Abstract
In this article, we make a theoretical and in silico study for uncovering and evaluating biomarkers in colon and rectal cancer (CRC) by the dynamic network biomarker (DNB) theory. We propose a strategy to employ the theoretical concept of UICC TNM classification in CRC. To reveal the critical transition of CRC, the DNB algorithm was implemented to analyze the genome-wide dynamic network through temporal gene expression data. The relationship between gene sets and clinical features was evaluated by weighted gene co-expression network analysis. The results show that MYC was significantly associated with tumor amplification, tumor immune cells, and survival times. The candidate tumor suppressor genes were ZBTB16, MAL, LIFR, and SLIT2. Protein–protein interaction (PPI) analysis shows that these candidate tumor suppressor genes were significant in immune cells. Data from the Human Protein Atlas showed that a high expression of these candidate tumor suppressor genes was associated with favorable prognosis in TNM stages I–IV. In conclusion, this work provides significant and novel information regarding the TNM stage, cause, and consequences of elevated MYC expression in CRC. MYC expression levels had significant negative correlations with tumor suppressor genes and immune cells.
Keywords: dynamic network biomarker, colon and rectal cancer, time-course gene expression data, tumor suppressor genes, immune infiltrates
Introduction
Colon and rectal cancer (CRC) is one of the most frequently diagnosed tumor in terms of incidence, and its incidence among young people has increased in recent years (Brenner et al., 2019; Dang et al., 2020). Over 1.8 million of new CRC cases and 881,000 deaths were estimated to occur in 2018 (Bray et al., 2018). Despite the advance technology in CRC screening and treatment strategies such as molecular biomarkers and immune therapy, poor prognosis remains significant (Wang et al., 2019). Lots of complex factors cause the occurrence and the progression of CRC, such as age, family history, gender, region, and tumor stage (Center et al., 2009; Peng et al., 2018; Tong et al., 2018).
Colon and rectal cancer is a genetically heterogeneous disease in which several key biomarkers in different tumor stages could play an important function in tumor initiation, growth, and progression (Ciardiello et al., 2019). It is reported that 40% of CRC patients have disease metastases (Tsikitis et al., 2014); 80% of them metastasize to the liver, and the remaining 20% metastasize to other visceral organs (Manfredi et al., 2006), so cancers diagnosed in early stage are important for the prognosis of CRC, but patients diagnosed at later stages are the majority (Dupaul-Chicoine et al., 2015).
Immune infiltrates in cancer play the key function in different tumor stages from tumorigenesis to tumor development (Balkwill and Coussens, 2004; Castell and Larsson, 2015). With a better understanding of tumor cells and the immune system, a new tumor treatment method, immunotherapy, brings hope to tumor patients (Ciardiello et al., 2019).
Recently, high-throughput omics, molecular biology, and computer technology, have provided us a computable perspective, revealing the molecular characteristics of occurrence, development, and prognosis in CRC. Theoretical and in silico studies have been predominantly applied for providing novel insights and strategies for cancer biomarker prediction and treatment. Traditionally, molecular biomarkers were identified as tumor samples from normal samples in clinical practices. There are some limitations in molecular biomarker screening (Wen et al., 2014; Xiaoping et al., 2016). To overcome these weaknesses, a network-based algorithm for identifying biomarkers attracts much interest and shows good performance (Liu et al., 2014). However, molecular markers and network-based markers are static analysis methods that distinguish biomarkers from normal samples and tumor samples (Liu et al., 2014). These methods cannot satisfy for predicting pre-disease or the inflection point of tumor stage transition, so there is a need for the prediction method with the function of dynamic prediction characteristics in pre-disease development and critical time–life cycle evolution.
In this study, we adopt the dynamic network biomarker (DNB) algorithm to identify the DNBs. The foundation of DNB algorithm was based on nonlinear dynamical theory and complex network theory (Wong et al., 2017). Biomarkers play a critical role in living organisms, which can be used to indicate and evaluate the biological process or drug targets for diagnosis or treatment. The math model for detecting DNBs can be summarized as the following conditions (Chen et al., 2015): from the system perspective, DNB is a system that nears the tipping point, which exists as a dominant group and is called DNB. The DNB model should satisfy the following four critical conditions (Liu et al., 2014, 2018; Zhang et al., 2019):
-
1.
Deviation for each DNB members is becoming highly fluctuating (SDin: standard deviation for input).
-
2.
Correlations among all members of DNB are increasing drastically (PCCin: Pearson correlation coefficients in absolute values for input).
-
3.
Correlations between any DNB members and non-DNB members rapidly decrease (PCCout: Pearson correlation coefficient in absolute values for output).
-
4.
There are no drastic deviations or correlations among all non-DNB members in the system.
The input standard deviation (SDin) can be defined as:
where xd is the expression of gene x in the new sample d, and is the average expression of gene x in the n reference samples.
Given n reference samples, the Pearson correlation coefficient (PCC) between gene x and gene y in the reference sample data can be calculated as:
where xi and yi are the expression values for gene x and y for the ith sample in the reference samples, respectively. At the same time, and are the average gene expression values of genes x and y in the reference samples, respectively.
In order to detect a reliable and clear signal of the pre-disease state, the composite index (CI) was used to evaluate the DNB score as follows:
Materials and Methods
Samples
The GSE21510 microarray dataset including gene expression profiles was acquired from the Gene Expression Omnibus (GEO) database. The GSE21510 dataset was employed for identifying a novel biomarker or a target of treatment for CRC using laser microdissection and oligonucleotide microarray analysis (Tsukamoto et al., 2011). The validation dataset was obtained from The Cancer Genome Atlas (TCGA) database. In these datasets, the samples were divided by the TNM staging system associated with CRC (Table 1).
TABLE 1.
Samples for TNM staging system associated with colon and rectal cancer.
| Dataset type | Dataset | Stage | Patient samples |
| Training | GSE21510 | Stage I | 15 |
| Stage II | 46 | ||
| Stage III | 39 | ||
| Stage IV | 23 | ||
| Validation | The Cancer Genome Atlas colon and rectal cancer gene expression profile | Stage I | 6 |
| Stage II | 36 | ||
| Stage III | 34 | ||
| Stage IV | 59 |
Analysis for Molecular Biomarkers in CRC
In order to search the critical genes in CRC, we utilized limma R package for screening differentially expressed genes (DEGs) between tumor and normal samples divided into different tumor stages. Two of the cutoff criteria of screening were false discovery rate < 0.05 and log2-fold change (| log2FC|) > 2.0.
Furthermore, STRING online database was used for constructing a protein–protein interaction network to investigate the relationships or connections among the critical genes (Szklarczyk et al., 2019). Because the PPI network was composed of nodes, edges, and degrees, analyzing the topology of the PPI network can help us identify hub genes and key communities. In our research, the PPI networks were analyzed by Cytoscape software with cytoHubba plugin (Shannon, 2003; Chin et al., 2014).
Network-Based Analysis for Screening Biomarkers in CRC
In this article, the TCGA Colon and Rectal Cancer gene expression profiles were used for evaluating the relationship between DNBs and different tumor stages by combining clinical information in CRC (Zhang and Horvath, 2005). The weighted gene correlated network analysis (WGCNA) method can facilitate the construction of free-scale gene expression networks, and the “WCCNA” R package was used to calculate a standard scale-free network (Langfelder and Horvath, 2008).
Validate DNBs by Multi-Dataset
To validate the DNBs calculated in GSE21510 and TCGA Colon and Rectal Cancer gene expression profiles, public databases were used as cross-validation. All the databases in this article are listed in Table 2.
TABLE 2.
