Paravalvular leak (PVL) remains a key limitation of transseptal transcatheter mitral valve replacement (TMVR) using the SAPIEN 3 valve (Edwards Lifesciences, Irvine, California), especially in prior surgical rings (VIR) or mitral annular calcification (VIM) (1,2). PVL often results from valve malalignment and imperfect implantation depth that allow leakage around the fabric skirt. Valve alignment is determined mainly by the position of the rigid delivery guidewire in the left ventricular (LV) apex.
We hypothesized that improving SAPIEN 3 valve coaxiality during VIR and VIM procedures would decrease PVL incidence. Specifically, the mitral annular-to-LV apical “Emory angle” on cardiac computed tomography (CT) predicts the noncoaxial S3 valve canting angle after implantation (Figure 1A). For each SAPIEN 3 valve, there is a maximum intrinsic canting angle after which there will be PVL, related to the external skirt height and valve width (Figures 1B and 1C), calculated as:
FIGURE 1. The Emory TMVR Angle.
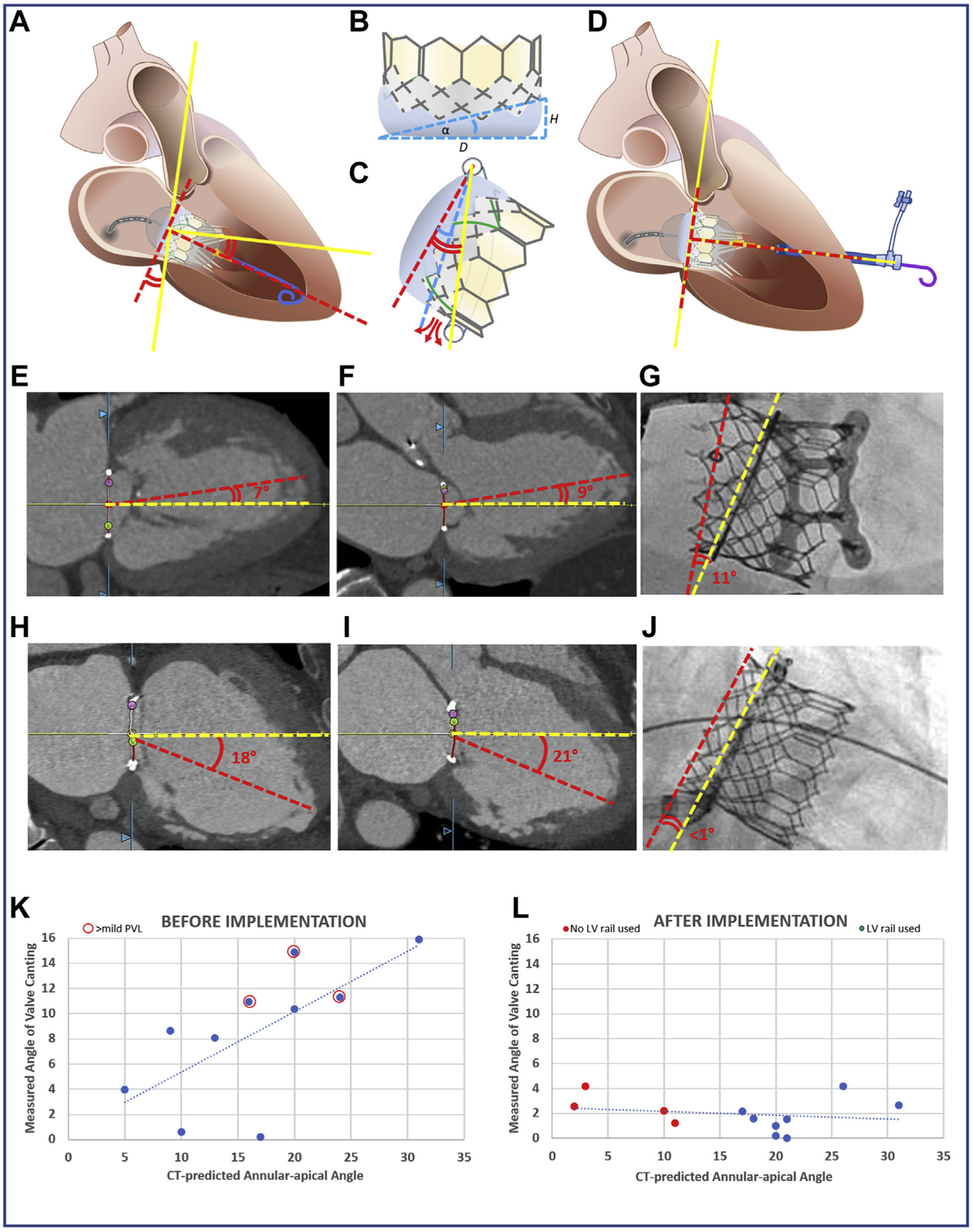
(A) Discordance between mitral annular centerline (yellow lines) and valve orientation imposed by guidewire position within the LV apex (red dashed lines). (B) Valve intrinsic angle α determined by external skirt height. (C) When annular-apical “Emory” angle (red curves) exceeds α (blue curve), annular skirt apposition is not possible, causing PVL (red arrows). (D) The valve is oriented perpendicular to the annual plane using an exteriorized apical guidewire (solid yellow line). Two representative cases demonstrate the canting angle before (E to G) and after (H to J) adopting the new strategy. (E to G) CT-derived Emory angles in (E) bicommissural (medial-lateral) and (F) LV outflow tract (anteroposterior) planes with (G) noncoaxial (canted) deployment evident on fluoroscopy in a case before we adopted selective guidewire exteriorization. (H to J) A case that illustrates improved valve alignment despite large Emory