Abstract
Throughout the last years, gut-resident Foxp3+ regulatory T (Treg) cells have been associated with a growing number of tissue-specific functions in the intestine, comprising various aspects of gut immunity and physiology. Treg cells have pivotal roles in intestinal tolerance induction and host defense by actively controlling immune responses towards harmless dietary antigens and commensal microorganisms as well as towards invading pathogens. In addition to these immune-related roles, it has become increasingly clear that intestinal Treg cells also exert important non-immune functions in the gut, such as promoting local tissue repair and preserving the integrity of the epithelial barrier. Thereby, intestinal Treg cells critically contribute to the maintenance of tissue homeostasis. In order to account for this functional diversity, gut-resident Treg cells have specifically adapted to the intestinal tissue microenvironment. In this Review, we discuss the specialization of Treg cells in the intestine. We survey the different populations of gut-resident Treg cells focussing on their unique functions, phenotypes and distinct transcription factor dependencies.
Keywords: Treg cell, functions, phenotypes, intestinal tolerance, microbiota, diet, tissue homeostasis, intestinal epithelial cells
Introduction
One of the major functions of Foxp3+ Treg cells residing in non-lymphoid tissues is to control local inflammation. Given the overwhelming load of microbial and food antigens in the intestine, a cardinal function of gut-resident Foxp3+ Treg cells is to contain inflammatory immune responses to the microbiota and dietary factors, thereby establishing and maintaining intestinal immune tolerance. This essential role of gut-resident Foxp3+ Treg cells is highlighted by the development of immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome in patients who lack functional Foxp3+ Treg cells (1, 2). IPEX syndrome results in spontaneous inflammation of many organs, yet IPEX patients most frequently suffer from severe gastrointestinal disorders and food allergies (3, 4), emphasizing the key role of Foxp3+ Treg cells in establishing tolerance within the intestine. In addition to maintaining tolerance towards environmental antigens, gut-resident Foxp3+ Treg cells also shape immunity against invading intestinal pathogens by either suppressing or promoting inflammatory anti-pathogen immune responses, thus determining host susceptibility to intestinal infections. Furthermore, there is growing evidence that intestinal Foxp3+ Treg cells regulate many non-immunological processes in the gut. Indeed, important roles of Treg cells in e.g. local tissue repair and promotion of epithelial barrier functions are now emerging. These non-traditional roles have a profound impact on gut homeostasis and physiology and should therefore be considered as important facets of gut-resident Foxp3+ Treg cell function.
In accordance with this remarkable functional heterogeneity, gut-resident Foxp3+ Treg cells have acquired unique phenotypes, governed by specific transcriptional networks, that are tailored to the diverse challenges of the intestinal tissue microenvironment. In fact, the existence of functionally distinct Treg cell subsets in the gut, enabling a certain division of labour, can be considered as one of the key factors underlying intestinal homeostasis. In this regard, two developmental origins have been described for intestinal Foxp3+ Treg cells (5). The first occurs in the thymus, where thymus-derived Treg (tTreg) cells are generated following recognition of self-antigen by the T cell receptor. The second pathway of Treg cell generation is in peripheral tissues, such as the gut, where, under certain conditions, naïve CD4+ T cells develop into peripherally-derived Treg (pTreg) cells upon recognition of their cognate antigen, which is regarded as being non-self. Thus, intestinal pTreg cells are thought to be mainly responsible for tolerance to non-self-antigens, such as environmental antigens, whereas tTreg cells would be preferentially involved in controlling autoreactive responses. Phenotypically, expression of the markers Helios and Neuropilin 1 (Nrp1) by tTreg cells but not by pTreg cells can be used to distinguish these subsets (6–8), although this distinction is known to have exceptions (9–11).
In summary, in this Review, we will discuss the current understanding of Foxp3+ Treg cell adaptation in the intestine, including their specific functions, phenotypes and distinct transcription factor dependencies.
Treg Cells Mediate Tolerance to Environmental Antigens
Control of T Cell Responses to Microbial Antigens
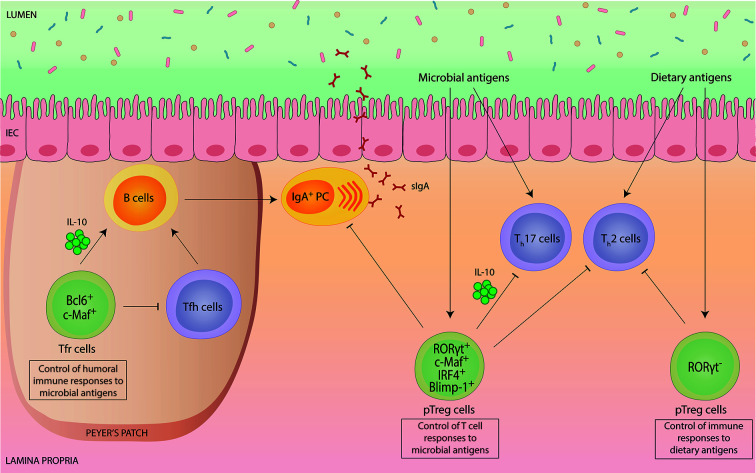
Since their initial discovery, Foxp3+ Treg cells were recognized as potent suppressors of T cell responses. Accordingly, gut-resident Treg cells play a pivotal role in suppressing effector T cell responses to the microbiota (Figure 1). A subpopulation of Foxp3+ Treg cells found primarily in the large intestine, characterized by co-expression of the RAR-related orphan receptor γt (RORγt), has been suggested to specifically mediate tolerance to the microbiota (12). Indeed, induction and maintenance of RORγt+ Treg cells critically depend on the microbiota (13–15) and/or specific metabolites thereof, such as microbial secondary bile acids (16–18) or short chain fatty acids (SCFA) (15, 19–21), although the specific role of SCFA for RORγt+ Treg cells is controversial (14). In addition to microbial metabolites, food-derived vitamin A seems to specifically drive RORγt+ Treg cells in the intestine (15). RORγt+ Treg cells comprise the majority of the Helios- Nrp1- Foxp3+ pTreg cells in the intestine that differentiate locally in response to commensal microbes in an antigen-specific manner (22–25). Consistently, RORγt+ pTreg cells are selectively decreased in germ-free and antibiotic-treated mice (14, 15). Likewise, during postnatal development, the generation of RORγt+ pTreg cells coincides with the increased uptake of luminal antigens and diversification of the microbiota during weaning, which is critical for the development of tolerance to gut bacteria (26, 27).
