Abstract
Background
Fabry disease is an inherited disorder of glycolipid metabolism with progressive involvement of multiple organs, including the gastrointestinal tract, in classically affected male patients. Clinical presentations in males with later-onset Fabry phenotypes are more heterogeneous and largely dependent on the level of residual α-galactosidase A activity.
Methods
We assessed agalsidase beta treatment outcomes of gastrointestinal symptoms in adult males with classic or later-onset Fabry disease. Self-reports of abdominal pain and diarrhea (‘present’/’not present’ since previous assessment) at last clinical visit (≥0.5 year of follow-up) were compared with treatment-baseline.
Results
Classic male patients were considerably younger at first treatment than the fewer males with later-onset phenotypes (36 vs. ~47 years) and reported gastrointestinal symptoms more frequently at baseline (abdominal pain: 56% vs. 13%; diarrhea: 57% vs. 23%). As compared with baseline, significantly fewer classic patients reported abdominal pain after a median of 4.7 years of treatment (N = 171, 56% vs. 41%, P < 0.001). Moreover, significantly fewer patients reported diarrhea after 5.5 years of follow-up (N = 169, 57% vs. 47%, P < 0.05). Among the males with later-onset phenotypes, albeit statistically non-significant, abdominal pain reports reduced after a median of 4.2 years (N = 48, 13% vs. 4%) and diarrhea reports reduced after a median of 4.4 years of treatment (N = 47, 23% vs. 13%).
Conclusions
Sustained treatment with agalsidase beta was associated with improvement in abdominal pain and diarrhea in a significant proportion of classic male Fabry patients. Males with later-onset phenotypes reported gastrointestinal symptoms much less frequently at baseline as compared with classic patients, and non-significant reductions were observed.
Keywords: Abdominal pain, Agalsidase beta, Diarrhea, Fabry disease, Fabry Registry, Phenotypes
Abbreviations: α-Gal A, α-galactosidase A; EOW, every other week; ERT, enzyme replacement therapy; GI, gastrointestinal; GL-3, globotriaosylceramide; lyso-GL-3, globotriaosylsphingosine
Highlights
-
•
GI symptoms significantly reduced in classic male Fabry patients on agalsidase beta.
-
•
Treatment was started long after symptoms began; earlier initiation is recommended.
-
•
Fabry-unrelated causes should be excluded if GI symptoms persist on treatment.
-
•
GI symptoms were much less prevalent among males with later-onset Fabry disease.
1. Introduction
Fabry disease (OMIM #301500) is a progressive, X-linked, lysosomal storage disorder caused by pathogenic variants in the GLA gene encoding α-galactosidase A (α-Gal A) [1,2]. Males with the severe, classic phenotype have absent or markedly deficient α-Gal A activity and extensive lysosomal accumulation of glycosphingolipids (in particular globotriaosylceramide [GL-3]) in many cell types [1,2]. Plasma levels of globotriaosylsphingosine (lyso-GL-3), the deacylated form of GL-3, are usually strikingly high in adult male patients [3]. Symptom onset typically occurs in childhood or adolescence [4,5] and manifestations of gastrointestinal (GI) tract dysfunction may include abdominal pain, diarrhea, nausea, vomiting, constipation, early satiety, and bloating [4,[6], [7], [8]]. As patients age, they are at high risk of developing serious organ complications (e.g. renal failure, cardiomyopathy, arrhythmia, stroke) [1,2,9].
Males with GLA variants associated with later-onset Fabry phenotypes have variable levels of residual α-Gal A activity and heterogeneity in clinical presentations [9,10]. Males who have very low levels of residual activity may develop pathology similar to pathology observed in classically affected males. If α-Gal A activity levels are significant, some tissues (e.g. vascular endothelium, most kidney cell types) may be protected from progressive GL-3 accumulation, but levels may be inadequate to prevent accumulation in myocardial cells and podocytes [11]. Plasma lyso-GL-3 levels are generally elevated but lower compared with those in classical patients [9]. Patients who have the relatively common p.Asn215Ser later-onset GLA variant often present later in life with complications primarily, although not exclusively, confined to the heart (e.g. left ventricular hypertrophy, arrhythmia) [[12], [13], [14]]. The prevalence of symptoms associated with Fabry disease, including GI symptoms, has not been determined in this patient population.
