et al. (2018). Carotid atherosclerotic plaque instability and cognition determined by ultrasound-measured plaque strain in asymptomatic patients with significant stenosis. J Neurosurg, 128(1), 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Brooks DJ, Okello A, & Edison P (2017). An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain, 140(3), 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall SL, Tran PL, Martin K, Blizzard L, & Srikanth V (2012). Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke, 43(7), 1982–1987. [DOI] [PubMed] [Google Scholar]
- Goldberg I, Auriel E, Russell D, & Korczyn AD (2012). Microembolism, silent brain infarcts and dementia. J Neurol Sci, 322(1–2), 250–253. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, et al. (2017). Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke, 48(10), e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardigan T, Hernandez C, Ward R, Hoda MN, & Ergul A (2017). TLR2 knockout protects against diabetes-mediated changes in cerebral perfusion and cognitive deficits. Am J Physiol Regul Integr Comp Physiol, 312(6), R927–R937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Enmi J, Kitamura A, Yamamoto Y, Saito S, Takahashi Y, et al. (2015). A novel mouse model of subcortical infarcts with dementia. J Neurosci, 35(9), 3915–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2017). The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron, 96(1), 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail R, Parbo P, Madsen LS, Hansen AK, Hansen KV, Schaldemose JL, et al. (2020). The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J Neuroinflammation, 17(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, et al. (2012). Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther, 342(2), 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Prakash R, Li W, Pillai B, Hafez S, Coucha M, et al. (2013). Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol, 304(6), H806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MB, Hafez S, Hoda MN, Baban B, Wagner J, Awad ME, et al. (2018). Chronic Remote Ischemic Conditioning Is Cerebroprotective and Induces Vascular Remodeling in a VCID Model. Transl Stroke Res, 9(1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A, Fujita Y, Oishi N, Kalaria RN, Washida K, Maki T, et al. (2012). Selective white matter abnormalities in a novel rat model of vascular dementia. Neurobiol Aging, 33(5), 1012 e1025–1035. [DOI] [PubMed] [Google Scholar]
- Lam CK, Yoo T, Hiner B, Liu Z, & Grutzendler J (2010). Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature, 465(7297), 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro C, & Limatola C (2020). Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front Immunol, 11, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laza C, Popescu BO, Popa M, Roceanu AM, Tiu C, Antochi FA, et al. (2013). Microemboli detection in patients with carotid artery stenting--a potential marker for future cognitive impairment? J Neurol Sci, 326(1–2), 96–99. [DOI] [PubMed] [Google Scholar]
- Lee J, Hamanaka G, Lo EH, & Arai K (2019). Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther, 25(12), 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prakash R, Chawla D, Du W, Didion SP, Filosa JA, et al. (2013). Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol, 304(11), R1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Valenzuela JP, Ward R, Abdelbary M, Dong G, Fagan SC, et al. (2019). Post-stroke neovascularization and functional outcomes differ in diabetes depending on severity of injury and sex: Potential link to hemorrhagic transformation. Exp Neurol, 311, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ward R, Valenzuela JP, Dong G, Fagan SC, & Ergul A (2017). Diabetes Worsens Functional Outcomes in Young Female Rats: Comparison of Stroke Models, Tissue Plasminogen Activator Effects, and Sexes. Transl Stroke Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan JB, Wilcock DM, & Hainsworth AH (2016). Vascular Contributions to Cognitive Impairment and Dementia: Topical Review of Animal Models. Stroke, 47(7), 1953–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TE, Khoury JC, Alwell KA, Moomaw CJ, Demel SL, Flaherty ML, et al. (2018). Sex differences in cardiovascular risk profiles of ischemic stroke patients with diabetes in the Greater Cincinnati/Northern Kentucky Stroke Study. J Diabetes, 10(6), 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam A, Poisson L, Nemutlu E, Datta I, Denic A, Dzeja P, et al. (2013). Profile of Circulatory Metabolites in a Relapsing-remitting Animal Model of Multiple Sclerosis using Global Metabolomics. J Clin Cell Immunol, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio D, Alonso N, & Gratacos M (2020). Chronic Diabetes Complications: The Need to Move beyond Classical Concepts. Trends Endocrinol Metab, 31(4), 287–295. [DOI] [PubMed] [Google Scholar]