angle α by externalizing the guidewire through a percutaneous para-apical sheath. The graphs show predicted (horizontal-axis) and observed (vertical-axis) valve canting angles before (K) and after (L) adopting the exteriorized LV guidewire strategy for cases when the Emory angle approached 15°. The incidence of >mild PVL (red circles) is reduced. CT = computed tomography; LV = left ventricular; PVL = paravalvular leak; TMVR = transcatheter mitral valve replacement.
For all SAPIEN 3 sizes, this angle is ~15°, with minor variation at different balloon inflation volumes, and 20° on SAPIEN 3 Ultra models.
When the Emory angle approaches the valve a, we tested whether guidewire exteriorization by percutaneous LV access improves valve alignment and reduces PVL (Figure 1D).
We retrospectively analyzed the relationship between baseline annular-to-apical angle and post-procedure valve canting angle in SAPIEN 3 VIR or VIM implantations (Figures 1E to 1G). We began incorporating the Emory angle into pre-procedure planning, and when it approached the intrinsic SAPIEN 3 angle, we exteriorized the delivery guidewire using CT-guided para-apical percutaneous LV access (Figures 1H to 1J). We measured post-procedure valve canting angle on the basis of final valve heights above the annular plane on transesophageal imaging and/or fluoroscopy in both anteroposterior and medial-lateral planes. We compared valve coaxiality (canting angles, 0° being ideal) achieved before and after systematically implementing this selective wire exteriorization strategy, and determined the impact on PVL.
Guidewires were exteriorized after transthoracic micropuncture needle access to the LV and exchange for 6-Fr sheaths (3). We selected para-apical LV access sites using CT and surface echocardiography along the centerline orthogonal to the mitral annulus or ring. After TMVR, the LV access site was closed with a 6/4 Amplatzer second-generation duct occluder (ADO2, Abbott, Abbott Park, Illinois).