Figure 1.
Intestinal Foxp3+ Treg cells mediate tolerance to environmental antigens.Intestinal Foxp3+ Treg cells are potent suppressors of immune responses to environmental antigens, such as commensal microbes and harmless dietary antigens. Peripherally-induced Helios- Nrp1- Foxp3+ Treg (pTreg) cells co-expressing RORγt differentiate specifically in response to microbial antigens and have a crucial role in the suppression of microbiota-specific Th17 cell and IgA responses. In addition, RORγt+ pTreg cells also contribute to the suppression of aberrant intestinal Th2 cell responses. Molecularly, RORγt+ pTreg cell differentiation and function, such as production of immunosuppressive IL-10, depends on the transcriptional regulators c-Maf, IRF4 and Blimp-1. pTreg cells induced by dietary antigens lack RORγt expression. This Treg cell subset has a specific role in installing tolerance to ingested antigens by controlling food-specific Th cell responses. Within Peyer’s Patches, specialized Foxp3+ T follicular regulatory (Tfr) cells depend on Bcl6 and c-Maf and exhibit a dual role in controlling the germinal center reaction and subsequent IgA production. While Tfr cells exert a suppressive effect on T follicular helper (Tfh) cell expansion and function, they can also promote B cell-mediated IgA secretion via IL-10.
Functionally, RORγt+ pTreg cells express particularly high levels of IL-10, CTLA-4 and ICOS, indicative of a superior suppressive capacity (13, 28). Especially, secretion of the anti-inflammatory cytokine IL-10 by Treg cells has proven to be essential for maintaining intestinal tolerance, as evidenced by the development of spontaneous colitis upon genetic deletion of IL-10 selectively in Foxp3+ cells (29). RORγt+ pTreg cells were shown to control intestinal inflammation in different models of colitis (13–15), although the specific role of RORγt+ pTreg cells has remained unclear, with different studies reporting different conclusions. Whereas one study proposed that RORγt+ pTreg cells are crucial in controlling aberrant Th2 cell responses (15), a finding that is consistent with the selective Th2 cell dysregulation observed in mice that specifically lack pTreg cells (30), another report observed selective control of Th1 and Th17 cells (14). This suggests that the function of RORγt+ pTreg cells is highly context-dependent and most likely influenced by the indigenous microbiota.
Our own work as well as that of others demonstrated a specific role of gut-resident Foxp3+ Treg cells in controlling intestinal microbiota-specific Th17 cell responses (31–34). Importantly, we identified the transcription factor c-Maf to be essential for gut-resident Treg cells to differentiate into RORγt+ pTreg cells, to express IL-10 and to maintain intestinal tolerance (31–34). Notably, in comparison to RORγt, c-Maf appears to have a more substantial role for the control of microbiota-specific T cell responses, as inflammatory Th17 cell accumulation and spontaneous intestinal inflammation was only observed upon Treg cell-specific deletion of c-Maf but not of RORγt (31, 32). Consistent with this, c-Maf-deficiency in Treg cells also resulted in gut dysbiosis and breakdown of host-microbiota homeostasis (32).
In accordance with the fact that expression of c-Maf (and RORγt) in Treg cells is dependent on STAT3 activation (15, 32, 35), uncontrolled intestinal Th17 cell responses and spontaneous colitis were also detected in Treg cell-specific STAT3-deficient mice (36). In addition to c-Maf, RORγt+ pTreg cells also co-express high levels of the transcription factor Blimp-1 (37). Blimp-1, together with IRF4, critically contributes to the control of IL-10 production in Treg cells (38, 39), although Foxp3+ Treg cell-specific deletion of Blimp-1 was not sufficient to cause severe chronic intestinal inflammation as it was observed in CD4+ T cell-specific Blimp-1-deficient mice (40).
Importantly, although tolerance induction to microbial antigens has been mainly attributed to pTreg cells, there is evidence that also naturally occurring tTreg cells contribute to this process (41).
Control of Humoral Immune Responses to Microbial Antigens
In addition to the control of microbiota-specific T cell responses, gut-resident Foxp3+ Treg cells also play an important role in regulating humoral immune responses to the microbiota, such as intestinal immunoglobulin A (IgA) production and selection (Figure 1). IgA is the most abundant antibody in mucosal secretions and essential to intestinal homeostasis by both maintaining non-invasive commensal bacteria and neutralizing invasive pathogens (42). Early reports demonstrated a supportive role of Treg cells for intestinal IgA production based on the findings that depletion of Treg cells resulted in a rapid loss of intestinal IgA (43), and that Treg cells can contribute to the germinal center (GC) reaction in Peyer’s Patches (PPs) by conversion into T follicular helper (Tfh) cells (44). Later, a specialized subset of Foxp3+ Treg cells within follicles, termed T follicular regulatory (Tfr) cells, was identified (45–47). Tfr cells share many characteristics with Tfh cells, including the expression of PD-1, CXCR5, and dependency on the transcription factor Bcl6, which allows them to gain access to GCs while maintaining their suppressive capacity (45–47). Thus, Tfr cells can specifically suppress excessive Tfh cell-mediated B cell responses. Consistent with this, lack of Tfr cells was shown to result in dysregulated Tfh cells and IgA selection in PPs, thereby precipitating intestinal microbial dysbiosis (48).