Validation databases for colon and rectal cancer.
| Dataset name | Data link | Reference/s |
| GEPIA | http://gepia.cancer-pku.cn/ | (Tang et al., 2017) |
| Human protein Atlas | https://www.proteinatlas.org/ | (Uhlén et al., 2015; Thul et al., 2017; Uhlen et al., 2017) |
| cBioProtal | https://www.cbioportal.org/ | (Cerami et al., 2012; Gao et al., 2013) |
| TIMER | http://cistrome.dfci.harvard.edu/TIMER/ | (Li, 2016, 2017) |
| Ualcan | http://ualcan.path.uab.edu/ | (Chandrashekar et al., 2017) |
| Oncomine | https://www.oncomine.org/ | (Selamat et al., 2012) |
Results
The Identified DNBs for Critical Stage in CRC
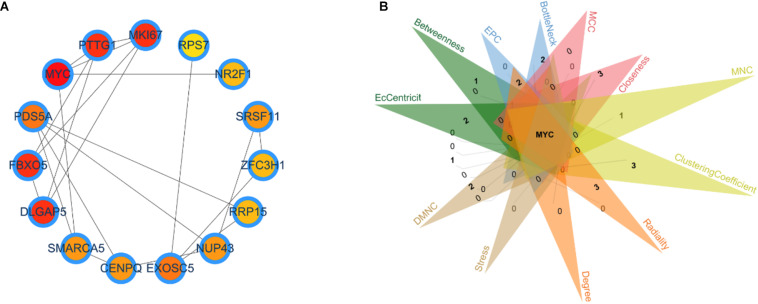
Identifying the different critical transition state in CRC from stage I to IV is crucial for clarifying the molecular mechanism that regulates tumor development. In this article, the DNB algorithm was implemented to search the DNBs. The DNB scores at four stage time points are shown in Table 3. The DNB score increased sharply from stage III and reached the peak at stage IV, so stage III is designated as a critical transition state, which corresponds to the development of a specific stage in CRC. Ultimately, the 98 DNBs from stage I to stage IV were divided into four sampling time points in Figure 1. Then, PPI analysis was constructed by Cytoscape with cytoHubba plug-in. CytoHubba can provide 12 topological analysis methods including betweenness, bottleneck, closeness, clustering coefficient, degree, DMNC, EcCentricity, EPC, MCC, MNC, radiality, and stress based on shortest paths (Chin et al., 2014). Lastly, the Venn diagram analysis showed that the DNB was MYC.
TABLE 3.
Dynamic network biomarker scores in different tumor stages in colon and rectal cancer.
| TNM stage | Score | Genes |
| Stage I | 0.42378583 | 98 |
| Stage II | 0.14771798 | 98 |
| Stage III | 0.09225563 | 98 |
| Stage IV | 0.647891324 | 98 |
FIGURE 1.
Identification of the critical transition state in colon and rectal cancer. (A) Key dynamic network biomarkers (DNBs) screened by DNB algorithms and visualized by protein–protein interaction analysis. (B) Venn diagram for 12 types of topological algorithms.
Molecular Biomarker Analysis for Different Tumor Stages in CRC
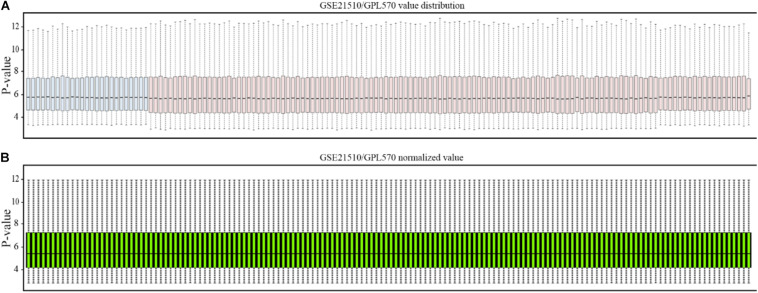
The affymetrix gene expression profiles (Figure 2A) were analyzed by online GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) tool, and the normalized results are shown in Figure 2B.
FIGURE 2.
Normalization of gene profiling data. (A) Distribution of GSE21510 gene profiling data. (B) Value-normalized distribution of GSE21510 gene profiling data.
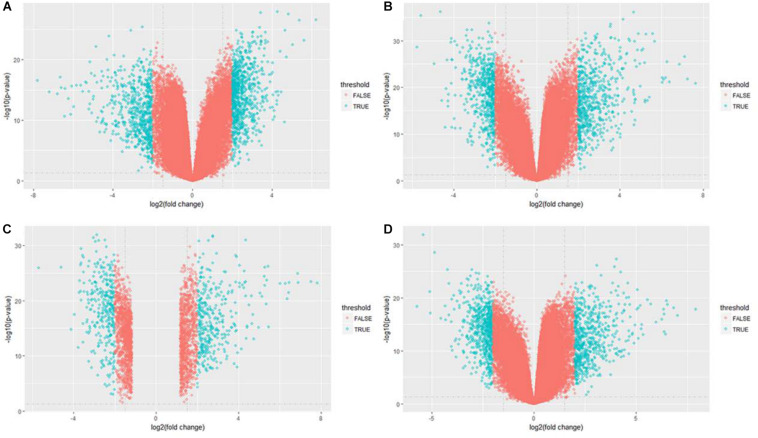
After data normalization and removal of batch effects, a total of 5,343 DEGs were identified, of which 2,738 were up-regulated genes and 2,605 were down-regulated genes (Figure 3). The detailed results of the differential expression are shown in Table 4.
FIGURE 3.
Volcano plot of differentially expressed genes in GSE21510 gene profiling data. (A) Stage I. (B) Stage II. (C) Stage III. (D) Stage IV.
TABLE 4.
Up-regulated and down-regulated genes in stages I, II, III, and IV associated with colon and rectal cancer in GSE21510 dataset.
| Stage | Up-regulated | Down-regulated |
| Stage I | 807 | 640 |
| Stage II | 634 | 607 |
| Stage III | 674 | 715 |
| Stage IV | 623 | 643 |
Weighted Gene Correlated Network Analysis for Different Tumor Stages in CRC
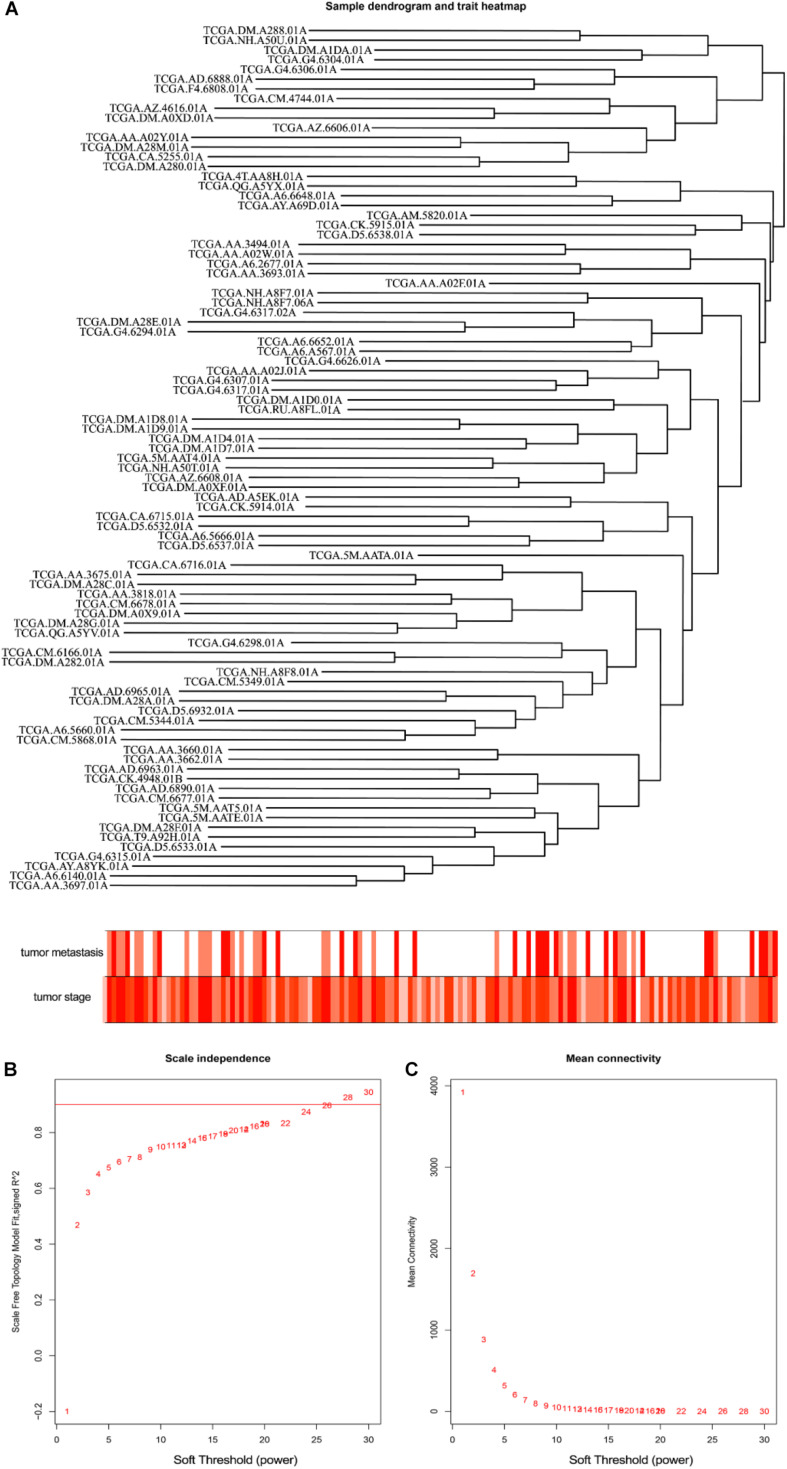
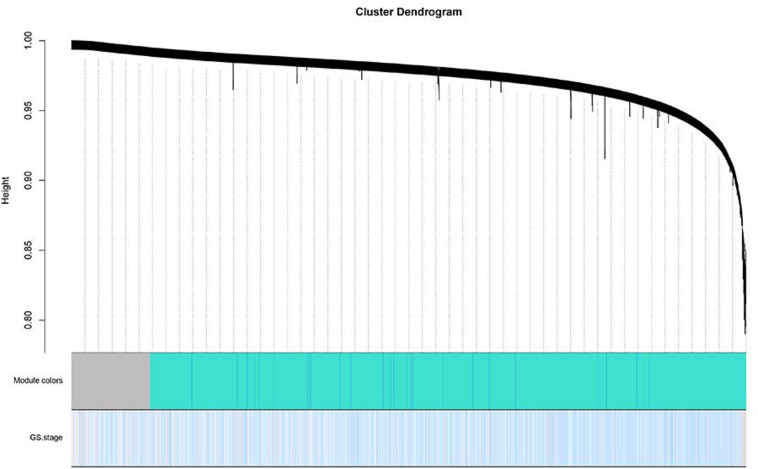
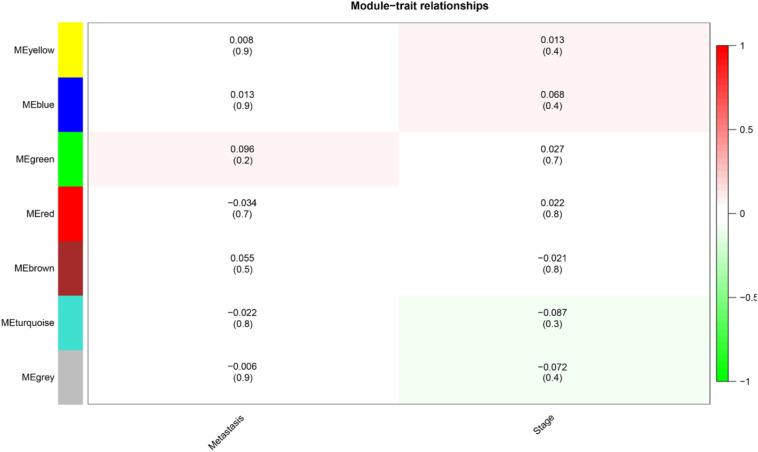
