Abstract
Chronic subdural hematoma (cSDH) is a common disease process associated with significant morbidity that occurs most often in elderly patients. Asymptomatic patients are typically treated conservatively, with surgical intervention reserved for patients with symptomatic and/or large hematomas that cause brain compression. However, conservatively managed cSDH cases frequently progress, and surgical evacuation of cSDH is associated with high rates of complication and recurrence. Recently, successful treatment of cSDH via middle meningeal artery (MMA) embolization has been reported in small case series and case reports. This article reviews the existing literature on MMA embolization for cSDH and discusses the need for randomized control trials and/or large prospective studies to establish the efficacy of MMA embolization for this disease.
Keywords: chronic subdural hematoma, cSDH, middle meningeal artery embolization, endovascular cSDH treatment, MMA embolization
Introduction
Chronic subdural hematoma (cSDH) is one of the most common neurosurgical pathologies that largely affects elderly patients and is associated with significant morbidity and mortality (1–3). cSDH is thought to evolve from a prior traumatic acute subdural hemorrhage that develops between the dura and arachnoid layer (2, 4, 5). Although this acute hematoma may resolve completely, in many cases the processes of inflammation, fibrinolysis, and/or angiogenesis lead to formation of a vascularized neomembrane that results in fluid exudation and subsequent hemorrhage, ultimately leading to volume expansion and neurological deficits (2, 4, 6). Histologically, the outer neomembrane is composed of friable sinusoidal neovessels that easily rupture spontaneously, which causes recurring hemorrhage (7–9). These neovessels derive their blood supply from the middle meningeal artery (MMA), which transverses the dura to connect to these fragile vessels (7, 8). Evidence in support of this theory comes from imaging studies reporting ipsilateral enlargement of the MMA in patients with cSDH (2, 8).
For symptomatic, refractory, and/or large cSDH causing brain compression, surgical evacuation and placement of a drain are commonly performed (10). On the other hand, asymptomatic and/or small cSDHs without brain compression are typically treated conservatively and followed-up closely with serial imaging. Successful MMA embolization for cSDH has been described in small case reports and series over the past several years (11–18). More recently, Ng et al. (19) in 2020 conducted a randomized controlled trial comparing surgery with and without MMA embolization in patients with cSDH. The authors reported one recurrence in each group. However, patients who underwent MMA had a higher hematoma reabsorption rate than those with surgical treatment alone (mean difference, 17.5 mL; 95% CI, 3.87–31.16 mL; p = 0.02). Embolization of the MMA is hypothesized to occlude the subdural membrane neovessels to inhibit the recurrent rupture of these vessels, thus facilitating reabsorption of the accumulated subdural fluid (20). Postembolization CT suggests that both particles and liquid embolisate can penetrate into the neomembranes, further strengthening this theory (20). Moreover, several systematic reviews and a meta-analysis have shown promising results with MMA embolization, including low rates of complication and recurrence (21, 22).
This review will discuss MMA embolization for cSDHs, focusing on the major literature studies and future directions needed to establish the efficacy of this technique as a stand-alone and/or adjunct alternative to surgical drainage.
Discussion
Demographic Characteristics and Treatment
cSDH typically occurs in elderly patients with or without a recognized preceding traumatic event and can be associated with the use of anticoagulant or antiplatelet medications (1–3, 10, 23–25). The occurrence of cSDHs has been steadily increasing, and with an ever-increasing elderly population, 60,000 new cases are projected each year over the next 10 years (1–3, 12, 24). Clinical manifestations of cSDH are protean; therefore, the diagnosis should be considered in any elderly patient with an altered mental status or new neurological deficit (10).