Enzyme replacement therapy (ERT) with either agalsidase beta (1 mg/kg every other week [EOW]) or agalsidase alfa (0.2 mg/kg EOW) is available for treatment of Fabry patients [2,15]. Pharmacological chaperone therapy is restricted to patients with amenable GLA variants according to results from an in vitro assay [2,16].
Therapeutic outcomes of GI symptoms among male patients stratified by Fabry phenotype have not yet been reported. The present Fabry Registry study evaluated the outcomes of abdominal pain and diarrhea among males with classic or later-onset Fabry phenotypes after sustained treatment with agalsidase beta.
2. Material and methods
The Fabry Registry (NCT00196742, sponsor: Sanofi Genzyme) is a multicenter, international, longitudinal, observational program designed to monitor the natural history and treatment outcomes of patients with Fabry disease. Patient and investigator involvement is voluntary. Recommended schedules of clinical assessments are available, but treating physicians determine assessment frequency according to each patient's individual need for medical care. Regarding GI symptoms, the Fabry Registry collects patient data on abdominal pain and diarrhea.
The present analysis included male Fabry patients aged ≥18 years at initiation of agalsidase beta as their initial Fabry disease-specific treatment. They received an average dose at or near the licensed dose of 1 mg/kg EOW (range 0.9–1.1 mg/kg EOW). Eligible males were required to have had a baseline (−12 to +1 months of first treatment) and a last on-treatment assessment of abdominal pain and/or diarrhea after a minimum of 0.5 year of follow-up. The analysis was restricted to patients with GLA variants categorized as being associated with either the classic or a later-onset phenotype of Fabry disease (https://dbfgp.org/dbFgp/fabry/); herein patients are referred to as ‘classic patients’ and ‘later-onset patients’. Registry data entered up to January 8th, 2019, were analyzed. Measured outcomes were patient self-reports of abdominal pain and diarrhea, using binary responses of ‘yes’ (present) or ‘no’ (not present) to the question ‘did you experience [symptom] since the last clinical visit’. Patients were differentiated according to Fabry phenotypes.
Usable GI symptom responses were defined as ‘yes’ or ‘no’ responses excluding ‘unknown’ or missing answers. P values were calculated using McNemar's test to compare the responses at last clinical visit with responses at treatment initiation. A P value <0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software v.9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
The demographics of the male patients who started agalsidase beta treatment aged ≥18 years are summarized by phenotype in Table 1. Classic male patients were considerably younger at onset of the first Fabry symptom (any) (10 vs. 36 years), diagnosis (~32 vs. 45 years) and first agalsidase beta infusion (36 vs. ~47 years) than the fewer later-onset male patients. The most prevalent GLA variants associated with later-onset phenotypes included p.Asn215Ser (occurring in approximately one-third of the later-onset patients), p.Arg112His, p.Arg301Gln, p.Arg356Trp, c.639+919G>A, and p.Arg363His.
Table 1.
Demographics of the classic and later-onset male patients who had initiated agalsidase beta aged ≥18 years and were included in the analysis of gastrointestinal symptoms.