The level 3 RNAseqV2 data of colorectal adenocarcinoma (COAD) were downloaded from the TCGA database. The gene expression profiles were generated using the Illumina HiSeq 2000 sequencing platforms. There were 475 samples in total, including 340 tumor and 135 normal samples; these samples were classified into four different stages (Table 5). In order to explore the association between gene sets and clinical features, WGCNA was used to construct free-scale gene co-expression networks (Tang et al., 2018). The samples of COAD were clustered using average linkage method and Pearson’s correlation method (Figure 4A). In this study, the power of β = 6 (scale free R2 = 0.95) was selected as the soft-thresholding criteria to construct a free-scale network (Figure 4B). A total of seven module eigengenes were identified based on the average linkage hierarchical clustering (Figure 5). The yellow module was found to have the highest correlation with tumor stage (Figure 6), so the yellow module was selected as a clinically significant module for identifying hub genes.
TABLE 5.
Gene expression profiles in different stages for The Cancer Genome Atlas colon and rectal cancer gene expression profile (the detailed clinical information of colon adenocarcinoma is in Supplementary Table S1).
| Stage | TCGA samples | Tumor/normal |
| Stage I | 78 | 72/6 |
| Stage II | 189 | 153/36 |
| Stage III | 135 | 100/35 |
| Stage IV | 73 | 15/58 |
FIGURE 4.
Clustering of colorectal adenocarcinoma (COAD) for soft-thresholding power. (A) Clustering of COAD samples. (B) Calculating the scale-free fit index of soft-thresholding power. (C) Calculating the mean connectivity of soft-thresholding power.
FIGURE 5.
Dendrogram of all differentially expressed genes clustered based on a dissimilarity measure. Dynamic tree cutting was applied to identify modules by dividing the dendrogram at significant branch points. Modules associated with tumor stage are displayed with different colors in the horizontal bar.
FIGURE 6.
Heat map of the correlation between module eigengenes and clinical subtypes (metastasis and stage) in colorectal adenocarcinoma. Each column showed the corresponding correlation and P-value.
PPI Network and Module Analysis for MYC
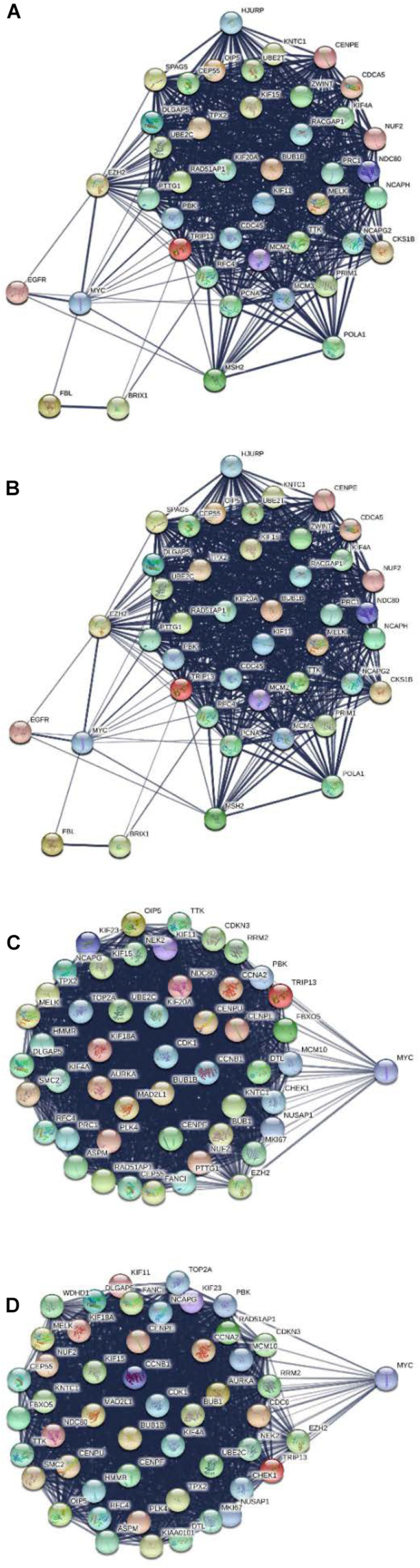
All DGEs, yellow module, and DNBs were uploaded to the STRING database for constructing the PPI network. Then, all the nodes and edges were divided by ClusterONE and analyzed by Cytoscape with cytoHubba. The results show that MYC can serve as a dynamic biomarker for CRC (Figure 7).
FIGURE 7.
Protein–protein interaction network for MYC in different stages. (A) MYC showed significance in stage I. (B) MYC showed significance in stage II. (C) MYC showed significance in stage III. (D) MYC showed significance in stage IV.
Validation of the Dynamic Network Biomarker Gene in TCGA Database Pan-Cancer Analysis for MYC
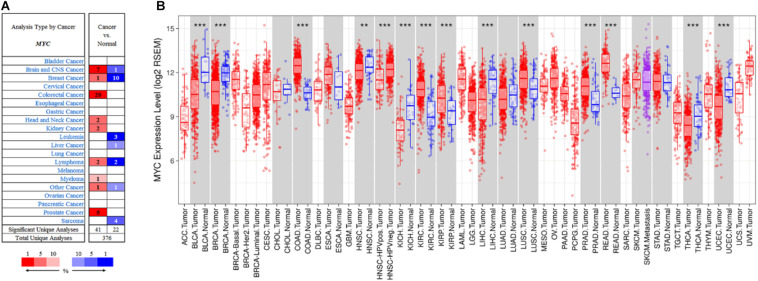
The expression of MYC level in different types of cancers was conducted by the Oncomine database (Rhodes et al., 2007). The Oncomine database analysis revealed that MYC mRNA expression of CRC had increased in 20 datasets compared to the normal tissues (Figure 8A). In addition, MYC mRNA expression was lower in breast cancer, head and neck cancer, kidney cancer, lymphoma, and myeloma cancer. Higher expression was observed in brain and CNS cancer, colorectal cancer, and prostate cancer. Pan-cancer analysis showed that MYC expression was significantly higher in colon adenocarcinoma and rectum adenocarcinoma compared with adjacent normal tissues (Figure 8B).