Besides the suppressive effect of Tfr cells on GC, there is growing evidence that Tfr cells can also act as “helper” cells for humoral immune responses (49). Mechanistically, this positive effect of Tfr cells on GC is associated with Tfr cell-derived IL-10 production (50). Indeed, IL-10 is known to promote the proliferation of activated B cells and subsequent IgA production (51, 52), as well as the development and maintenance of intestinal microbiota-dependent IgA+ plasma cells (53). However, the relative contribution of Treg cell-derived IL-10 production for intestinal IgA production has remained unclear. We and others recently showed that intestinal Foxp3+ Treg cells require the transcription factor c-Maf to produce IL-10 and to adopt a Tfr cell phenotype (32, 33). Interestingly, Treg cell-specific deletion of c-Maf resulted in strongly elevated frequencies of lamina propria IgA+ plasma cells (32). While c-Maf clearly controls multiple Treg cell functions beyond their ability to produce IL-10, we also observed a slight increase in intestinal IgA levels in Treg cell-specific IL-10-deficient mice (32).
A very recent report discovered that microbiota-dependent RORγt+ pTreg cells and IgA+ B cells can regulate each other in a double-negative feedback loop that is transmitted through multiple generations (54). While these findings suggest that intestinal IgA level are also critically controlled by Foxp3+ Treg cells outside of follicles, the cellular and molecular entities involved in this reciprocal regulation remain to be defined. Notably, given that RORγt+ pTreg cell differentiation is dependent on c-Maf, these results also suggest that the hyper IgA phenotype of Treg cell-specific c-Maf-deficient mice is at least partially driven by the lack of direct suppression of RORγt+ pTreg cells on IgA (32). Collectively, these findings suggest a highly context-dependent function of Foxp3+ Treg cells for intestinal IgA regulation. Clearly, more work is needed to precisely define the role of Treg cells in regulating humoral immunity in the gut.
Control of Immune Responses to Dietary Antigens
Aside from microbial antigens, dietary antigens represent a major source of natural antigenic stimulation in the gut. Tolerance to food antigens is characterized by the absence and/or suppression of antigen-specific immune responses, a phenomenon known as oral tolerance. Foxp3+ Treg cells play a central role in installing oral tolerance, as evidenced by the fact that loss-of-function mutations affecting Foxp3 in mice and humans result in spontaneous severe allergic inflammation, such as food allergies (FA) (4, 55). Likewise, inducible depletion or functional impairment of Foxp3+ Treg cells in mice tolerant to ovalbumin was shown to be sufficient to abolish oral tolerance, demonstrating a dominant role of antigen-specific Treg cells in conferring tolerance to ingested antigens (56, 57).
Among the intestinal Foxp3+ Treg cell populations, pTreg cells but not tTreg cells, appear to be essential for oral tolerance induction (5, 30, 58) (Figure 1). More specifically, analysis of germ-free mice fed with an elemental diet devoid of dietary antigens identified a specific pTreg cell population that was unaffected by the absence of the gut microbiota but disappeared upon antigen-free diet (59). These food-induced pTreg cells were distinguishable from microbiota-induced pTreg cells by their lack of RORγt expression (59). Importantly, without this population, mice showed an increased susceptibility to FA (59). Notably, although directed against the microbiota, ablation of RORγt+ pTreg cells also rendered mice more susceptible to FA (60). Vice versa, FA patients manifest dynamic microbial dysbiosis and RORγt+ pTreg cell-inducing microbiota therapy in mice promoted restoration of oral tolerance in FA (60), demonstrating a hitherto unrecognized mechanistical link between Treg cell-mediated tolerance induction to microbial and dietary antigens.
Other examples of how nutritional signals impact on mucosal immune responses stem from studies focussing on the manipulation of the host nutritional status (61–63). Intermittent fasting, for instance, was shown to strongly affect the abundance and functionality of intestinal lymphocytes, including Treg cells, as well as the susceptibility to inflammatory diseases (61, 62), highlighting the close link between diet, Treg cells and intestinal immune homeostasis.
Treg Cells Control Intestinal Inflammation and Host Defense
Control of Intestinal Inflammation and Tissue Damage
Gut-resident Foxp3+ Treg cells not only operate during homeostasis to establish and maintain a tolerogenic environment in the intestine. In fact, Treg cells are able to specifically sense inflammatory signals, which leads to their activation and heightening of their suppressive capacity to counteract e.g. inflammation and inflammation-driven tissue damage (64, 65).
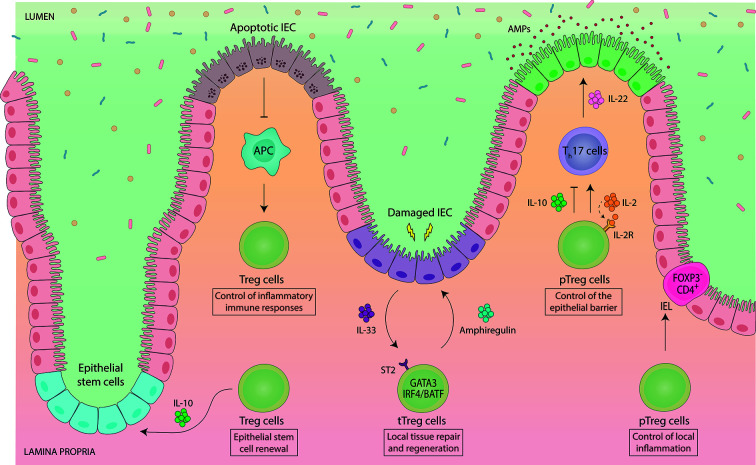
A substantial fraction of intestinal Foxp3+ Treg cells has a phenotypic signature specifically linked to tissue repair, such as expression of ST2, the receptor for the alarmin IL-33, and the growth factor amphiregulin (66) (Figure 2). In addition, enhanced production and activation of IL-10 and TGF-ß has been detected in ST2+ Treg cells, demonstrating their highly activated and suppressive phenotype (67). ST2+ Treg cells co-express the canonical transcription factor of type 2 immunity GATA3, are mostly Helios+/Nrp1+ and are unaffected by the absence of the gut microbiota, indicative of a thymic origin (15). GATA3 directly interacts with Foxp3 both on protein and gene level to regulate expression of Foxp3 itself as well as the downstream Foxp3-dependent transcriptional program (68, 69). Developmentally, ST2+ tTreg cells rely on IRF4 and BATF for their differentiation (70–72).