| Classic male patients | Later-onset male patients | |
|---|---|---|
| Abdominal pain | ||
| ‘Yes’/’No’ data, n | 171 | 48 |
| Age at first Fabry symptom (any), n Median (25th–75th) |
144 10.0 (7.1–14.6) |
35 36.2 (25.9–47.5) |
| Age at Fabry diagnosis, n Median (25th–75th) |
170 31.7 (22.3–39.9) |
48 45.4 (35.7–53.0) |
| Age at first agalsidase beta, n Median (25th–75th) |
171 36.2 (28.9–43.7) |
48 47.6 (38.9–54.0) |
| Agalsidase beta follow-up time, n Median (25th–75th) |
171 4.7 (2.8–9.7) |
48 4.2 (1.7–6.8) |
| Diarrhea | ||
| ‘Yes’/’No’ data, n | 169 | 47 |
| Age at first Fabry symptom (any), n Median (25th–75th) |
142 9.9 (6.9–14.5) |
34 36.0 (25.9–45.8) |
| Age at Fabry diagnosis, n Median (25th–75th) |
167 31.3 (22.3–39.3) |
47 45.2 (35.5–52.9) |
| Age at first agalsidase beta, n Median (25th–75th) |
169 35.8 (27.9–43.3) |
47 47.1 (37.8–53.7) |
| Agalsidase beta follow-up time, n Median (25th–75th) |
169 5.5 (3.1–9.8) |
47 4.4 (1.7–6.9) |
Ages and follow-up time shown in years; 25th–75th, 25th–75th percentile.
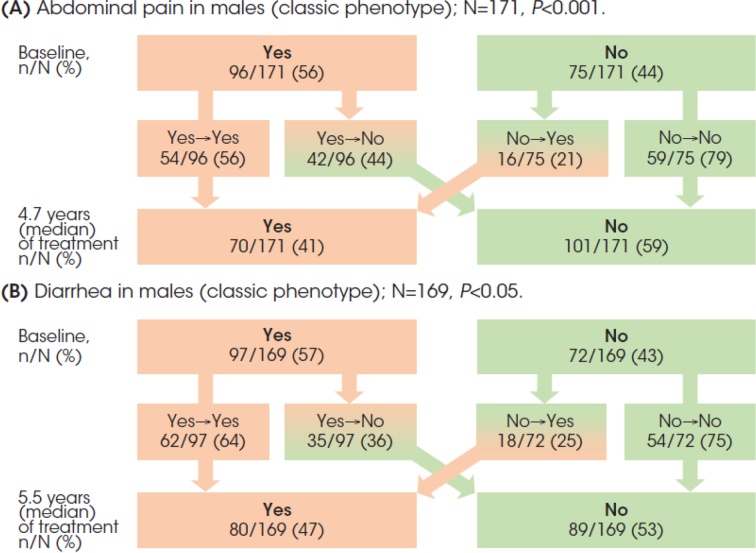
Among 171 classic male patients, 96 (56%) reported abdominal pain at start of treatment (Fig. 1A). The response had shifted from ‘yes’ to ‘no’ in 42 of these 96 (44%) patients after 4.7 years (median) of treatment, and from ‘no’ to ‘yes’ in 16 of 75 (21%) patients who initially had a ‘no’ response. Overall, there was a significant reduction in the proportion of patients reporting abdominal pain from 56% to 41% at last follow-up (P < 0.001).
Fig. 1.
Males with the classic phenotype: Abdominal pain (A) and diarrhea (B) responses (‘yes’ or ‘no’) at baseline and after long-term agalsidase beta treatment follow-up.
Diarrhea was reported by 97 of 169 (57%) classic patients (Fig. 1B). The response had shifted from ‘yes’ to ‘no’ in 35 (36%) of these patients after 5.5 years of follow-up, and from ‘no’ to ‘yes’ in 18 of 72 (25%) patients. The reduction in the proportion of patients reporting diarrhea from 57% to 47% was statistically significant (P < 0.001).