FIGURE 8.
Pan-cancer analysis for MYC. (A) MYC expression in Oncomine database. (B) MYC expression in TIMER database. The cell color in (A) is determined by the best gene rank percentile for the analyses within the cell. Red: tumor. Blue: normal. The box plot colors in (B) indicate tumor and normal expression. Red: tumor. Blue: normal. P-value significance codes: 0 ≤ *** < 0.001 ≤ ** < 0.01 ≤ * < 0.05 ≤. < 0.1).
MYC Expression and Prognosis in CRC
MYC expression was evaluated by the PrognoScan and was notably found to significantly impact prognosis in CRC. Four cohorts (GSE12945, GSE17536, GSE14333, and GSE17537), including different tumor stages of CRC, showed that high MYC expression was associated with poor prognosis (Table 6).
TABLE 6.
Survival analysis of MYC mRNA in colon and rectal cancer patients (the PrognoScan).
| Dataset | Endpoint | Number | ln(HRhigh/HRlow) | Cox P-value | ln(HR) | HR (95% CIlow–CIupp) |
| GSE12945-HG-U133_Plus_2 | Disease-free survival | 51 | 15.43 | 0.875782 | 0.09 | 1.09 (0.36–3.35) |
| Overall survival | 62 | −15.38 | 0.647096 | −0.16 | 0.86 (0.44–1.67) | |
| GSE17536-HG-U133_Plus_2 | Disease-free survival | 145 | −0.65 | 0.601886 | −0.15 | 0.86 (0.50–1.50) |
| Disease-free survival | 145 | −1.11 | 0.01759 | −3.58 | 0.03 (0.00–0.54) | |
| Disease-specific survival | 177 | −0.97 | 0.115903 | −0.36 | 0.70 (0.45–1.09) | |
| Overall survival | 177 | −0.73 | 0.216913 | −0.24 | 0.78 (0.53–1.15) | |
| Overall survival | 177 | −0.64 | 0.021105 | −2.34 | 0.10 (0.01–0.70) | |
| Disease-specific survival | 177 | −0.69 | 0.020065 | −2.8 | 0.06 (0.01–0.64) | |
| GSE14333-HG-U133_Plus_2 | Disease-free survival | 226 | −0.44 | 0.272903 | −0.23 | 0.79 (0.52–1.20) |
| Disease-free survival | 226 | −0.67 | 0.52951 | −0.1 | 0.90 (0.66–1.24) | |
| GSE17537-HG-U133_Plus_2 | Disease-free survival | 55 | −1.23 | 0.010481 | −5.84 | 0.00 (0.00–0.25) |
| Disease-specific survival | 49 | −1.81 | 0.043557 | −5.94 | 0.00 (0.00–0.84) | |
| Overall survival | 55 | −0.65 | 0.823679 | −0.06 | 0.94 (0.56–1.59) | |
| Overall survival | 55 | −2.11 | 0.001411 | −7.34 | 0.00 (0.00–0.06) | |
| Disease-free survival | 55 | 0.88 | 0.681468 | 0.13 | 1.14 (0.62–2.09) | |
| Disease-specific survival | 49 | −0.68 | 0.96835 | −0.02 | 0.99 (0.47–2.07) |
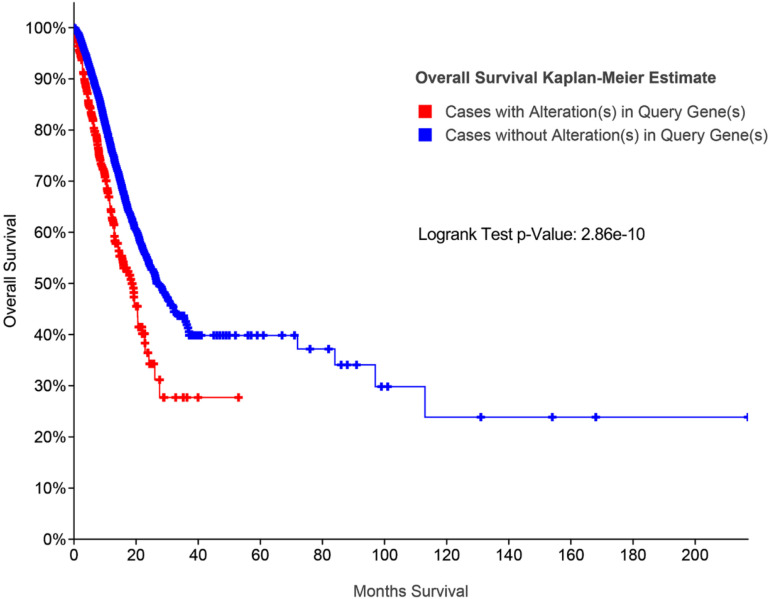
Additionally, survival analysis shows that high MYC alteration was associated with poor prognosis in CRC (Figure 9).
FIGURE 9.
Survival analysis for MYC by cBioPortal database.
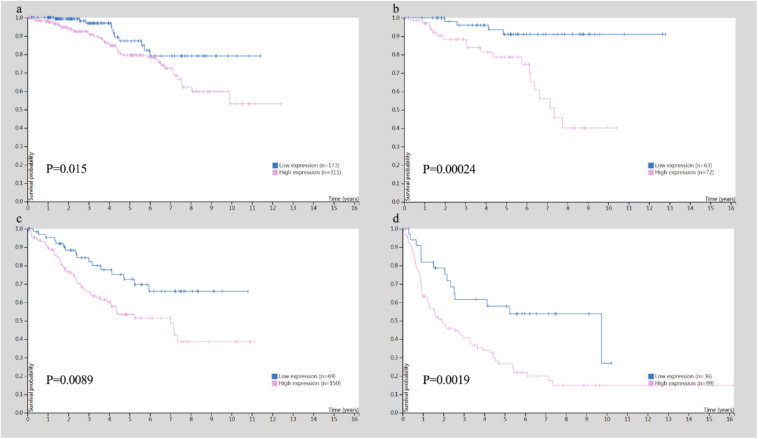
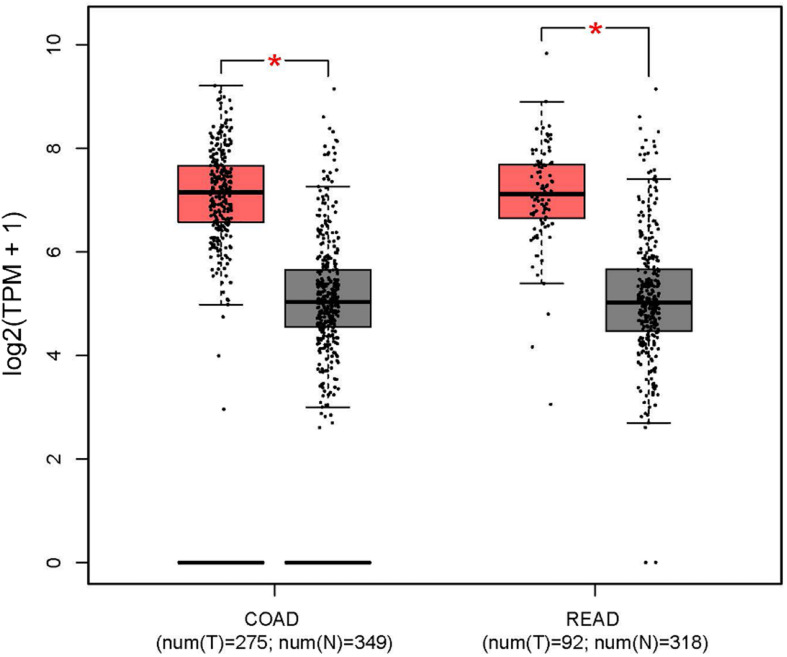
In addition, the Human Protein Atlas database was utilized to confirm the MYC prognostic value according to TNM stage (Figure 10). The data in the Human Protein Atlas database are mainly extracted from TCGA. The MYC expression was associated with a gradually decreased survival from stage I (5-year survival high 80%, 5-year survival low 87%), stage II (5-year survival high 79%, 5-year survival low 92%), stage III (5-year survival high 51%, 5-year survival low 69%) to stage IV (5-year survival high 19%, 5-year survival low 41%). The results showed that the MYC mRNA expression level was remarkably higher in CRC (Figure 11), so it is conceivable that a high MYC expression could serve as a risk factor for poor prognosis in CRC.