- Nie Q, Zhu L, Zhang L, Leng B, & Wang H (2019). Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and Calpain-1 activation. Life Sci, 232, 116662. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Schaffer CB, Friedman B, Lyden PD, & Kleinfeld D (2007). Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A, 104(1), 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, et al. (2010). A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke, 41(6), 1278–1284. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, & Ihara F (2003). Microvasculature of the human cerebral white matter: arteries of the deep white matter. Neuropathology, 23(2), 111–118. [DOI] [PubMed] [Google Scholar]
- Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, et al. (2010). Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol, 67(2), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol, 9(7), 689–701. [DOI] [PubMed] [Google Scholar]
- Prakash R, Johnson M, Fagan SC, & Ergul A (2013). Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PLoS One, 8(2), e56264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, & Sorond F (2012). Transcranial Doppler ultrasound: technique and application. Semin Neurol, 32(4), 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp JH, Hollenbeck K, & Pan XM (2008). An experimental model of lacunar infarction: embolization of microthrombi. J Vasc Surg, 48(1), 196–200. [DOI] [PubMed] [Google Scholar]
- Rapp JH, Pan XM, Neumann M, Hong M, Hollenbeck K, & Liu J (2008). Microemboli composed of cholesterol crystals disrupt the blood-brain barrier and reduce cognition. Stroke, 39(8), 2354–2361. [DOI] [PubMed] [Google Scholar]
- Rincon F, & Wright CB (2013). Vascular cognitive impairment. Curr Opin Neurol, 26(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, et al. (2011). Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol, 55(1–3), 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohtani R, Ihara M, & Tomimoto H (2004). White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke, 35(11), 2598–2603. [DOI] [PubMed] [Google Scholar]
- Silva R, Miranda CM, Liu T, Tse G, & Roever L (2019). Atrial Fibrillation and Risk of Dementia: Epidemiology, Mechanisms, and Effect of Anticoagulation. Front Neurosci, 13, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratoulias V, Venero JL, Tremblay ME, & Joseph B (2019). Microglial subtypes: diversity within the microglial community. EMBO J, 38(17), e101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, & Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nat Neurosci, 21(10), 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. (2019). Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement, 15(1), 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, & Zlokovic BV (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev, 99(1), 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, & Ungvari Z (2017). Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol, 312(1), H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigiani LJ, & Hamel E (2017). An endothelial link between the benefits of physical exercise in dementia. J Cereb Blood Flow Metab, 37(8), 2649–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, Geerlings MI, Biessels GJ, Nederkoorn PJ, & Kloppenborg RP (2018). White Matter Hyperintensities and Cognition in Mild Cognitive Impairment and Alzheimer’s Disease: A Domain-Specific Meta-Analysis. J Alzheimers Dis, 63(2), 515–527. [DOI] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, et al. (2017). White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging, 50, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic-Cvetkovic V (2012). Microembolus detection by transcranial Doppler sonography: review of the literature. Stroke Res Treat, 2012, 382361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, et al. (2017). Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J Neurosci, 37(11), 2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Iliff JJ, Liao Y, Chen MJ, Shinseki MS, Venkataraman A, et al. (2012). Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci, 32(50), 17948–17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, & Ergul A (2018). Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. Am J Physiol Heart Circ Physiol, 315(5), H1402–H1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. (2020). Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol, 16(3), 137–153. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol, 12(8), 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washida K, Hattori Y, & Ihara M (2019). Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate. Int J Mol Sci, 20(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Hoffmann A, Wintermark M, Pan X, Tu R, & Rapp JH (2012). Do microemboli reach the brain penetrating arteries? J Surg Res, 176(2), 679–683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.