Conservative management is often reserved for asymptomatic patients without significant brain compression or midline shift (10). Such management includes frequent follow-up imaging, reversal and discontinuation of anticoagulation, and often the administration of corticosteroids (10, 26–34). Steroids are thought to inhibit the formation of the neomembrane and neovessels by suppressing both fibrinolysis and inflammation (35). However, treatment of cSDHs with corticosteroids has yielded mixed results. One systematic review found that 83–97% of patients treated with corticosteroids alone returned to neurological baseline, and only 4–27.8% would need further treatment, defined as a second round of steroids or surgical evacuation (36). A larger meta-analysis of more than 30,000 patients that included both observational studies and randomized control trials found that higher morbidity was associated with the use of corticosteroids to treat cSDHs, with no therapeutic benefit (37). Finally, a small recent randomized control trial comparing corticosteroids to placebo among patients with cSDH found that steroids may decrease the risk of requiring surgical evacuation (38). Larger randomized clinical trials are underway to investigate this therapy (39–41).
Statins are another class of medication that may be considered as conservative treatment of cSDH. This class of medication has demonstrated efficacy in reducing inflammation at the endothelium, which can also help prevent neomembrane formation (42). In addition, the role of statins in enhancing the functionality of endothelial progenitor cells (43) results in vascular protection within the brain (44). One retrospective study observed patients who were found to have a cSDH without an indication for surgery for 3 months. Chan et al. (45) found that those who were receiving atorvastatin had a 16.7% chance of experiencing deteriorating mental status, requiring bur hole drainage, compared with 58.3% of those who were not receiving atorvastatin. Finally, a recent clinical trial that included 200 patients demonstrated that among patients who had cSDH and no indication for surgery, 8 weeks of atorvastatin was associated with a significantly greater reduction in hematoma volume and improvement in neurological outcome, compared with patients who were treated without a statin. Additionally, 11.2% of those receiving atorvastatin required surgical evacuation during the study, compared with 23.5% who were not receiving a statin (46).
For patients with cSDH causing symptoms, significant midline shift, and/or neurological deficits, surgical intervention is often performed via twist drill craniotomy, bur hole craniostomy, or standard or mini craniotomy (10). Although surgical interventions are typically successful and result in clinical improvement in most patients, surgical mortality rates as high as 32% have been reported (4, 10, 24, 47, 48). Furthermore, complications and recurrence following surgical intervention occur in up to 9% and 28% of patients, respectively (10). Although the use of a subdural drain following evacuation has been reported to decrease the risk of recurrence by up to 50%, the rates of complications, recurrences, morbidity, and mortality still remain substantial (10, 49). Recently, Soleman et al. (50) conducted a randomized, controlled trial that found that subperiosteal drains are associated with an 8% rate of recurrence following bur hole drainage and lower rates of iatrogenic morbidity than subdural drain placement. Similarly, Santarius et al. (51) in 2009 conducted a randomized control trial on the use of drains vs. no drain after bur hole evacuation of cSDHs and found a low rate of recurrence of 9% among patients with a drain vs. 24% among those without a drain. Nonetheless, given the substantial risk of recurrence reported across the various surgical techniques, alternative treatment modalities, either in isolation or in combination with surgical intervention, are warranted.
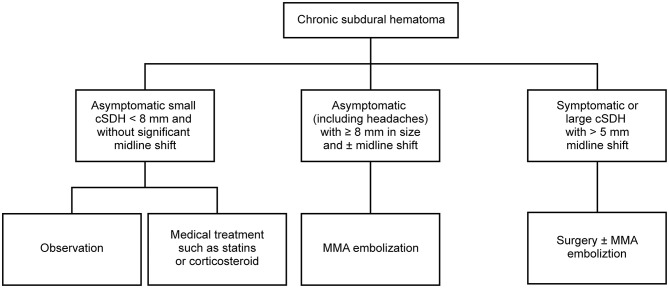
MMA embolization has been shown to be effective, particularly in patients with recurrent cSDH or patients for whom surgical intervention is associated with a particularly high risk (e.g., due to receipt of anticoagulation medication or advanced comorbid conditions), and may significantly reduce the morbidity and mortality associated with cSDH; Table 1 (11, 16, 18, 22, 52–55) illustrates the major published case series in the current literature (11–18, 22, 52–55). Figure 1 shows a proposed treatment algorithm for cSDHs with the use of MMA embolization. However, level 1 evidence for the use of MMA embolization is lacking, with the majority of literature consisting of case reports or small case series.