Figure 2.
Intestinal Foxp3+ Treg cells engage in a functional crosstalk with intestinal epithelial cells. Gut-resident Foxp3+ Treg cells support tissue physiology by maintaining intestinal epithelial cell (IEC) homeostasis. Vice versa, IEC-derived signals control the abundance and functionality of intestinal Treg cells. Treg cell-derived IL-10 promotes the renewal of intestinal epithelial stem cells, while apoptotic IEC negatively regulate the proliferation and abundance of intestinal Treg cells. Thymically-induced Helios+ Nrp1+ Foxp3+ Treg (tTreg) cells co-expressing GATA3 have been implicated in local tissue repair and regeneration. GATA3+ tTreg cells express ST2, by which they can sense IL-33, an alarmin, which is produced by IEC e.g. upon infection-induced damage. In response, ST2+ GATA3+ tTreg cells get activated, expand and produce the growth factor amphiregulin. Developmentally, ST2+ GATA3+ tTreg cells rely on IRF4 and BATF for their differentiation. pTreg cells indirectly contribute to the maintenance of the epithelial barrier by controlling the abundance of IL-22-producing Th17 cells. IL-22 directly acts on IEC to control IEC growth, permeability, production of mucus and antimicrobial proteins (AMPs). While Treg cells are mostly presented as suppressors of Th17 cells, they can also promote Th17 cell responses via consumption of IL-2 during mucosal infections. Intestinal pTreg cells also show intra-tissue specialization. Upon migration to the IEC barrier, pTreg cells downregulate Foxp3 and become CD4+ Foxp3- intraepithelial (IEL) T cells in order to control local inflammation, demonstrating a dominant role of the IEC microenvironment in controlling Treg cell lineage stability and plasticity.
Since IL-33 is primarily produced by intestinal epithelial cells upon local damage (73), and ST2+ tTreg cells exhibit high expression of the gut-homing receptors CCR9 and α4β7 (67, 74), the prevailing model for ST2+ tTreg cell function is that they specifically home to sites of damage in the intestine and mediate repair, although this has not been formally proven yet. In support of a specific role of ST2+ GATA3+ tTreg cells in controlling local inflammation, it was shown that GATA3 expression was not required at steady state, but was essential under inflammatory conditions to enable Treg cell accumulation at inflammatory sites (75). Furthermore, Treg cell-specific GATA3 deletion led to spontaneous inflammation in mice, including development of intestinal pathologies (68, 69), although these disorders were not present in young mice, but only observed upon aging (ca. after 6 months) (68, 69, 75, 76). Notably, since Helios+ Nrp1+ tTreg cells are thought to be positively selected against self-antigens, they may also be involved in preventing autoimmune inflammation in the gut. In an experimental system, in which a model self-antigen was specifically expressed in the intestinal epithelium, activation and expansion of autoreactive T cells was inhibited by self-antigen-specific tTreg cells (77).
While ST2+ GATA3+ tTreg cells are clearly linked to the regulation of type 2 inflammation, type 1 inflammatory immune responses appear to be specifically controlled by Foxp3+ Treg cells co-expressing the transcription factor T-bet (78, 79). In fact, type 1 inflammation selectively induces T-bet expression in Treg cells via IFN-γ- or IL-27-dependent signalling to endow Treg cells with the homeostatic and migratory properties required for the suppression of type 1 immune responses (76, 78–80). Functionally, T-bet+ Treg cells were shown to limit Th1-mediated autoimmune- or infection-induced pathology in the intestine but also at extra-intestinal sites (76, 78–80). However, T-bet+ Foxp3+ Treg cells may also acquire pro-inflammatory IFN-γ co-expression during intestinal inflammation, thereby promoting gut inflammatory diseases (81, 82).
Control of Host Defense Against Intestinal Pathogens
Foxp3+ Treg cells are also important in regulating host defense against invading intestinal pathogens. In this context, it has become clear that the functional role of Treg cell-mediated control of immune responses to infectious agents is highly context-dependent, ranging from detrimental to advantageous outcomes for the host.
For instance, during intestinal helminth infection, Foxp3+ Treg cells are actively induced by the pathogen leading to a state of hyporesponsiveness, which is key for parasite persistence (83, 84). However, expansion of Treg cells not only enhances parasite survival but also protects the host from excessive type 2 inflammatory immune responses against the pathogen, thereby minimizing `collateral damage` to the gut tissue (85, 86). Notably, upon helminth infection, selective expansion of Foxp3+ Helios+ tTreg as well as Foxp3+ Helios- pTreg cells has been described (87), suggesting that control of anti-helminth immunity involves multiple pathways of Treg cell recruitment. Indeed, ST2+ tTreg cells, activated and expanded by helminth-induced epithelial damage-mediated release of IL-33 (88), as well as pTreg cells, induced by helminth-derived secretory products (89), were shown to contribute to the control of mucosal helminth infection.