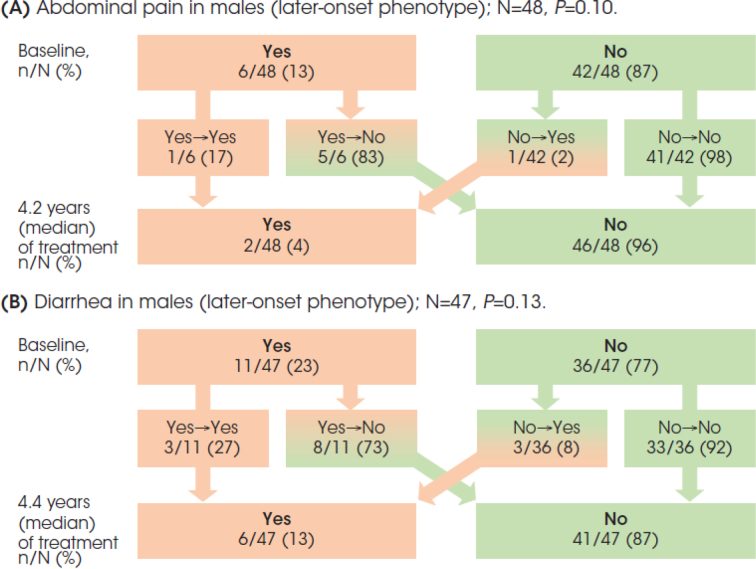
Among 48 later-onset patients, only 6 (13%) reported abdominal pain at treatment initiation (Fig. 2A). Five (83%) of these patients had a change in response from ‘yes’ to ‘no’ after 4.2 years (median) of treatment. All but one (98%) of the 42 patients who initially had a ‘no’ response did not report abdominal pain at last follow-up. Albeit not statistically significant, there was a reduction in the percentage of patients reporting abdominal pain from 13% to 4% at last follow-up.
Fig. 2.
Males with a later-onset phenotype: Abdominal pain (A) and diarrhea (B) responses (‘yes’ or ‘no’) at baseline and after long-term agalsidase beta treatment follow-up.
Diarrhea was reported by 11 of 47 (23%) later-onset patients (Fig. 2B). The response changed from ‘yes’ to ‘no’ in 8 (73%) of these patients after 4.4 years of treatment. The vast majority (92%) of the 36 patients who had a ‘no’ response at treatment initiation did not report diarrhea at last follow-up. The overall reduction in the percentage of patients reporting diarrhea from 23% to 13% was statistically non-significant.
4. Discussion
To date, outcomes of GI symptoms among adult male Fabry patients receiving long-term disease-specific treatment stratified by Fabry disease phenotypes have not been assessed. This Fabry Registry analysis compared the proportion of adult males with classic or later-onset phenotypes reporting abdominal pain and/or diarrhea at baseline and after sustained treatment with agalsidase beta (0.9–1.1 mg/kg EOW). We found a statistically significant reduction in the proportion of classic patients reporting abdominal pain (by 15%) and/or diarrhea (by 10%) after median treatment durations of 4.7 and 5.5 years, respectively. Most later-onset patients had reported absence of GI symptoms at treatment initiation, and almost all of these patients reported their absence at last follow-up. The reductions in percentages of the later-onset patients who did report presence of symptoms did not attain statistical significance.
The primary pathology in classic Fabry disease, cellular GL-3 accumulation, is believed to induce enteric small fiber neuropathy and ganglionopathy (disrupting autonomic functions), vasculopathy (causing ischemic injury) and inflammatory processes, which may collectively lead to manifestations of GI tract dysfunction (e.g. regional rapid bowel transit time, reduced peristalsis, intestinal stasis, bacterial overgrowth, malabsorption, pancreatic insufficiency, gastroparesis) [6,8,17]. In addition, high levels of plasma lyso-GL-3 may contribute to the pathology by inhibiting α-Gal A activity (promoting GL-3 storage), inducing proliferation of vascular smooth muscle cells, sensitization of peripheral nociceptive neurons, or even modifying the gut microbiota [3,[18], [19], [20]]. The complexity of the underlying pathology is probably responsible for the variability in descriptions of abdominal pain by Fabry patients (e.g. burning pain in the mid/lower abdomen, cramping, bloating, abdominal skin tenderness, and mid-abdominal discomfort increasing with [changes in] food intake or stress). Diarrhea may be related to meals or food intake, is often associated with significant urgency, and can be very frequent [6,8].