Table 1.
Major published studies of middle meningeal artery (MMA) embolization for chronic subdural hematoma (cSDH).
| Study | Year | Study type | No. of patients | Embolysate used | Major findings |
|---|---|---|---|---|---|
| Ishihara et al. (52) | 2007 | Case series | 7 | NBCA | One of the earliest studies in which patients with cSDH were treated with MMA embolization following either a third recurrence or after a second recurrence and major bleeding risk. No recurrences were found in these patients at last follow-up (up to 15 months). |
| Kim (53) | 2017 | Prospective cohort | 20 | PVA | Analyzed 20 patients with recurrent cSDH treated with MMA embolization and found 85% to have a mRS <2 on follow-up; however, found no difference in mRS scores when comparing patients with traditional treatment for recurrent cSDH. Also found a significant increase in brain re-expansion, decreased hematoma recurrence, and similar complication rates to the conventional treatment group. |
| Ban et al. (11) | 2018 | Prospective cohort | 72 | PVA | Largest study to date; reported no complications associated with MMA embolization and found a statistically lower treatment failure rate (1.4%) than 469 conventionally treated patients (28% failure rate). |
| Farkas et al. (54) | 2018 | Case series | 10 | NR | Follow-up CT at 128 days showed a 45% reduction in cSDH size following MMA embolization in 10 patients (5 primary treatment vs. 5 for recurrent treatment). |
| Matsumoto et al. (18) | 2018 | Case series | 4 | NBCA | Four patients underwent combined MMA embolization and bur hole drainage for refractor cSDH, and none required an additional treatment vs. 2 of 10 patients who required another intervention following conventional therapy for a refractory cSDH. |
| Link et al. (16) | 2019 | Case series | 49 | PVA | Total of 60 SDHs embolized in 49 patients (42 primary therapy, 8 recurrences, and 10 prophylaxis prior to surgery). Reported a 91% long-term success rate, measured as either stable or decreased size of cSDH and avoidance of additional surgery. |
| Waquas et al. (22) | 2019 | Case series | 8 | Onyx | Six procedures were for primary therapy and two were after surgical recurrence. No retreatments recorded after embolization with complete resolution in 3 patients. All patients had a mRS <2 with an average follow-up of 3 months. |
| Okuma et al. (55) | 2019 | Case series | 17 | NBCA and microspheres | Nine of 17 patients with mRS ≤2 with average follow-up of 26 months. |
CT, computed tomography; mRS, modified Rankin Scale; NBCA, n-butyl cyanoacrylate; NR, not reported; PVA, polyvinyl alcohol.
Figure 1.
Proposed treatment algorithm for chronic subdural hematoma (cSDH) with the use of middle meningeal artery (MMA) embolization. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
MMA Embolization Techniques
Descriptions of the technique of MMA embolization remain scarce, with the majority of existing literature reporting the injection of microparticles (11, 16, 17, 22, 56, 57). Some authors have discussed the potential advantages of liquid embolisate for these procedures. These potentially include the depth of penetration, larger volume of embolisate, quicker procedures, increased durability, and improved visualization of the embolic agent (12). To date, no comparative studies have been performed, and further research is warranted to determine the most effective embolic agent for this procedure.
Complications
The reported complication rate among patients who undergo MMA embolization for cSDH is low (12). The two largest series, those of Link et al. (16) (N = 49 patients) and Ban et al. (11) (N = 72 patients), reported no procedural complications attributable to MMA embolization. The recently published meta-analysis by Srivatsan et al. (21) analyzed three different double-arm studies comparing embolization and conventional surgery. The authors found a complication rate of 2% in the embolization group vs. 4% in the conventional surgical group (21).