Importantly, in addition to suppressing anti-pathogen immunity, Foxp3+ Treg cells can also directly promote host-protective immune responses. Upon mucosal infections with Citrobacter rodentium or Candida albicans, Treg cells were shown to support protective Th17 cell responses by consumption of IL-2 (90–92), a potent inhibitor of Th17 cell differentiation (93). This supportive role stands in opposition to the suppressive function of Treg cells for microbiota-specific Th17 cell responses during homeostasis (see section above) (31–34, 36). Nevertheless, recent data indicate that Treg cells also participate in the inhibition of inflammatory pathogen-specific Th17 cell responses. For instance, susceptibility to infection with the intestinal protozoan parasite Giardia lamblia correlated with increased RORγt+ pTreg to Th17 cell ratios, suggesting that RORγt+ pTreg cells also contribute to the suppression of Th17 cells during intestinal infection, thereby hampering protective immunity (94). Similarly, induction of RORγt+ pTreg cells in response to the pathobiont Helicobacter hepaticus prevented expansion of pathogenic antigen-specific Th17 cells, thus enabling immunological tolerance (31).
Treg Cells Preserve Gut Physiology
Control of Epithelial Barrier Functions
A novel concept in immunology is that tissue-resident immune cells not only mediate immune homeostasis and host defense but also critically contribute to the maintenance of organismal physiology. In this regard, essential roles of Foxp3+ Treg cells in sustaining homeostasis of diverse tissues are now emerging, although much knowledge about these non-canonical tissue-specific functions is still to be obtained (95). In the intestine, Foxp3+ Treg cells are involved in preserving the function and homeostasis of intestinal epithelial cells (IEC) (Figure 2). Positioned as a physical barrier between the intestinal lumen and the immune cells in the lamina propria, IEC spatially segregate host and microbiota (96). At the same time IEC facilitate the crosstalk between microbes and host cells by sensing and responding to immune as well as microbial stimuli (96).
Foxp3+ Treg cells promote IEC homeostasis by supporting epithelial stem-cell renewal (97). In an in vitro organoid system, addition of Treg cells or their major effector cytokine IL-10 supported stem-cell renewal (97). In vivo, depletion of Treg cells decreased intestinal stem cell proportions while higher differentiation rates of IEC were observed (97). Interestingly, IL-10 was also shown to maintain IEC function by regulating their fucosylation and by protecting IEC from endoplasmic reticulum stress as well as from Fas-mediated apoptosis (98–101), although the precise cellular source of IL-10 was not elucidated in these studies. In addition to direct effects on IEC, intestinal Foxp3+ Treg cells shape IEC function also indirectly by controlling e.g. the abundance of IL-22-producing Th17 cells in the gut (31–34, 36, 90). IEC constitutively express the IL-22 receptor, and IL-22 signalling in IEC is critical for maintaining the integrity of the mucosal barrier (102).
Conversely to the effect of Treg cells on IEC, signals derived from IEC also influence the function and abundancy of Foxp3+ Treg cells in the lamina propria, thereby establishing a reciprocal regulatory circuit (Figure 2). For instance, intestinal ST2+ GATA3+ tTreg cell function is boosted by the release of IL-33 upon IEC damage (see section above) (66, 67). Another example comes from a study analysing the effects of IEC apoptosis on intestinal Treg cell homeostasis, in which apoptotic IEC reduced the abundancy of gut-resident Foxp3+ Treg cells, thus lowering the threshold for inflammatory immune responses (103). Even expansion of intestinal Treg cells induced by direct antigen-driven interaction with IEC has been suggested (104, 105), although the role of IEC antigen presentation in shaping intestinal immunity has not been thoroughly explored so far. Recently, another unconventional interaction of Treg cells with IEC has been identified. Upon migration to the epithelium, intestinal pTreg cells were shown to downregulate Foxp3 and convert to intraepithelial (IEL) CD4+ T cells in order to control intestinal inflammation (106). These findings reveal an unprecedented phenotypic and functional adaptability of intestinal Treg cells. Moreover, they demonstrate a dominant role of the IEC microenvironment in controlling Treg cell lineage stability and plasticity, highlighting the close interdependence between Treg cells and IEC (106).
Concluding Remarks
It is now well established that intestinal Foxp3+ Treg cells are critical for the tolerance to commensal microbes, the induction of oral tolerance and for host defense against enteric pathogens, thereby installing gut immune homeostasis. Beyond these classical immune-related functions, novel roles of Treg cells in gut organismal homeostasis are emerging, unrevealing a greater functional and phenotypic diversity of the intestinal Treg cell compartment than was previously recognized. Given these non-canonical functions in tissue maintenance, regeneration and repair, intestinal Treg cells can be considered not only as mediators of immunological tolerance but also of disease tolerance, a concept, which encompasses multiple mechanisms that help decrease host susceptibility to tissue damage during pro-inflammatory immune responses (107, 108).
Clearly, we are just beginning to understand the impact of Treg cells on gut physiology and much remains to be uncovered about the relationship and crosstalk between Treg cells and distinct intestinal tissue cells, such as endothelial, epithelial, stromal or neuronal populations. From a translational point of view, impaired intestinal Treg cell functionality is associated with chronic inflammatory diseases, such as inflammatory bowel disease and food allergy. Thus, further explorations into the characteristics, dependencies and targets of different intestinal Treg cell subsets will undoubtedly help to develop more targeted manipulation strategies, aiming at a selective enhancement or inhibition of Treg cell function in a context- and tissue-specific manner.