FIGURE 10.
Survival analysis for TNM stage in colon and rectal cancer. (A) Survival analysis for MYC in stage I. (B) Survival analysis for MYC in stage II. (C) Survival analysis for MYC in stage III. (D) Survival analysis for MYC in stage IV.
FIGURE 11.
MYC expression level in colon and rectal cancer from the GEPIA database (red box: tumor; gray box: normal). COAD, colon adenocarcinoma; READ, rectum adenocarcinoma.
Correlation Analysis Between MYC and Infiltrating Immune Cells
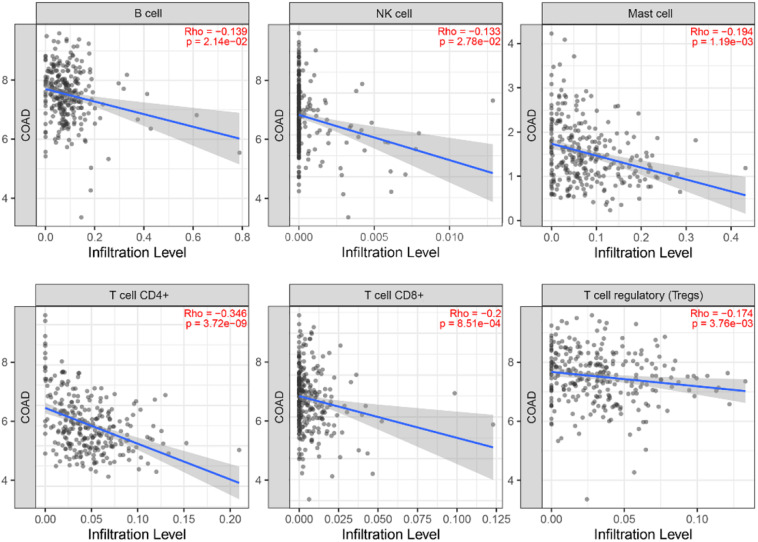
In order to investigate the status of tumor-infiltrating lymphocytes, TIMER database was employed to explore whether the MYC expression was correlated with immune infiltration levels in CRC. The relationship between MYC expression and infiltrating immune cells is shown in Figure 12. The MYC expression levels showed a correlation with B, NK, Master, CD4+ T, CD8+ T, and regulatory T cell, and a high expression level of MYC was negatively correlated with CD8+ T cells.
FIGURE 12.
Correlation of MYC expression with immune infiltration level in the TIMER database.
Screening of Tumor Suppressor Genes
Then, four candidate target genes in stage I, stage II, stage III, and stage IV were obtained by overlapping DNBs, down-regulated genes from stage I to IV, and human tumor suppressor genes (Table 7).
TABLE 7.
Tumor suppressor genes in stage I to stage IV associated with colon and rectal cancer.
| Stage | Tumor suppressor genes |
| Stage I | ZBTB16, MAL, LIFR, SLIT2 |
| Stage II | ZBTB16, MAL, LIFR, SLIT2 |
| Stage III | ZBTB16, MAL, LIFR, SLIT2 |
| Stage IV | ZBTB16, MAL, LIFR, SLIT2 |
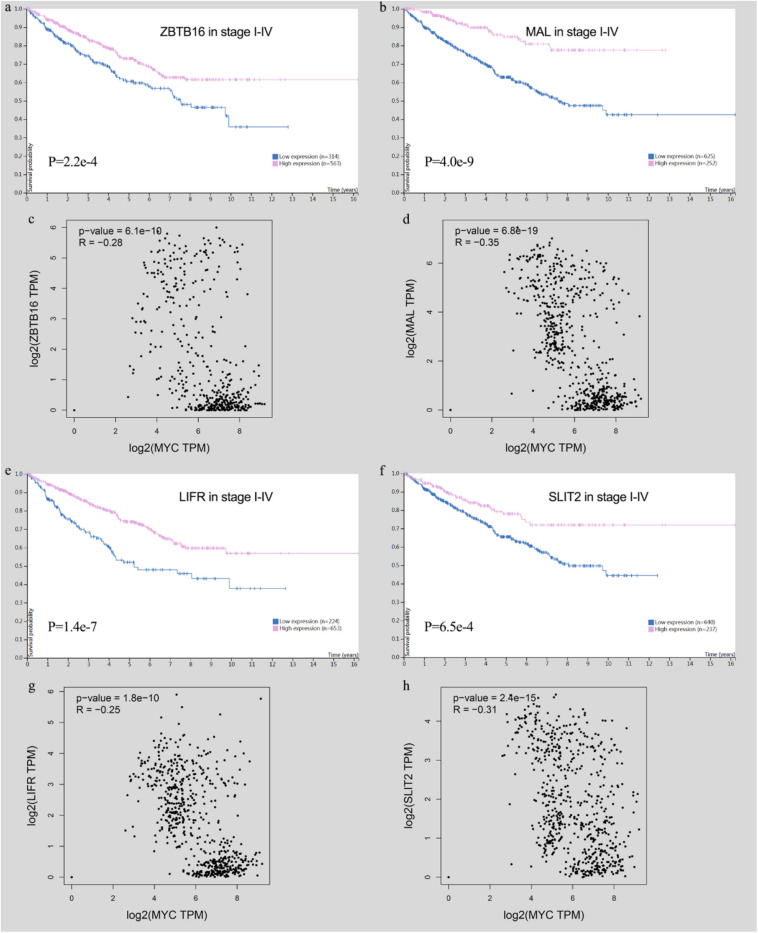
High-expression tumor suppressor genes show a good prognosis in CRC, and they have a significant negative correlation with MYC in immune cells (Figure 13).
FIGURE 13.
Survival analysis of tumor suppressor genes and the relationship with MYC). (A) ZBTB16 expression was correlated with high survival probability. (B) MAL expression was correlated with high survival probability. (C) ZBTB16 was negatively correlated with MYC. (D) MAL was negatively correlated with MYC. (E) LIFR expression was correlated with high survival probability. (F) SLIT2 expression was correlated with high survival probability. (G) LIFR was negatively correlated with MYC. (H) SLIT2 was negatively correlated with MYC.