Recurrences
With a reported recurrence rate as high as 28% among patients with surgically treated cSDH, one of the main outcome measures analyzed in MMA embolization series is recurrence of the hemorrhage and the need for subsequent intervention. Along these lines, Link et al. (16) found that 9% of a series of 49 patients with 60 cSDHs undergoing MMA embolization required further intervention. This series included 42 hemorrhages treated for the first time, 8 postsurgical recurrences of hemorrhage, and 10 hemorrhages that occurred before surgery (16). In the largest study to date, Ban et al. (11) analyzed 72 patients with cSDH who underwent MMA embolization, including 27 asymptomatic patients who underwent embolization as sole treatment and 45 previously symptomatic patients who had previously undergone hematoma evacuation for symptomatic relief. Ban et al. (11) found a treatment failure rate (remaining or reaccumulated hematoma >1 cm in diameter at 6-month follow-up and/or the need for additional surgical procedures) of only 1.4%. Furthermore, the same authors found the treatment failure rate among 469 conventionally treated patients (402 with initial surgical evacuation and 67 with conservative management) to be significantly higher at 28% (11). Similarly, in the meta-analysis by Srivatsan et al. (21) the authors found a significantly higher hematoma recurrence rate among conventionally treated patients (28%) relative to those undergoing embolization.
Neurological Outcomes
Although the majority of series of patients who underwent MMA embolization used the primary end point of resolution of the hematoma on follow-up imaging, four studies also reported neurological outcomes. In a series of 8 patients, Waqas et al. (22) observed that all patients had a modified Rankin Scale (mRS) score of <2 following a minimum 2-month follow-up (mean follow-up, 3 months). Similarly, Matsumoto et al. (18) published a small series of four patients with refractory cSDH who were treated with MMA embolization and found that all patients exhibited a follow-up mRS of 0. Meanwhile, Okuma et al. (55) reported 9 of 17 patients with an mRS score of ≤2 after long-term follow-up of an average of 26 months (only 3 of 17 patients had an mRS score ≤2 at admission). Lastly, Kim et al. (53) found that 17 of 20 (85%) patients with a recurrent cSDH treated with MMA embolization had an mRS <2 on follow-up. However, in the same study, 20 of 23 (87%) patients with conventional treatment exhibited similarly low mRS scores (53). Likewise, in the meta-analysis by Srivatsan et al. (21), no significant difference was found between mRS scores among patients treated with embolization embolized vs. patient who received conventional treatment.
Future Direction
Several randomized control trials investigating the efficacy, safety, and utility of MMA embolization for cSDHs are underway (15, 16, 58–63). Additionally, various embolisates for MMA embolization are currently being studied. The SQUID Trial for the Embolization of the Middle Meningeal Artery for Treatment of Chronic Subdural Hematoma (STEM) is a randomized control trial that is investigating the safety and efficacy of SQUID for the management of cSDHS (61). Another embolisate currently being analyzed is Onyx, which is being evaluated in the Embolization of the Middle Meningeal Artery with ONYX Liquid Embolic System for Subacute and Chronic Subdural Hematoma (EMBOLISE) (62). Both of these trials are comparing medical management alone to MMA embolization, and surgical treatment with embolization to surgical treatment alone. Because the literature on MMA embolization of cSDHs includes a large number of patients who also received surgical intervention, randomized control trials will need to be conducted in a manner to also elucidate the appropriate patient selection for either MMA embolization alone or in combination with surgical intervention.
Conclusion
MMA embolization for cSDH represents an emerging treatment modality, with a rapidly increasing number of studies analyzing this innovative and largely successful technique. However, many questions remain, including appropriate patient selection, efficacy as a stand-alone procedure, optimal embolization techniques, and timing of embolization with regard to surgical intervention in symptomatic patients. Furthermore, the majority of literature on MMA embolization for cSDH reports cases studies and small case series, whereas several randomized control trials have shown efficacy with surgical interventions. Hence, many randomized clinical trials using MMA as a treatment for cSDH are underway at several centers in the United States and Europe (58–65). These studies will ultimately provide insight on the safety, efficacy, and use of this novel technique in the treatment of cSDHS.