The exact mechanisms that may yield improvement in GI symptoms with Fabry disease-specific therapy have yet to be fully elucidated. It has been hypothesized that GL-3 in cells in the GI tract may be (partially) cleared with Fabry-specific treatment, which could lead to preservation of function or amelioration of dysfunction of these cells [21]. Agalsidase beta has been shown to clear microvascular endothelial accumulation of GL-3 from the kidneys, heart and skin [22], and ERT has been shown to improve nervous system outcomes [15]. Moreover, ERT decreased plasma lyso-GL-3 levels in an agalsidase dose-dependent manner [23,24], which paralleled changes in GI symptoms [24]. Limited data suggested that treatment with chaperone therapy was associated with improvements in diarrhea in male patients with amenable GLA variants, as measured using the Gastrointestinal Symptom Rating Scale [25].
A previous Fabry Registry study reported changes in GI symptoms among 168 females with Fabry disease receiving agalsidase beta [21]. That study did not stratify female patients by GLA variant phenotype classification (56% had a variant classified as “classic”) but reported overall results that were comparable to our findings in classic male patients; 45% reporting abdominal pain and 39% reporting diarrhea at treatment initiation, with reductions by 14% and 12%, respectively, after a mean agalsidase beta treatment duration of 5.7 years. Of note, clinical presentations associated with GLA variants are more heterogeneous in female patients than in male patients and depend in part on the X-chromosome inactivation profiles in the various organs [26].
As our study included only agalsidase beta-treated male patients stratified by phenotype, the prevalence of GI symptoms cannot be directly compared with the lower prevalence found in studies on the natural history of Fabry disease [7,27]. Abdominal pain (56%) and diarrhea (57%) were common at treatment initiation (median age 36 years) among classic patients in our study. A Fabry Outcome Survey study among 139 untreated male patients who had documented GI symptom data (mean age 32 years) reported a prevalence of 28% and 26% for abdominal pain and diarrhea, respectively, but the study lacked stratification by phenotype [7]. Among later-onset patients in our study, 13% and 23% of the patients reported abdominal pain or diarrhea, respectively, at treatment initiation (median age 47 years). Published observational studies exploring phenotypic presentations of later-onset Fabry disease patients are limited and have primarily focused on patients with the p.Asn215Ser [[12], [13], [14]] and c.639+919G>A [[28], [29], [30]] GLA variants and have not identified GI symptoms as being a frequent problem in patients having these variants.
Most classic patients who had GI symptoms at treatment baseline did not report complete resolution of these symptoms. A factor that may have contributed to this observation is that epidemiological studies in normal male populations, although scarce, suggest that GI symptoms are common. For example, a national, cross-sectional, telephone survey of US households (2510 subjects) reported that 18% of adult men aged 40–59 years reported abdominal pain or discomfort, and 26% diarrhea or loose stools within the month before the interview [31]. Therefore, some of the GI symptoms reported by patients included in the current analysis are likely not Fabry disease-related and unlikely to respond to Fabry-specific treatment. In these cases, patients should be investigated for other, treatable causes of cramping and diarrhea such as irritable bowel syndrome (aggravated by Fabry anxiety), inflammatory bowel disease, celiac disease, lactose intolerance and bacterial overgrowth. Another factor is that agalsidase beta treatment was initiated relatively late in most classic patients (median age 36 years), long after symptoms began, while earlier initiation has been recommended [2,32,33] and is generally associated with better clinical outcomes [15].
Our observations suggest an overall reduction in the proportion of male patients with classic Fabry disease reporting GI symptoms after sustained treatment with agalsidase beta. However, several limitations in our study must be recognized. First, the use of rigorous binary endpoints of ‘yes’ (present) and ‘no’ (not present) to describe self-reported outcomes, rather than validated GI symptom rating scales [6], precluded any symptom improvements from being analyzed, as data on changes in intensity and frequency of symptoms were insufficient. Second, subjective measures may be biased by factors such as patient perception and lack of a standardized definition. Thirdly, although all patients had received an average agalsidase beta dose at or near 1 (range 0.9–1.1) mg/kg every other week, strict adherence to the biweekly infusion regimen during the entire follow-up period cannot be confirmed. Finally, co-medication use that might affect the outcomes and comorbid conditions associated with GI symptoms could not be analyzed due to limited available data.