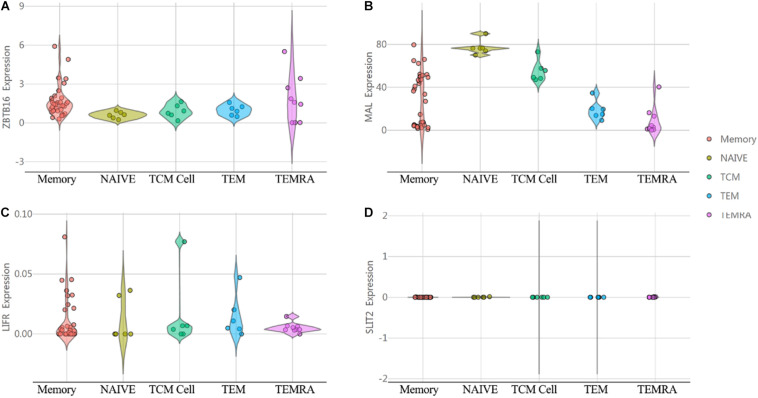
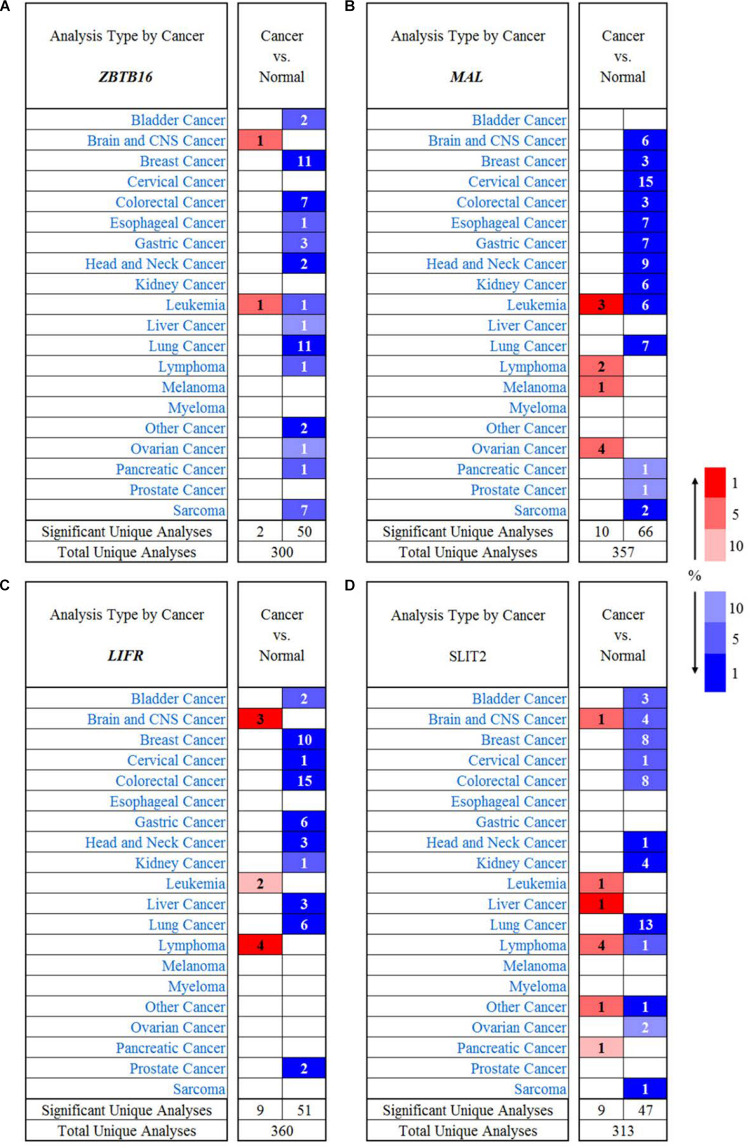
In order to investigate the immunological mechanisms of tumor suppressor genes, the 10KIP (10,000 Immunomes Project) database (Zalocusky et al., 2018) was used to validate these tumor suppressor genes (Figure 14). Other than SLIT2 expression, ZBTB16, MAL, and LIFR expressions have a significant expression level with memory, naive, tcm, tem, and temra cell. Furthermore, the Oncomine database shows that the expression of tumor suppressor genes was significantly high in CRC (Figure 15).
FIGURE 14.
The expression of tumor suppressor genes in the human immune system. (A) ZBTB16. (B) MAL. (C) LIFR. (D) SLIT2.
FIGURE 15.
The expression of tumor suppressor genes in different types of human cancers. (A) ZBTB16 in different cancer data sets compared with normal tissues in the Oncomine database. (B) MAL in different cancer data sets compared with normal tissues in the Oncomine database. (C) LIFR in different cancer data sets compared with normal tissues in the Oncomine database. (D) SLIT2 in different cancer data sets compared with normal tissues in the Oncomine database.
Discussion
Cancers are often initiated by some key genetic events, such as activated proto-oncogenes or inactive tumor suppressor genes (Casey et al., 2017), so elucidating the underlying molecular mechanism for these events is critically important for diagnosis and treatment in CRC. Although microarray and high-throughput sequencing has been widely used to predict the potential therapeutic targets for CRC, there is little research on identifying the biomarkers for the critical transition stage. In this study, we conducted a theoretical and in silico analysis of CRC development using the DNB algorithm. By applying the DNB algorithm in RStudio 1.1.453, we identified 98 DNBs that may herald the imminent critical transition in different tumor stages and provide new insights for the theory and practice studies in CRC. Moreover, the key member of the DNBs was calculated by PPI analysis and validated by public bioinformatics databases.
This study attempts to identify the DNBs for CRC. Dynamic network biomarker theory was performed in this study to recognize the key state of CRC from the time of stage I to stage IV. A joint bioinformatics analysis method was used for indicating tumor suppressor genes associated with DNBs in CRC; this may provide a set of useful targets for future investigation into the molecular mechanisms and biomarkers. Lastly, the critical DNBs and tumor suppressor genes in different tumor stages were detected and validated. The DNBs show that MYC might serve as a biomarker for the precise diagnosis and treatment of CRC in the future. The tumor suppressor genes were ZBTB16, MAL, LIFR, and SLIT2. These may contribute to the finding of molecular mechanisms underlying the initiation and development of CRC.
MYC proto-oncoproteins, including c-MYC, MYCN, and MYCL, perform their functions in cell proliferation, growth, death, angiogenesis, invasion, transformation, and metastasis (Wahlström and Arsenian Henriksson, 2015). MYC is a transcription factor that controls the expression of a large number of genes involved in cell proliferation, growth, angiogenesis, invasion, and metastasis (Walz et al., 2014). There is controversy about whether c-MYC can predict the prognosis of CRC (He et al., 2018). In all cancers, CRC seems to be particularly dependent on c-Myc activity. The transcription target c-Myc of the Wnt signaling pathway is up-regulated in up to 80% of CRC, which is mainly due to the loss of the Apc tumor suppressor (Myant and Sansom, 2011). Most coding mutations affect the stability of MYC proteins, thereby increasing their levels rather than altering the basic role of MYC (Elisha-Feil et al., 2006; Wierstra and Alves, 2008). It is reported that spermine synthase (SMS), in cooperation with MYC, can maintain CRC cell survival, so the combined inhibition of SMS and MYC signaling may be a promising strategy for inducing synergistic apoptosis and tumor regression (Guo et al., 2020).
MYC does not require changes to the protein sequence to cause cancer; it instead becomes an oncogene through abnormal expression (Love et al., 2012). MYC is a positive effector of tissue inflammation and can cause immune diseases (Williamson et al., 2006; Wang et al., 2011; Mckeown and Bradner, 2014; Gilhus et al., 2016). In T cells, it is involved in the proliferation, function, and lytic capacity of CD8 T lymphocytes (Ferrari et al., 1985; Grausz et al., 1986; Gamble et al., 1990; Selvatici et al., 1990; Nie et al., 2012). In activated primary T lymphocytes, MYC drives metabolic reprogramming through a MYC-dependent metabolic pathway that links glutamine breakdown to the biosynthesis of polyamines. However, MYC can also induce anti-inflammatory responses through macrophage function (Wang et al., 2011; Mckeown and Bradner, 2014). c-MYC has been shown to be expressed in humans as a macrophage classified as M2 (Pello et al., 2012) and plays a key role in macrophage polarization and tumor-associated macrophage function (Kim et al., 2015). Exposure to an anti-inflammatory response results in “alternatively activated” macrophage-M2 cells (Mosser and Edwards, 2008; Martinez et al., 2009), which is associated with anti-inflammatory properties, angiogenesis, and tissue remodeling (Kelly-Spratt et al., 2011). The c-MYC protein represents an early molecular marker of cell cycle activity, which may be important in the development of immune diseases (Trop-Steinberg and Azar, 2018). A study on immunohistochemical staining for metastatic colorectal cancer found that patients with a high expression level of c-MYC had a significantly lower progression-free survival time and overall survival than those with reduced c-MYC expression (Strippoli et al., 2020). In summary, these findings indicate that the uncertain role of MYC function warrants further investigation (Yunos et al., 2020).
ZBTB16 (zinc finger and BTB domain containing 16) is a member of the Krueppel C2H2 zinc finger protein family and encodes a zinc finger transcription factor that contains nine Kruppel zinc finger domains at the carboxy terminus. The protein is located in the nucleus, participates in the cell cycle process, and interacts with histone deacetylase. The specific situation of abnormal gene rearrangement at this locus is related to acute promyelocytic leukemia (The National Center for Biotechnology Information, 2020b). ZBTB16 acts as a transcriptional repressor (Lin et al., 2013). It plays a key role in the development and/or maintenance of myeloid maturity and other differentiated tissues. A potential substrate recognition component of the E3 ubiquitin protein ligase complex can mediate the ubiquitination of target proteins and subsequent proteasome degradation (Furukawa et al., 2003).