Author Contributions
JC: manuscript writing, editing, and literature review. CN and AW: manuscript writing and literature review. FA: editing. AD: editing and final approval. All authors contributed to the article and approved the submitted version.
Conflict of Interest
AD is a consultant for Medtronic, Stryker, Cerenovus, Penumbra, and Koswire. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Neuroscience Publications staff at Barrow Neurological Institute for help with manuscript preparation.
Glossary
Abbreviations
- cSDH
chronic subdural hematoma
- MMA
middle meningeal artery
- mRS
modified Rankin Scale.
References
- 1.Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States veterans administration and civilian populations. J Neurosurg. (2015) 123:1209–15. 10.3171/2014.9.JNS141550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foreman P, Goren O, Griessenauer CJ, Dalal SS, Weiner G, Schirmer CM. Middle meningeal artery embolization for chronic subdural hematomas: cautious optimism for a challenging pathology. World Neurosurg. (2019) 126:528–9. 10.1016/j.wneu.2019.03.160 [DOI] [PubMed] [Google Scholar]
- 3.Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. (2011) 114:72–6. 10.3171/2010.8.JNS10298 [DOI] [PubMed] [Google Scholar]
- 4.Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. (2014) 10:570–8. 10.1038/nrneurol.2014.163 [DOI] [PubMed] [Google Scholar]
- 5.Lee KS. Natural history of chronic subdural haematoma. Brain Inj. (2004) 18:351–8. 10.1080/02699050310001645801 [DOI] [PubMed] [Google Scholar]
- 6.Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. (2017) 14:108. 10.1186/s12974-017-0881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagahori T, Nishijima M, Takaku A. [Histological study of the outer membrane of chronic subdural hematoma: possible mechanism for expansion of hematoma cavity]. No Shinkei Geka. (1993) 21:697–701. [PubMed] [Google Scholar]
- 8.Takizawa K, Sorimachi T, Ishizaka H, Osada T, Srivatanakul K, Momose H, et al. Enlargement of the middle meningeal artery on MR angiography in chronic subdural hematoma. J Neurosurg. (2016) 124:1679–83. 10.3171/2015.5.JNS1567 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Kaimori M. [Histological study of vascular structure between the dura mater and the outer membrane in chronic subdural hematoma in an adult]. No Shinkei Geka. (1999) 27:431–6. [PubMed] [Google Scholar]
- 10.Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. (2018) 50:7–15. 10.1016/j.jocn.2018.01.050 [DOI] [PubMed] [Google Scholar]
- 11.Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. middle meningeal artery embolization for chronic subdural hematoma. Radiology. (2018) 286:992–9. 10.1148/radiol.2017170053 [DOI] [PubMed] [Google Scholar]
- 12.Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg. (2019) 11:912–5. 10.1136/neurintsurg-2019-014730 [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. (2013) 4:104. 10.4103/2152-7806.116679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J, Whang K, Hong SK, Pyen JS, Cho SM, Kim JY, et al. Middle meningeal artery embolization in recurrent chronic subdural hematoma combined with arachnoid cyst. Korean J Neurotrauma. (2015) 11:187–90. 10.13004/kjnt.2015.11.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: a case series. Operat Neurosurg. (2018) 14:556–62. 10.1093/ons/opx154 [DOI] [PubMed] [Google Scholar]
- 16.Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. (2019) 85:801–7. 10.1093/neuros/nyy521 [DOI] [PubMed] [Google Scholar]