Before adopting the Emory angle measurements, we performed SAPIEN 3 TMVR in 10 patients (VIR n = 4, VIM n = 6). After adoption of the strategy, we performed TMVR in 12 patients (VIR n = 5, VIM n = 7). Before adoption, percutaneous LV access was used in 0% of cases (median Emory angle 16.5° (10.75° to 20°). After adoption, LV access was used in 67% of cases (3 of 5 VIR and 5 of 7 VIM). Overall median Emory angle was 19° (10.75° to 21°); 6.5° (2.75° to 10.25°) in those without LV access and 20.5° (19.5° to 22.25°) in cases employing LV access. Before adoption, deployed valves were 9.5° off ideal (range 0° to 16°), whereas after selectively adopting LV access, median valve canting angle was reduced to only 1.9° (range 0° to 4°); p = 0.0006, 2.4° in those without LV access, and 1.6° in those with LV access (Figures 1K and 1L). Two of the 7 patients (both VIM) undergoing LV access had significant pericardial effusions at procedure conclusion and underwent on-table pericardiocentesis without clinical sequelae. There were no other complications of para-apical LV access.
The incidence of >mild PVL due to valve malalignment or associated intervention was 30% before adoption compared with 0% afterward (p = 0.08). Event-free 30-day survival was 70% before adoption and 92% afterward (p = 0.30).
Significant PVL remains a limitation of VIR and VIM SAPIEN 3 TMVR and contributes to poor outcomes compared with VIV procedures. Many aspects of the procedure including features of the annular landing zone (type and size of surgical ring, extent and distribution of calcium) and implantation depth contribute to PVL. Valve malalignment after transseptal implantation is also a key contributor and appears driven largely by guidewire-enforced trajectories. A mitral annular–LV apical angle approaching 15° predicts this PVL during TMVR with SAPIEN 3 valves.
Although limited by small sample size and in need of larger prospective validation, selective use of this CT-predicted, percutaneous guidewire externalization strategy appears to achieve more coaxial SAPIEN 3 implantation with attendant reduction in PVL. Given the need for pericardial drainage in 2 of 7 patients, the technique requires comfort with managing apical access, closure, and its potential complications.
ACKNOWLEDGMENTS
The authors are grateful to Drs. Jose F. Condado and Isida Byku for their clinical contributions and thoughtful comments.
Supported by Emory Structural Heart and Valve program intramural funds, and National Institutes of Health grant Z01-HL006040. This work was approved by the Emory University Institutional Review Board. Informed consent was not required for this retrospective analysis. Dr. A.B. Greenbaum has been a proctor for Edwards Lifesciences and Medtronic; and is a consultant with equity in Transmural Systems. His employer has research contracts for investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr. Paone has been a consultant and proctor for Edwards Lifesciences. Dr. Grubb has been a speaker and proctor for Edwards Lifesciences, Boston Scientific, and Medtronic; is an investigator for Edwards Lifesciences and Medtronic; and is an advisory board member for Boston Scientific and Medtronic. Dr. Lederman’s employer (NIH) and Edwards Lifesciences have a cooperative research and development agreement (CRADA) on transcatheter modification of the mitral valve. Dr. Babaliaros has been a consultant for Edwards Lifesciences and Transmural Systems; and has equity in Transmural Systems. His employer has research contracts for investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Interventions author instructions page.
REFERENCES
- 1.Yoon SH, Whisenant BK, Bleiziffer S, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 2019;40:441–51. [DOI] [PubMed] [Google Scholar]
- 2.Guerrero M, Vemulapalli S, Viang Q, et al. Thirty-day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve-in-valve), failed surgical rings (valve-in-ring), and native valve with severe mitral annular calcification (valve-in-mitral annular calcification) in the United States. Circ Cardiovasc Interv 2020;13:e008425. [DOI] [PubMed] [Google Scholar]
- 3.Eng MH, Kherallah RY, Guerrero M, et al. Complete percutaneous apical access and closure: Short and intermediate term outcomes. Catheter Cardiovasc Interv 2020;96:481–7. [DOI] [PubMed] [Google Scholar]