Author Contributions
CC and CN wrote the manuscript. CC generated the figures. CN provided the overall design and guidance for this Review. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet (2001) 27:18–20. 10.1038/83707 [DOI] [PubMed] [Google Scholar]
- 2. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet (2001) 27:20–1. 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- 3. Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol (2003) 15:430–5. 10.1097/00002281-200307000-00010 [DOI] [PubMed] [Google Scholar]
- 4. Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, et al. Severe Food Allergy as a Variant of IPEX Syndrome Caused by a Deletion in a Noncoding Region of the FOXP3 Gene. Gastroenterology (2007) 132:1705–17. 10.1053/j.gastro.2007.02.044 [DOI] [PubMed] [Google Scholar]
- 5. Curotto de Lafaille MA, Lafaille JJ. Natural and Adaptive Foxp3+ Regulatory T Cells: More of the Same or a Division of Labor? Immunity (2009) 30:626–35. 10.1016/j.immuni.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 6. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros Transcription Factor Family Member, Differentiates Thymic-Derived from Peripherally Induced Foxp3+ T Regulatory Cells. J Immunol (2010) 184:3433–41. 10.4049/jimmunol.0904028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosagenerated induced Foxp3+ T reg cells. J Exp Med (2012) 209:1723–42. 10.1084/jem.20120914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med (2012) 209:1713–22. 10.1084/jem.20120822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One (2011) 6:e24226. 10.1371/journal.pone.0024226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottschalk RA, Corse E, Allison JP. Expression of Helios in Peripherally Induced Foxp3 + Regulatory T Cells. J Immunol (2012) 188:976–80. 10.4049/jimmunol.1102964 [DOI] [PubMed] [Google Scholar]
- 11. Pratama A, Schnell A, Mathis D, Benoist C. Developmental and cellular age direct conversion of CD4+ T cells into RORγ+ or Helios+ colon Treg cells. J Exp Med (2020) 217(1):e20190428. 10.1084/jem.20190428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohnmacht C. Tolerance to the Intestinal Microbiota Mediated by ROR(γt)+ Cells. Trends Immunol (2016) 37:477–86. 10.1016/j.it.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 13. Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, et al. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol (2016) 9:444–57. 10.1038/mi.2015.74 [DOI] [PubMed] [Google Scholar]
- 14. Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Sci (80- ) (2015) 349:993–7. 10.1126/science.aaa9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Sci (80- ) (2015) 349:989–93. 10.1126/science.aac4263 [DOI] [PubMed] [Google Scholar]
- 16. Hang S, Paik D, Yao L, Kim E, Jamma T, Lu J, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature (2019) 576:143–8. 10.1038/s41586-019-1785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature (2020) 581:475–9. 10.1038/s41586-020-2193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature (2020) 577:410–5. 10.1038/s41586-019-1865-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly -Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Sci (80- ) (2013) 341:569–73. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature (2013) 504:446–50. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 21. Arpaia N, Campbell C, Fan X, Dikiy S, Van Der Veeken J, Deroos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature (2013) 504:451–5. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature (2011) 478:250–4. 10.1038/nature10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon BD, Hsieh C-S. Antigen-Specific Development of Mucosal Foxp3 + RORγt + T Cells from Regulatory T Cell Precursors. J Immunol (2016) 197:3512–9. 10.4049/jimmunol.1601217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishio J, Baba M, Atarashi K, Tanoue T, Negishi H, Yanai H, et al. Requirement of full TCR repertoire for regulatory T cells to maintain intestinal homeostasis. Proc Natl Acad Sci U S A (2015) 112:12770–5. 10.1073/pnas.1516617112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR, et al. Rapid and Efficient Generation of Regulatory T Cells to Commensal Antigens in the Periphery. Cell Rep (2016) 17:206–20. 10.1016/j.celrep.2016.08.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Coughlin PE, McCrate S, et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol (2017) 2:aao1314. 10.1126/sciimmunol.aao1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Déjardin F, et al. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity (2019) 50:1276–1288.e5. 10.1016/j.immuni.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 28. Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J Exp Med (2008) 205:1381–93. 10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T Cell-Derived Interleukin-10 Limits Inflammation at Environmental Interfaces. Immunity (2008) 28:546–58. 10.1016/j.immuni.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 30. Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature (2012) 482:395–9. 10.1038/nature10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature (2018) 554:373–7. 10.1038/nature25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, et al. c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host–microbiota homeostasis. Nat Immunol (2019) 20:471–81. 10.1038/s41590-019-0316-2 [DOI] [PubMed] [Google Scholar]
- 33. Wheaton JD, Yeh C-H, Ciofani M. Cutting Edge: c-Maf Is Required for Regulatory T Cells To Adopt RORγt + and Follicular Phenotypes. J Immunol (2017) 199:3931–6. 10.4049/jimmunol.1701134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imbratta C, Leblond MM, Bouzourène H, Speiser DE, Velin D, Verdeil G, et al. Maf deficiency in T cells dysregulates T reg - TH17 balance leading to spontaneous colitis. Sci Rep (2019) 9:1–13. 10.1038/s41598-019-42486-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussein H, Denanglaire S, Van Gool F, Azouz A, Ajouaou Y, El-Khatib H, et al. Multiple Environmental Signaling Pathways Control the Differentiation of RORγt-Expressing Regulatory T Cells. Front Immunol (2020) 10:3007. 10.3389/fimmu.2019.03007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a stat3-dependent manner. Sci (80- ) (2009) 326:986–91. 10.1126/science.1172702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogawa C, Bankoti R, Nguyen T, Hassanzadeh-Kiabi N, Nadeau S, Porritt RA, et al. Blimp-1 Functions as a Molecular Switch to Prevent Inflammatory Activity in Foxp3+RORγt+ Regulatory T Cells. Cell Rep (2018) 25:19–28.e5. 10.1016/j.celrep.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol (2011) 12:304–12. 10.1038/ni.2006 [DOI] [PubMed] [Google Scholar]