- 17.Link TW, Rapoport BI, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: endovascular technique and radiographic findings. Interv Neuroradiol. (2018) 24:455–62. 10.1177/1591019918769336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto H, Hanayama H, Okada T, Sakurai Y, Minami H, Masuda A, et al. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. (2018) 49:40–7. 10.1016/j.jocn.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 19.Ng S, Derraz I, Boetto J, Dargazanli C, Poulen G, Gascou G, et al. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: a pilot study assessing hematoma volume resorption. J Neurointerv Surg. (2020) 12:695–9. 10.1136/neurintsurg-2019-015421 [DOI] [PubMed] [Google Scholar]
- 20.Saito H, Tanaka M, Hadeishi H. Angiogenesis in the septum and inner membrane of refractory chronic subdural hematomas: consideration of findings after middle meningeal artery embolization with low-concentration n-butyl-2-cyanoacrylate. NMC Case Rep J. (2019) 6:105–10. 10.2176/nmccrj.cr.2018-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. (2019) 122:613–9. 10.1016/j.wneu.2018.11.167 [DOI] [PubMed] [Google Scholar]
- 22.Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg. (2019) 126:228–36. 10.1016/j.wneu.2019.02.208 [DOI] [PubMed] [Google Scholar]
- 23.Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. (2002) 78:71–5. 10.1136/pmj.78.916.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. (2012) 35:155–69. 10.1007/s10143-011-0349-y [DOI] [PubMed] [Google Scholar]
- 25.Foelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochir. (1975) 32:247–50. 10.1007/BF01405457 [DOI] [PubMed] [Google Scholar]
- 26.Decaux O, Cador B, Dufour T, Jego P, Cazalets C, Laurat E, et al. [Nonsurgical treatment of chronic subdural hematoma with steroids: two case reports]. Rev Med Interne. (2002) 23:788–91. 10.1016/S0248-8663(02)00676-8 [DOI] [PubMed] [Google Scholar]
- 27.Delgado-Lopez PD, Martin-Velasco V, Castilla-Diez JM, Rodriguez-Salazar A, Galacho-Harriero AM, Fernandez-Arconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia. (2009) 20:346–59. 10.1016/S1130-1473(09)70154-X [DOI] [PubMed] [Google Scholar]
- 28.Dran G, Berthier F, Fontaine D, Rasenrarijao D, Paquis P. [Effectiveness of adjuvant corticosteroid therapy for chronic subdural hematoma: a retrospective study of 198 cases]. Neurochirurgie. (2007) 53:477–82. 10.1016/j.neuchi.2007.09.146 [DOI] [PubMed] [Google Scholar]
- 29.Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg. (2005) 19:327–33. 10.1080/02688690500305332 [DOI] [PubMed] [Google Scholar]
- 30.Thotakura AK, Marabathina NR. Nonsurgical treatment of chronic subdural hematoma with steroids. World Neurosurg. (2015) 84:1968–72. 10.1016/j.wneu.2015.08.044 [DOI] [PubMed] [Google Scholar]
- 31.Inzelberg R, Neufeld MY, Reider I, Gari P. Non surgical treatment of subdural hematoma in a hemodialysis patient. Clin Neurol Neurosurg. (1989) 91:85–9. 10.1016/S0303-8467(89)80014-9 [DOI] [PubMed] [Google Scholar]
- 32.Rudiger A, Ronsdorf A, Merlo A, Zimmerli W. Dexamethasone treatment of a patient with large bilateral chronic subdural haematomata. Swiss Med Wkly. (2001) 131:387. [DOI] [PubMed] [Google Scholar]
- 33.Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. (1974) 31:73–9. 10.1001/archneur.1974.00490380021001 [DOI] [PubMed] [Google Scholar]
- 34.Pichert G, Henn V. [Conservative therapy of chronic subdural hematomas]. Schweiz Med Wochenschr. (1987) 117:1856–62. [PubMed] [Google Scholar]
- 35.Thotakura AK, Marabathina NR. The role of medical treatment in chronic subdural hematoma. Asian J Neurosurg. (2018) 13:976–83. 10.4103/ajns.AJNS_13_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berghauser Pont LME, Dirven CMF, Dippel DWJ, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol. (2012) 19:1397–403. 10.1111/j.1468-1331.2012.03768.x [DOI] [PubMed] [Google Scholar]