5. Conclusions
In our analysis of binary responses, sustained treatment with agalsidase beta was associated with improvement of abdominal pain and diarrhea in a significant proportion of male patients with classic Fabry disease. GI symptoms were substantially less prevalent at treatment initiation among males with later-onset Fabry phenotypes, and the vast majority of those patients remained free of symptoms.
Compliance with ethical standards
The Fabry Registry protocol, informed consent form and any authorization documents that are locally required for entering patient information into the Fabry Registry are in accordance with the Declaration of Helsinki, and are reviewed and approved by the local, fully constituted Institutional Review Board or Independent Ethics Committee unless a specific site provides evidence that approval is not required or that this requirement has been waived by a particular Institutional Review Board or Independent Ethics Committee.
Informed consent
Each Fabry Registry site is independent and responsible for obtaining informed written consent from patients to submit their health data to the Fabry Registry and use/disclose their anonymized data in analyses.
Funding
This work was supported by Sanofi Genzyme, the sponsor of the Fabry Registry, including the medical writing/editing support in the preparation of this manuscript from Emma Butterworth of Excerpta Medica. Hans Ebels of Sanofi Genzyme provided editorial support and Badari Gudivada of Sanofi Genzyme provided programming support. The authors are responsible for the content of this manuscript and the decision to submit the manuscript for publication. AO was supported by ISCIII Intensificación de la Actividad Investigadora.
CRediT authorship contribution statement
Robert J. Hopkin: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editing. Ulla Feldt-Rasmussen: Investigation, Writing - review & editing. Dominique P. Germain: Investigation, Writing - review & editing. Ana Jovanovic: Investigation, Writing - review & editing. Ana Maria Martins: Investigation, Writing - review & editing. Kathleen Nicholls: Investigation, Writing - review & editing. Alberto Ortiz: Investigation, Writing - review & editing. Juan Politei: Investigation, Writing - review & editing. Elvira Ponce: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editing. Carmen Varas: Investigation, Writing - review & editing. Frank Weidemann: Investigation, Writing - review & editing. Meng Yang: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editing. William R. Wilcox: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editing.
Declaration of Competing Interest
RJH has received consulting honoraria from Amicus Therapeutics, Protalix Corporation, Sanofi Genzyme and Takeda, was an investigator in clinical trials sponsored by Amicus Therapeutics, Protalix Corporation, Sanofi Genzyme and Takeda, and received research funding from Amicus Therapeutics, Protalix Corporation and Sanofi Genzyme; these activities have been monitored and found to be in compliance with the conflict of interest policies at Cincinnati Children's Hospital Medical Center. UFR has received Advisory Board honoraria from Amicus Therapeutics, Freeline, Sanofi Genzyme and Takeda, and speaker honoraria from Amicus Therapeutics, Sanofi Genzyme and Takeda. DPG has received consulting honoraria from Sanofi Genzyme and Takeda, and speaker honoraria and travel support from Amicus Therapeutics, Sanofi Genzyme and Takeda. AJ has received a research grant from Amicus Therapeutics, Advisory Board honoraria from Amicus Therapeutics, Sanofi Genzyme and Takeda, speaker honoraria from Amicus Therapeutics, BioMarin and Sanofi Genzyme, and travel support from Amicus Therapeutics and Sanofi Genzyme. AMM has received Advisory Board honoraria from BioMarin and Sanofi Genzyme, and speaker honoraria and travel support from Alexion, BioMarin and Sanofi Genzyme. AO has received consulting honoraria and travel support from Sanofi Genzyme, and speaker honoraria from Amicus Therapeutics, Freeline, Sanofi Genzyme and Takeda. KN has served as an advisor for Amicus Therapeutics, Sanofi Genzyme and Takeda, has received research support from Amicus Therapeutics and Takeda, and has received travel support from Sanofi Genzyme. JP has received honoraria and travel support from Amicus Therapeutics, Protalix Corporation, Sanofi Genzyme and Takeda. EP and MY are full-time employees of Sanofi Genzyme. CV is a member of the Fabry Registry Board of Advisors. FW has received speaker honoraria and travel support from Amicus Therapeutics, Sanofi Genzyme and Takeda. WRW consults for Sanofi Genzyme and was an investigator in clinical studies and trials sponsored by Amicus Therapeutics, Protalix Corporation, Sanofi Genzyme and Takeda, and has received research funding from Amicus Therapeutics, Sanofi Genzyme, and Takeda; these activities are monitored and are in compliance with the conflict of interest policies at Emory University School of Medicine.