MAL (Mal, T cell differentiation protein) is a highly hydrophobic integral membrane protein that belongs to the MAL family of protein lipids. This protein is a candidate connexin in T cell signal transduction and has been located in the endoplasmic reticulum of T cells. In addition, the protein lipid is located in the tight myelin sheath of cells in the nervous system and is related to the biogenesis or function of the myelin sheath. The protein plays a role in the formation, stabilization, and maintenance of membrane microdomains rich in glycosphingolipids. The down-regulation of MAL is associated with a variety of human epithelial malignancies. Alternative splicing produces four transcript variants that differ from each other by the presence or the absence of alternately spliced exons 2 and 3 (The National Center for Biotechnology Information, 2020a).
LIFR (LIF receptor alpha) encodes proteins belonging to the type 1 cytokine receptor family. The protein combines with the high-affinity transformant subunit gp130 to form a receptor complex that mediates the action of a leukemia inhibitory factor, which is a multifunctional cytokine that participates in cell differentiation, proliferation, and, in adults and embryos, survival. Mutations in this gene cause Schwartz-Jampel syndrome type 2, which is a disease of curved bone dysplasia. Translocation involves the promoter of this gene. LIFR is a signaling molecule. The soluble form inhibits the biological activity of LIF by blocking the binding of LIF to the target cell receptor. Multiple splice variants encoding the same protein have been found for this gene (The National Center for Biotechnology Information, 2020a).
SLIT2 (slit-directed ligand 2) encodes a member of the slit family of secreted glycoproteins, which is a ligand of the Robo family of immunoglobulin receptors. Slot proteins play a highly conserved role in axon guidance and neuron migration and may also be functional in other cell migration processes, including leukocyte migration. Members of the SLIT family are characterized by an N-terminal signal peptide, four leucine-rich repeats, nine epidermal growth factor repeats, and a C-terminal cysteine junction. The proteolytic processing of this protein produces an N-terminal fragment containing four leucine-rich repeats, five epidermal growth factor repeats, and a C-terminal fragment containing four epidermal growth factor repeats and a cysteine junction. Both full-length and cleaved proteins are secreted extracellularly and can play a role in axonal rejection and other specific processes. Alternative splicing results in multiple transcript variants (The National Center for Biotechnology Information, 2020a). SLIT2 acts as a molecular guideline for cell migration during axonal navigation involving the ventral midline of the neural tube and axonal projection into different regions. SLIT2 appears to be necessary for midline guidance of the forebrain, and it acts as a repulsive signal to prevent the protruding axons of the olfactory bulb from improperly crossing the midline. In the development of the spinal cords, SLIT2 may play a key role in regulating the response to netrin as it guides the commissural axons to the floor. Only the commissural axons that cross the midline respond to SLIT2 in vitro.
However, there are several limitations that should be acknowledged in this study. The DNBs and the tumor suppressor genes were only validated in the TCGA database, so further molecular experiments are needed to better confirm the theoretical and in silico prediction. In conclusion, we hope that this study may provide some evidence for the future genomic treatment of CRC from new insights.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
YT and SD conceived the study. YS collected the data. CX reviewed the algorithm. YT wrote the R code and the manuscript. All the authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- CRC
colon and rectal cancer
- GEO
Gene Expression Omnibus
- DEG
differentially expressed genes
- PPI
protein–protein interaction
- COADREAD
TCGA colon and rectal cancer gene expression profile
- COAD
colon adenocarcinoma
- READ
rectum adenocarcinoma
- DNB
dynamic network biomarker
- WGCNA
weighted gene co-expression network analysis
- DAVID
Database for Annotation Visualization and Integrated Discovery
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- STRING
Search Tool for the Retrieval of Interacting Genes
- TCGA
The Cancer Genome Atlas
- BP
biological process
- BH
Benjamini and Hochberg
- log2FC
log2-fold change
- SD
standard deviation
- PCC
Pearson coefficient
- CI
composite index.
Footnotes
Funding. This work was supported by the Key Projects of 13th Five Year Plan of Chongqing Municipal Education Commission (2017-GX-028), the Basic Science and Frontier Project of Chongqing Municipal Science and Technology Commission (cstc2016jcyjA0582), and the Major Decision-Making Consulting Project of Chongqing (20182B-03).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.555540/full#supplementary-material
References
- Balkwill F., Coussens L. M. (2004). Cancer: an inflammatory link. Nature 431 405–406. 10.1038/431405a [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Brenner D. R., Heer E., Sutherland R. L. (2019). National Trends in Colorectal Cancer Incidence Among Older and Younger Adults in Canada. JAMA Network Open. 2 E22–E349. 10.1001/jamanetworkopen.2019.8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey S. C., Baylot V., Felsher D. W. (2017). MYC: Master Regulator of Immune Privilege. Trends Immunol. 38 298–305. 10.1016/j.it.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell A., Larsson L. G. (2015). Targeting MYC Translation in Colorectal Cancer. Cancer Discov. 5 701–703. 10.1158/2159-8290.cd-15-0660 [DOI] [PubMed] [Google Scholar]
- Center M. M., Jemal A., Smith R. A. (2009). Worldwide Variations in Colorectal Cancer. CA A Cancer J. Clin. 59 366–378. 10.3322/caac.20038 [DOI] [PubMed] [Google Scholar]
- Cerami E., Gao J., Dogrusoz U. (2012). The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2 401–404. 10.1158/2159-8290.cd-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar D. S., Bashel B., Balasubramanya S. A. H. (2017). UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 19 649–658. 10.1016/j.neo.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Liu R., Chen L., Aihara K. (2015). Identifying critical differentiation state of MCF-7 cells for breast cancer by dynamical network biomarkers. Front. Gen. 6:252. 10.3389/fgene.2015.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. H., Chen S. H., Wu H. H., Ho C. W., Ko M. T., Lin C. Y. (2014). cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8(Suppl. 4):S11. 10.1186/1752-0509-8-S4-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello D., Vitiello P. P., Cardone C. (2019). Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 76 22–32. 10.1016/j.ctrv.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Dang H. X., Krasnick B. A., White B. S. (2020). The clonal evolution of metastatic colorectal cancer. Sci. Adv. 6:eaay9691. 10.1126/sciadv.aay9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J., Arabzadeh A., Dagenais M. (2015). The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity 43 751–763. 10.1016/j.immuni.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Elisha-Feil Z., Weber A., Zhao K. (2006). The FUSE/FBP/FIR/TFIIH system is a molecular machine programming a pulse of c-myc expression. Eur. Mole. Biol. Organizat. J. 25 2119–2130. 10.1038/sj.emboj.7601101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Torelli U., Selleri L. (1985). Study of the levels of expression of two oncogenes, c-myc and c-myb, in acute and chronic leukemias of both lymphoid and myeloid lineage. Leuk. Res. 9 833–842. 10.1016/0145-2126(85)90304-2 [DOI] [PubMed] [Google Scholar]
- Furukawa M., He Y. J., Borchers C. (2003). Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat. Cell Biol. 5 1001–1007. 10.1038/ncb1056 [DOI] [PubMed] [Google Scholar]
- Gamble D. A., Schwab R., Weksler M. E. (1990). Decreased steady state c-myc mRNA in activated T cell cultures from old humans is caused by a smaller proportion of T cells that transcribe the c-myc gene. J. Immunol. 144 3569–3573. [PubMed] [Google Scholar]
- Gao J., Aksoy B. A., Dogrusoz U. (2013). Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhus N. E., Skeie G. O., Romi F. (2016). Myasthenia gravis– autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 12 259–268. 10.1038/nrneurol.2016.44 [DOI] [PubMed] [Google Scholar]
- Grausz J. D., Fradelizi D., Dautry F. (1986). Modulation of c-fos and c-Myc mRNA levels in normal human lymphocytes by calcium ionophore A23187 and phorbol ester. Eur. J. Immunol. 16 1217–1221. 10.1002/eji.1830161006 [DOI] [PubMed] [Google Scholar]