- 37.Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. (2014) 259:449–57. 10.1097/SLA.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 38.Prud'homme M, Mathieu F, Marcotte N, Cottin S. A pilot placebo controlled randomized trial of dexamethasone for chronic subdural hematoma. Can J Neurol Sci. (2016) 43:284–90. 10.1017/cjn.2015.393 [DOI] [PubMed] [Google Scholar]
- 39.Allison A, Edlmann E, Kolias AG, Davis-Wilkie C, Mee H, Thelin EP, et al. Statistical analysis plan for the dex-CSDH trial: a randomised, double-blind, placebo-controlled trial of a 2-week course of dexamethasone for adult patients with a symptomatic chronic subdural haematoma. Trials. (2019) 20:698. 10.1186/s13063-019-3866-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miah IP, Holl DC, Peul WC, Walchenbach R, Kruyt N, de Laat K, et al. Dexamethasone therapy versus surgery for chronic subdural haematoma (DECSA trial): study protocol for a randomised controlled trial. Trials. (2018) 19:575. 10.1186/s13063-018-2945-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolias AG, Edlmann E, Thelin EP, Bulters D, Holton P, Suttner N, et al. Dexamethasone for adult patients with a symptomatic chronic subdural haematoma (Dex-CSDH) trial: study protocol for a randomised controlled trial. Trials. (2018) 19:670. 10.1186/s13063-018-3050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buttmann M, Lorenz A, Weishaupt A, Rieckmann P. Atorvastatin partially prevents an inflammatory barrier breakdown of cultured human brain endothelial cells at a pharmacologically relevant concentration. J Neurochem. (2007) 102:1001–8. 10.1111/j.1471-4159.2007.04563.x [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Wei J, Hu L, Hu S. Beneficial effects of statins on endothelial progenitor cells. Am J Med Sci. (2012) 344:220–6. 10.1097/MAJ.0b013e31824998f9 [DOI] [PubMed] [Google Scholar]
- 44.Potey C, Ouk T, Petrault O, Petrault M, Berezowski V, Salleron J, et al. Early treatment with atorvastatin exerts parenchymal and vascular protective effects in experimental cerebral ischaemia. Br J Pharmacol. (2015) 172:5188–98. 10.1111/bph.13285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan DYC, Chan DTM, Sun TFD, Ng SCP, Wong GKC, Poon WS. The use of atorvastatin for chronic subdural haematoma: a retrospective cohort comparison study. Br J Neurosurg. (2017) 31:72–7. 10.1080/02688697.2016.1208806 [DOI] [PubMed] [Google Scholar]
- 46.Jiang R, Zhao S, Wang R, Feng H, Zhang J, Li X, et al. Safety and efficacy of atorvastatin for chronic subdural hematoma in chinese patients: a randomized clinicalTrial. JAMA Neurol. (2018) 75:1338–46. 10.1001/jamaneurol.2018.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivamoto HS, Lemos HP, Jr, Atallah AN. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg. (2016) 86:399–418. 10.1016/j.wneu.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 48.Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. (2003) 74:937–43. 10.1136/jnnp.74.7.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng D, Zhu Y. External drains versus no drains after burr-hole evacuation for the treatment of chronic subdural haematoma in adults. Cochrane Database Syst Rev. (2016) 2016:CD011402. 10.1002/14651858.CD011402.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soleman J, Lutz K, Schaedelin S, Kamenova M, Guzman R, Mariani L, et al. Subperiosteal vs subdural drain after burr-hole drainage of chronic subdural hematoma: a randomized clinical trial (cSDH-drain-trial). Neurosurgery. (2019) 85:E825–34. 10.1093/neuros/nyz095 [DOI] [PubMed] [Google Scholar]