References
- 1.Germain D.P. Fabry disease. Orphanet J. Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz A., Germain D.P., Desnick R.J. Fabry disease revisited: management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018;123:416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Aerts J.M., Groener J.E., Kuiper S. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkin R.J., Bissler J., Banikazemi M. Characterization of Fabry disease in 352 pediatric patients in the Fabry Registry. Pediatr. Res. 2008;64:550–555. doi: 10.1203/PDR.0b013e318183f132. [DOI] [PubMed] [Google Scholar]
- 5.Laney D.A., Peck D.S., Atherton A.M. Fabry disease in infancy and early childhood: a systematic literature review. Genet. Med. 2015;17:323–330. doi: 10.1038/gim.2014.120. [DOI] [PubMed] [Google Scholar]
- 6.Hilz M.J., Arbustini E., Dagna L. Non-specific gastrointestinal features: could it be Fabry disease? Dig. Liver Dis. 2018;50:429–437. doi: 10.1016/j.dld.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann B., Schwarz M., Mehta A., Keshav S. Gastrointestinal symptoms in 342 patients with Fabry disease: prevalence and response to enzyme replacement therapy. Clin. Gastroenterol. Hepatol. 2007;5:1447–1453. doi: 10.1016/j.cgh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Zar-Kessler C., Karaa A., Sims K.B., Clarke V., Kuo B. Understanding the gastrointestinal manifestations of Fabry disease: promoting prompt diagnosis. Ther. Adv. Gastroenterol. 2016;9:626–634. doi: 10.1177/1756283X16642936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arends M., Wanner C., Hughes D. Characterization of classical and nonclassical Fabry disease: a multicenter study. J. Am. Soc. Nephrol. 2017;28:1631–1641. doi: 10.1681/ASN.2016090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germain D.P., Oliveira J.P., Bichet D.G. Use of a rare disease registry for establishing phenotypic classification of previously unassigned GLA variants: a consensus classification system by a multispecialty Fabry disease genotype-phenotype workgroup. J. Med. Genet. 2020 doi: 10.1136/jmedgenet-2019-106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desnick R.J., Ioannou Y.A., Eng C.M. α-Galactosidase A deficiency: Fabry disease. In: Valle D., Antonarakis S., Ballabio A., Beaudet A., Mitchell G.A., editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY: 2020. http://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225546984 (Accessed July 20, 2020) [Google Scholar]
- 12.Germain D.P., Brand E., Burlina A. Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: a multicenter Fabry Registry study. Mol. Genet. Genomic Med. 2018;6:492–503. doi: 10.1002/mgg3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavalle L., Thomas A.S., Beaton B. Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193550. e0193550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oder D., Liu D., Hu K. α-Galactosidase A genotype N215S induces a specific cardiac variant of Fabry disease. Circ. Cardiovasc. Genet. 2017;10 doi: 10.1161/CIRCGENETICS.116.001691. e001691. [DOI] [PubMed] [Google Scholar]
- 15.Germain D.P., Elliott P.M., Falissard B. The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: a systematic literature review by a European panel of experts. Mol. Genet. Metab. Rep. 2019;19:100454. doi: 10.1016/j.ymgmr.2019.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin E.R., Della Valle M.C., Wu X. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet. Med. 2017;19:430–438. doi: 10.1038/gim.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Politei J., Durand C., Schenone A.B., Torres A., Mukdsi J., Thurberg B.L. Chronic intestinal pseudo-obstruction. Did you search for lysosomal storage diseases? Mol. Genet. Metab. Rep. 2017;11:8–11. doi: 10.1016/j.ymgmr.