- Guo Y., Ye Q., Deng P. (2020). Spermine synthase and MYC cooperate to maintain colorectal cancer cell survival by repressing Bim expression. Nat. Commun. 3243:3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W. L., Weng X. T., Wang J. L. (2018). Association Between c-Myc and Colorectal Cancer Prognosis: A Meta-Analysis. Front. Physiol. 9:1549. 10.3389/fphys.2018.01549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Spratt K. S., Pitteri S. J., Gurley K. E. (2011). Plasma Proteome Profiles Associated with Inflammation, Angiogenesis, and Cancer. PLoS One 6:e19721. 10.1371/journal.pone.0019721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Jeon Y. J., Cui R. (2015). Role of MYC-Regulated Long Noncoding RNAs in Cell Cycle Regulation and Tumorigenesis. JNCI J. Natl. Cancer Inst. 107 dju505–dju505. 10.1093/jnci/dju505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9:559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. (2016). Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 17:174. 10.1186/s13059-016-1028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. (2017). TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77 e108–e110. 10.1158/0008-5472.CAN-17-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. Y., Huang C. C., Hsieh Y. T. (2013). Analysis of the interaction between Zinc finger protein 179 (Znf179) and promyelocytic leukemia zinc finger (Plzf). J. Biomed. Sci. 20:98. 10.1186/1423-0127-20-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wang X., Aihara K. (2014). Early Diagnosis of Complex Diseases by Molecular Biomarkers, Network Biomarkers, and Dynamical Network Biomarkers. Med. Res. Rev. 34 455–478. 10.1002/med.21293 [DOI] [PubMed] [Google Scholar]
- Liu X., Chang X., Leng S. (2018). Detection for disease tipping points by landscape dynamic network biomarkers. Natl. Sci. Rev. 6 775–785. 10.1093/nsr/nwy162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love C., Sun Z., Jima D. (2012). The genetic landscape of mutations in Burkitt lymphoma. Nat. Genet. 44 1321–1325. 10.1038/ng.2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi S., Lepage C., Hatem C. (2006). Epidemiology and Management of Liver Metastases From Colorectal Cancer. Anna. Surg. 244 254–259. 10.1097/01.sla.0000217629.94941.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F. O., Helming L., Gordon S. (2009). Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 27 451–483. 10.1146/annurev.immunol.021908.132532 [DOI] [PubMed] [Google Scholar]
- Mckeown M. R., Bradner J. E. (2014). Therapeutic Strategies to Inhibit MYC. Cold Spring Harbor Perspect. Med. 4:a014266. 10.1101/cshperspect.a014266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M., Edwards J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8 958–969. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myant K., Sansom O. J. (2011). More, More, More: Downregulation of a MK5-FoxO3a-mir34b/c Pathway Further Increases c-Myc Levels in Colorectal Cancer. Mole. Cell 41 369–370. 10.1016/j.molcel.2011.01.028 [DOI] [PubMed] [Google Scholar]
- Nie Z., Hu G., Wei G. (2012). c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151 68–79. 10.1016/j.cell.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pello O. M., De Pizzol M., Mirolo M. (2012). Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 119 411–421. 10.1182/blood-2011-02-339911 [DOI] [PubMed] [Google Scholar]
- Peng L., Weigl K., Boakye D. H. (2018). Brenner, Risk scores for predicting advanced colorectal neoplasia in the average-risk population: a systematic review and metaanalysis. Am. J. Gastroenterol. 113 1788–1800. 10.1038/s41395-018-0209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. R., Kalyana-Sundaram S., Mahavisno V. (2007). Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9 166–180. 10.1593/neo.07112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selamat S. A., Chung B. S., Girard L., Zhang W. (2012). Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genom. Res. 22 1197–1211. 10.1101/gr.132662.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvatici R., Rubini M., Orlando P. (1990). c-fos, c-myc and IL-2R mRNA expression in PHA activated T lymphocytes treated with a monoclonal anti-HLA class I antibody (MAb 01.65). Biochem. Int. 22 397–403. [PubMed] [Google Scholar]
- Shannon P. (2003). Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Gen. Res. 13 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strippoli A., Cocomazzi A., Basso M. (2020). c-MYC Expression Is a Possible Keystone in the Colorectal Cancer Resistance to EGFR Inhibitors. Cancers 12:638. 10.3390/cancers12030638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A. L., Lyon D., Junge A. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucl. Acids Res. 47 D607–D613. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Kong D., Cui Q. (2018). Prognostic Genes of Breast Cancer Identified by Gene Co-expression Network Analysis. Front. Oncol. 8:374. 10.3389/fonc.2018.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Li C., Kang B. (2017). GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucl. Acids Res. 45 W98–W102. 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Center for Biotechnology Information (2020a). CERT1 ceramide transporter 1 [Homo sapiens (human)]. Maryland: The National Center for Biotechnology Information. [Google Scholar]
- The National Center for Biotechnology Information (2020b). CERT1 ceramide transporter 1 [Homo sapiens (human)]. Maryland: The National Center for Biotechnology Information. [Google Scholar]
- Thul P. J., Åkesson L., Wiking M. (2017). A subcellular map of the human proteome. Science 356:eaal3321. 10.1126/science.aal3321 [DOI] [PubMed] [Google Scholar]
- Tong S., Ling-Dong C., Yu-Hong L. (2018). Ube2v1-mediated ubiquitination and degradation of Sirt1 promotes metastasis of colorectal cancer by epigenetically suppressing autophagy. J. Hematol. Oncol. 11 1–16. 10.1186/s13045-018-0638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trop-Steinberg S., Azar Y. (2018). Is Myc an Important Biomarker? Myc Expression in Immune Disorders and Cancer. Am. J. Med. Sci. 355 67–75. 10.1016/j.amjms.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Tsikitis V. L., Larson D. W., Huebner M. (2014). Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer 14:336. 10.1186/1471-2407-14-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S., Ishikawa T., Iida S. (2011). Clinical significance of osteoprotegerin expression in human colorectal cancer. Clin. Cancer Res. 17 2444–2450. 10.1158/1078-0432.ccr-10-2884 [DOI] [PubMed] [Google Scholar]
- Uhlén M., Fagerberg L., Hallström B. M. (2015). Tissue-based map of the human proteome. Science 347 1260419–1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- Uhlen M., Zhang C., Lee S. (2017). A pathology atlas of the human cancer transcriptome. Science 357:eaan2507. 10.1126/science.aan2507 [DOI] [PubMed] [Google Scholar]
- Wahlström T., Arsenian Henriksson M. (2015). Impact of MYC in regulation of tumor cell metabolism. Biochim. Biophys. Acta. 1849 563–569. 10.1016/j.bbagrm.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Walz S., Lorenzin F., Morton J. (2014). Corrigendum: Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature 511:440 10.1038/nature14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Duan W., Yan S. (2019). Circulating long non-coding RNA colon cancer-associated transcript 2 protected by exosome as a potential biomarker for colorectal cancer. Biomed. Pharmacother. 113:108758. 10.1016/j.biopha.2019.108758 [DOI] [PubMed] [Google Scholar]
- Wang R., Dillon C. P., Shi L. Z. (2011). The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35 871–882. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Wanwei Z., Zeng T. (2014). MCentridFS: a tool for identifying module biomarkers for multi-phenotypes from high-throughput data. Mole. Biosyst. 10 2870–2875. 10.1039/c4mb00325j [DOI] [PubMed] [Google Scholar]
- Wierstra I., Alves J. (2008). The c-myc promoter: still MysterY and challenge. Adv. Cancer Res. 99 113–333. 10.1016/s0065-230x(07)99004-1 [DOI] [PubMed] [Google Scholar]
- Williamson L., Raess N. A., Caldelari R. (2006). Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. Eur. Mole. Biol. Organizat. J. 25 3298–3309. 10.1038/sj.emboj.7601224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. W. H., Pastrello C., Kotlyar M. (2017). Modeling tumor progression via the comparison of stage-specific graphs. Methods 132 34–41. 10.1016/j.ymeth.2017.06.033 [DOI] [PubMed] [Google Scholar]
- Xiaoping L., Zhi-Ping L., Xing-Ming Z. (2016). Identifying disease genes and module biomarkers by differential interactions. J. Am. Med. Inform. Assoc. JAMIA 19 241–248. 10.1136/amiajnl-2011-000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunos R. I. M., Mutalib N. S. A., Tieng F. Y. F. (2020). Actionable Potentials of Less Frequently Mutated Genes in Colorectal Cancer and Their Roles in Precision Medicine. Biomolecules 10:476. 10.3390/biom10030476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalocusky K. A., Kan M. J., Hu Z., Dunn P., Thomson E., Wiser J., et al. (2018). The 10,000 Immunomes Project: Building a Resource for Human Immunology. Cell Rep. 25 513.e–522.e. 10.1016/j.celrep.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Horvath S. (2005). A General Framework For Weighted Gene Co-Expression Network Analysis. Statist. Appl. Gen. Mole. Biol. 4:17. 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
- Zhang F., Liu X., Zhang A. (2019). Genome-wide dynamic network analysis reveals a critical transition state of flower development in Arabidopsis. BMC Plant Biol. 19:11. 10.1186/s12870-018-1589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.