- 51.Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. (2009) 374:1067–73. 10.1016/S0140-6736(09)61115-6 [DOI] [PubMed] [Google Scholar]
- 52.Ishihara H, Ishihara S, Kohyama S, Yamane F, Ogawa M, Sato A, et al. Experience in endovascular treatment of recurrent chronic subdural hematoma. Int Neuroradiol. (2007) 13 (Suppl. 1):141–4. 10.1177/15910199070130S121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. (2017) 101:520–7. 10.1016/j.wneu.2017.02.070 [DOI] [PubMed] [Google Scholar]
- 54.Farkas N, Bo R, Arcot K, Tiwari A, Rurkel-Parrella D, Selas G, et al. Radiographic efficacy of middle meningeal artery embolization in treatment of chronic subdural hematoma (P6.213). Neurology. (2018) 90 (15 Suppl). Available online at: https://n.neurology.org/content/90/15_Supplement/P6.213/tab-article-info [Google Scholar]
- 55.Okuma Y, Hirotsune N, Sato Y, Tanabe T, Muraoka K, Nishino S. Midterm follow-up of patients with middle meningeal artery embolization in intractable chronic subdural hematoma. World Neurosurg. (2019) 126:e671–8. 10.1016/j.wneu.2019.02.121 [DOI] [PubMed] [Google Scholar]
- 56.Catapano JS, Fredrickson VL, Fujii T, Cole TS, Koester SW, Baranoski JF, et al. Complications of femoral versus radial access in neuroendovascular procedures with propensity adjustment. J Neurointerv Surg. (2019) 12:611–5. 10.1136/neurintsurg-2019-015569 [DOI] [PubMed] [Google Scholar]
- 57.Gore P, Theodore N, Brasiliense L, Kim LJ, Garrett M, Nakaji P, et al. The utility of onyx for preoperative embolization of cranial and spinal tumors. Neurosurgery. (2008) 62:1204–11. 10.1227/01.neu.0000333292.74986.ac [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health . Middle Meningeal Artery (MMA) Embolization Compared to Traditional Surgical Strategies to Treat Chronic Subdural Hematomas (cSDH). (2019). Available online at: https://ClinicalTrials.gov/show/NCT04095819 (accessed October 7, 2020).
- 59.National Institutes of Health . Dartmouth Middle Meningeal Embolization Trial (DaMMET). (2020). Available online at: https://ClinicalTrials.gov/show/NCT04270955 (accessed October 7, 2020).
- 60.National Institutes of Health . Embolization of the Middle Meningeal Artery for the Prevention of Chronic Subdural Hematoma Recurrence in High Risk Patients (EMPROTECT). (2020). Available online at: https://ClinicalTrials.gov/show/NCT04372147 (accessed October 7, 2020).
- 61.National Institutes of Health . The SQUID Trial for the Embolization of the Middle Meningeal Artery for Treatment of Chronic Subdural Hematoma (STEM). (2020). Available online at: https://ClinicalTrials.gov/show/NCT04410146 (accessed October 7, 2020).
- 62.National Institutes of Health . Embolization of the Middle Meningeal Artery With ONYX™ Liquid Embolic System for Subacute and Chronic Subdural Hematoma. (2020). Available online at: https://ClinicalTrials.gov/show/NCT04402632 (accessed October 7, 2020).
- 63.National Institutes of Health . Endovascular Embolization for Chronic Subdural Hematomas Following Surgical Evacuation. (2020). Available online at: https://ClinicalTrials.gov/show/NCT04272996 (accessed October 7, 2020).
- 64.National Institutes of Health . Middle Meningeal Artery Embolization for Treatment of Chronic Subdural Hematoma. (2017). Available online at: https://ClinicalTrials.gov/show/NCT03307395 (accessed October 7, 2020).
- 65.National Institutes of Health . Middle Meningeal Artery Embolization for Chronic Subdural Hematoma. (2019). Available online at: https://ClinicalTrials.gov/show/NCT04065113 (accessed October 7, 2020).