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilera-Correa J.J., Madrazo-Clemente P., Martínez-Cuesta M.D.C. Lyso-Gb3 modulates the gut microbiota and decreases butyrate production. Sci. Rep. 2019;9:12010. doi: 10.1038/s41598-019-48426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi L., Vernon J., Kopach O. The Fabry disease-associated lipid lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci. Lett. 2015;594:163–168. doi: 10.1016/j.neulet.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Niño M.D., Aguilera-Correa J.J., Politei J., Esteban J., Requena T., Ortiz A. Unraveling the drivers and consequences of gut microbiota disruption in Fabry disease: the lyso-Gb3 link. Future Microbiol. 2020;15:227–231. doi: 10.2217/fmb-2019-0249. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox W.R., Feldt-Rasmussen U., Martins A.M. Improvement of Fabry disease-related gastrointestinal symptoms in a significant proportion of female patients treated with agalsidase beta: data from the Fabry Registry. JIMD Rep. 2018;38:45–51. doi: 10.1007/8904_2017_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng C.M., Guffon N., Wilcox W.R. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 23.Goker-Alpan O., Gambello M.J., Maegawa G.H. Reduction of plasma globotriaosylsphingosine levels after switching from agalsidase alfa to agalsidase beta as enzyme replacement therapy for Fabry disease. JIMD Rep. 2016;25:95–106. doi: 10.1007/8904_2015_483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krämer J., Lenders M., Canaan-Kühl S. Fabry disease under enzyme replacement therapy—new insights in efficacy of different dosages. Nephrol. Dial. Transplant. 2018;33:1362–1372. doi: 10.1093/ndt/gfx319. [DOI] [PubMed] [Google Scholar]
- 25.Schiffmann R., Bichet D.G., Jovanovic A. Migalastat improves diarrhea in patients with Fabry disease: clinical-biomarker correlations from the phase 3 FACETS trial. Orphanet J. Rare Dis. 2018;13:68. doi: 10.1186/s13023-018-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Echevarria L., Benistan K., Toussaint A. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016;89:44–54. doi: 10.1111/cge.12613. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox W.R., Oliveira J.P., Hopkin R.J. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol. Genet. Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Chiang H.L., Wang N.H., Song I.W. Genetic epidemiological study doesn’t support GLA IVS4+919G>A variant is a significant mutation in Fabry disease. Mol. Genet. Metab. 2017;121:22–27. doi: 10.1016/j.ymgme.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Hsu T.R., Hung S.C., Chang F.P. Later onset Fabry disease, cardiac damage progress in silence: experience with a highly prevalent mutation. J. Am. Coll. Cardiol. 2016;68:2554–2563. doi: 10.1016/j.jacc.2016.09.943. [DOI] [PubMed] [Google Scholar]
- 30.Liu H.C., Perrin A., Hsu T.R. Age at first cardiac symptoms in Fabry disease: association with a Chinese hotspot Fabry mutation (IVS4+919G>A), classical Fabry mutations, and sex in a Taiwanese population from the Fabry Outcome Survey (FOS) JIMD Rep. 2015;22:107–113. doi: 10.1007/8904_2015_418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler R.S., Stewart W.F., Liberman J.N., Ricci J.A., Zorich N.L. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig. Dis. Sci. 2000;45:1166–1171. doi: 10.1023/a:1005554103531. [DOI] [PubMed] [Google Scholar]
- 32.Germain D.P., Fouilhoux A., Decramer S. Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clin. Genet. 2019;96:107–117. doi: 10.1111/cge.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkin R.J., Jefferies J.L., Laney D.A. The management and treatment of children with Fabry disease: a United States-based perspective. Mol. Genet. Metab. 2016;117:104–113. doi: 10.1016/j.ymgme.2015.10.007. [DOI] [PubMed] [Google Scholar]