- 39. Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol (2006) 7:457–65. 10.1038/ni1320 [DOI] [PubMed] [Google Scholar]
- 40. Bankoti R, Ogawa C, Nguyen T, Emadi L, Couse M, Salehi S, et al. Differential regulation of Effector and Regulatory T cell function by Blimp1. Sci Rep (2017) 7:12078. 10.1038/s41598-017-12171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature (2013) 497:258–62. 10.1038/nature12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev (2014) 260:76–85. 10.1111/imr.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A (2009) 106:19256–61. 10.1073/pnas.0812681106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3 + T cells in gut Peyer’s patches. Sci (80- ) (2009) 323:1488–92. 10.1126/science.1169152 [DOI] [PubMed] [Google Scholar]
- 45. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med (2011) 17:983–8. 10.1038/nm.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med (2011) 17:975–82. 10.1038/nm.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the Germinal Center Reaction by Foxp3 + Follicular Regulatory T Cells. J Immunol (2011) 187:4553–60. 10.4049/jimmunol.1101328 [DOI] [PubMed] [Google Scholar]
- 48. Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, et al. Foxp3+ T Cells Regulate Immunoglobulin A Selection and Facilitate Diversification of Bacterial Species Responsible for Immune Homeostasis. Immunity (2014) 41:152–65. 10.1016/j.immuni.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 49. Xie MM, Dent AL. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front Immunol (2018) 9:1536. 10.3389/fimmu.2018.01536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laidlaw BJ, Lu Y, Amezquita RA, Weinstein JS, Vander Heiden JA, Gupta NT, et al. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol (2017) 2(16):eaan4767. 10.1126/sciimmunol.aan4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A (1992) 89:1890–3. 10.1073/pnas.89.5.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J, et al. Interleukln 10 and transforming growth factor β cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med (1992) 175:671–82. 10.1084/jem.175.3.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kunisawa J, Gohda M, Hashimoto E, Ishikawa I, Higuchi M, Suzuki Y, et al. Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun (2013) 4:1772. 10.1038/ncomms2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramanan D, Sefik E, Galván-Peña S, Wu M, Yang L, Yang Z, et al. An Immunologic Mode of Multigenerational Transmission Governs a Gut Treg Setpoint. Cell (2020) 181:1276–90.e13. 10.1016/j.cell.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, et al. Allergic dysregulation and hyperimmunoglobulinemia e in Foxp3 mutant mice. J Allergy Clin Immunol (2005) 116:1106–15. 10.1016/j.jaci.2005.08.046 [DOI] [PubMed] [Google Scholar]
- 56. Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity (2011) 34:237–46. 10.1016/j.immuni.2011.01.016 [DOI] [PubMed] [Google Scholar]
- 57. Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-Cell-like lineage impairs oral tolerance and promotes food allergy. Immunity (2015) 42:512–23. 10.1016/j.immuni.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto De Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest (2005) 115:1923–33. 10.1172/JCI24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Sci (80- ) (2016) 351:858–63. 10.1126/science.aac5560 [DOI] [PubMed] [Google Scholar]
- 60. Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med (2019) 25:1164–74. 10.1038/s41591-019-0461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab (2018) 27:1222–35.e6. 10.1016/j.cmet.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nagai M, Noguchi R, Takahashi D, Morikawa T, Koshida K, Komiyama S, et al. Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell (2019) 178:1072–87.e14. 10.1016/j.cell.2019.07.047 [DOI] [PubMed] [Google Scholar]
- 63. Visekruna A, Hartmann S, Sillke YR, Glauben R, Fischer F, Raifer H, et al. Intestinal development and homeostasis require activation and apoptosis of diet-reactive T cells. J Clin Invest (2019) 129:1972–83. 10.1172/JCI98929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arvey A, Van Der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol (2014) 15:580–7. 10.1038/ni.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van der Veeken J, Gonzalez AJ, Cho H, Arvey A, Hemmers S, Leslie CS, et al. Memory of Inflammation in Regulatory T Cells. Cell (2016) 166:977–90. 10.1016/j.cell.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature (2014) 513:564–8. 10.1038/nature13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Siede J, Fröhlich A, Datsi A, Hegazy AN, Varga DV, Holecska V, et al. IL-33 receptor-expressing regulatory t cells are highly activated, Th2 biased and suppress CD4 T Cell proliferation through IL-10 and TGFβ Release. PLoS One (2016) 11(8):e0161507. 10.1371/journal.pone.0161507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Y, Su MA, Wan YY. An Essential Role of the Transcription Factor GATA-3 for the Function of Regulatory T Cells. Immunity (2011) 35:337–48. 10.1016/j.immuni.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rudra D, Deroos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol (2012) 13:1010–9. 10.1038/ni.2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hayatsu N, Miyao T, Tachibana M, Murakami R, Kimura A, Kato T, et al. Analyses of a Mutant Foxp3 Allele Reveal BATF as a Critical Transcription Factor in the Differentiation and Accumulation of Tissue Regulatory T Cells. Immunity (2017) 47:268–83.e9. 10.1016/j.immuni.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 71. Delacher M, Imbusch CD, Hotz-Wagenblatt A, Mallm JP, Bauer K, Simon M, et al. Precursors for Nonlymphoid-Tissue Treg Cells Reside in Secondary Lymphoid Organs and Are Programmed by the Transcription Factor BATF. Immunity (2020) 52:295–312.e11. 10.1016/j.immuni.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol (2015) 16:276–85. 10.1038/ni.3085 [DOI] [PubMed] [Google Scholar]
- 73. Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity (2015) 42:1005–19. 10.1016/j.immuni.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang C, Thangamani S, Kim M, Gu BH, Lee JH, Taparowsky EJ, et al. BATF is required for normal expression of gut-homing receptors by T helper cells in response to retinoic acid. J Exp Med (2013) 210:475–89. 10.1084/jem.20121088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wohlfert EA, Zhu J, Belkaid Y, Wohlfert EA, Grainger JR, Bouladoux N, et al. GATA3 controls Foxp3 + regulatory T cell fate during inflammation in mice. J Clin Invest (2011) 121:4503–15. 10.1172/JCI57456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol (2015) 16:197–206. 10.1038/ni.3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, et al. CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity (2015) 43:896–908. 10.1016/j.immuni.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol (2009) 10:595–602. 10.1038/ni.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Levine AG, Medoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature (2017) 546:421–5. 10.1038/nature22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hall AOH, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, et al. The Cytokines Interleukin 27 and Interferon-γ Promote Distinct Treg Cell Populations Required to Limit Infection-Induced Pathology. Immunity (2012) 37:511–23. 10.1016/j.immuni.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cao AT, Yao S, Stefka AT, Liu Z, Qin H, Liu H, et al. TLR4 regulates IFN-γ and IL-17 production by both thymic and induced Foxp3 + T regs during intestinal inflammation. J Leukoc Biol (2014) 96:895–905. 10.1189/jlb.3A0114-056RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Di Giovangiulio M, Rizzo A, Franzè E, Caprioli F, Facciotti F, Onali S, et al. Tbet Expression in Regulatory T Cells Is Required to Initiate Th1-Mediated Colitis. Front Immunol (2019) 10:2158. 10.3389/fimmu.2019.02158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol (2016) 138:666–75. 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. White MPJ, McManus CM, Maizels RM. Regulatory T-cells in helminth infection: induction, function and therapeutic potential. Immunology (2020) 160:248–60. 10.1111/imm.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. D’Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T Cells: A Role in the Control of Helminth-Driven Intestinal Pathology and Worm Survival. J Immunol (2009) 182:2340–8. 10.4049/jimmunol.0802767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rausch S, Huehn J, Loddenkemper C, Hepworth MR, Klotz C, Sparwasser T, et al. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol (2009) 39:3066–77. 10.1002/eji.200939644 [DOI] [PubMed] [Google Scholar]
- 87. Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: The regulators and the regulated. Trends Immunol (2012) 33:181–9. 10.1016/j.it.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 88. Obata-Ninomiya K, Ishiwata K, Nakano H, Endo Y, Ichikawa T, Onodera A, et al. CXCR6+ST2+ memory T helper 2 cells induced the expression of major basic protein in eosinophils to reduce the fecundity of helminth. Proc Natl Acad Sci U S A (2018) 115:E9849–58. 10.1073/pnas.1714731115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med (2010) 207:2331–41. 10.1084/jem.20101074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang Z, Friedrich C, Hagemann SC, Korte WH, Goharani N, Cording S, et al. Regulatory T cells promote a protective Th17-associated immune response to intestinal bacterial infection with C. rodentium. Mucosal Immunol (2014) 7:1290–301. 10.1038/mi.2014.17 [DOI] [PubMed] [Google Scholar]
- 91. Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernández-Santos N, et al. CD4+CD25+Foxp3+ Regulatory T Cells Promote Th17 Cells In Vitro and Enhance Host Resistance in Mouse Candida albicans Th17 Cell Infection Model. Immunity (2011) 34:422–34. 10.1016/j.immuni.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, et al. Foxp3+ regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity (2011) 34:409–21. 10.1016/j.immuni.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 93. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity (2007) 26:371–81. 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 94. Yordanova IA, Cortés A, Klotz C, Kühl AA, Heimesaat MM, Cantacessi C, et al. RORγt+ Treg to Th17 ratios correlate with susceptibility to Giardia infection. Sci Rep (2019) 9(1):20328. 10.1038/s41598-019-56416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol (2016) 34:609–33. 10.1146/annurev-immunol-032712-095948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med (2017) 49:e338. 10.1038/emm.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell (2018) 175:1307–20.e22. 10.1016/j.cell.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Goto Y, Lamichhane A, Kamioka M, Sato S, Honda K, Kunisawa J, et al. IL-10-producing CD4+ T cells negatively regulate fucosylation of epithelial cells in the gut. Sci Rep (2015) 5:2015. 10.1038/srep15918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, et al. Interleukin-10 Blocked Endoplasmic Reticulum Stress in Intestinal Epithelial Cells: Impact on Chronic Inflammation. Gastroenterology (2007) 132:190–207. 10.1053/j.gastro.2006.10.030 [DOI] [PubMed] [Google Scholar]
- 100. Hasnain SZ, Tauro S, Das I, Tong H, Chen AH, Jeffery PL, et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology (2013) 144(2):357–68.e9. 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 101. Bharhani MS, Borojevic R, Basak S, Ho E, Zhou P, Croitoru K. IL-10 protects mouse intestinal epithelial cells from Fas-induced apoptosis via modulating Fas expression and altering caspase-8 and FLIP expression. Am J Physiol - Gastrointest Liver Physiol (2006) 291(5):G820-9. 10.1152/ajpgi.00438.2005 [DOI] [PubMed] [Google Scholar]
- 102. Keir ME, Yi T, Lu TT, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med (2020) 217(3):e20192195. 10.1084/jem.20192195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nakahashi-Oda C, Udayanga KGS, Nakamura Y, Nakazawa Y, Totsuka N, Miki H, et al. Apoptotic epithelial cells control the abundance of T reg cells at barrier surfaces. Nat Immunol (2016) 17:441–50. 10.1038/ni.3345 [DOI] [PubMed] [Google Scholar]
- 104. Westendorf AM, Fleissner D, Groebe L, Jung S, Gruber AD, Hansen W, et al. CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut (2009) 58:211–9. 10.1136/gut.2008.151720 [DOI] [PubMed] [Google Scholar]
- 105. Thelemann C, Eren RO, Coutaz M, Brasseit J, Bouzourene H, Rosa M, et al. Interferon-γ induces expression of MHC class II on intestinal epithelial cells and protects mice from colitis. PLoS One (2014) 9(1):e86844. 10.1371/journal.pone.0086844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sujino T, London M, Van Konijnenburg DPH, Rendon T, Buch T, Silva HM, et al. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Sci (80- ) (2016) 352:1581–6. 10.1126/science.aaf3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science (2012) 335:936–41. 10.1126/science.1214935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol (2017) 17:83–96. 10.1038/nri.2016.136 [DOI] [PubMed] [Google Scholar]