Abstract
Background
To assess the association between serum lactate levels and intolerance to enteral nutrition (EN) in septic patients treated with vasopressors.
Methods
This retrospective study was conducted between January 1, 2015 and May 1, 2018 in an intensive care unit (ICU). Patients with sepsis who were given EN and treated with vasopressors were included. EmpowerStats software and R (version 3.3.2) was used to examine the association between serum lactate levels and intolerance to EN.
Results
Among the 132 septic patients (age, 60.6±18.1 years) enrolled, 35 (26.5%) patients suffered intolerance to EN. Multiple logistic regression analysis demonstrated that an elevated lactate level was an independent risk factor for EN intolerance [odds ratio (OR): 2.7; 95% confidence interval (CI): 1.6–4.4; P<0.001]. The area under the receiver operating characteristic (ROC) curve for serum lactate levels was 0.764 (95% CI: 0.664–0.864). Stratified analysis suggested that age was the most prominent interactive factor for serum lactate levels in EN intolerance. Serum lactate levels were closely correlated to EN intolerance in elderly patients (age ≥65 years) (OR: 9.5; 95% CI: 2.1–42.4; P=0.0261 for interaction), while no such association was identified in younger patients (age <65 years; OR: 1.7; 95% CI: 1.0–2.9; P=0.052).
Conclusions
Serum lactate levels were associated with an increased risk of EN intolerance in patients with sepsis, especially in elderly individuals. An elevated serum lactate level may be an early predictor of EN intolerance in elderly septic patients treated with vasopressors. However, further studies are called for to verify these findings.
Keywords: Serum lactate level, enteral nutrition (EN), intolerance, sepsis
Introduction
In patients with sepsis, enteral nutrition (EN) feeding can preserve the function of the intestinal barrier, promote the recovery of gastrointestinal function, and play a role in metabolic conditioning and support. Early EN plays a key role in reducing the incidence of infection, resulting in shorter length of hospital stay, decreased hospitalisation costs, and lower mortality (1-3). Nutritional guidelines recommend that EN support is started within 24 to 48 hours of admission to the intensive care unit (ICU) to improve the prognosis of critically ill patients (4-6). However, septic patients often suffer from hemodynamic instability, which results in gastrointestinal dysfunction and the inability to tolerate EN, leading to reflux aspiration and increasing the fatality rate and length of ICU stay (7).
Lavrentieva et al. (8) showed that approximately 35% of septic patients develop feeding intolerance during EN. Early EN in septic patients may increase the risk of digestive complications compared with early isocaloric parenteral nutrition (9). The guidelines of 2016 Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) for critical nutrition in American adults (10) recommend delaying EN for patients with hemodynamic instability until adequate resuscitation or hemodynamic stability has been achieved. EN can be carefully initiated or reinitiated for patients who have been treated with a reduced dosage of vasoactive drugs. Therefore, the early prediction of EN feeding intolerance is of particularly importance for septic patients who are treated with vasopressors (11).
The pathogenesis of intestinal nutritional intolerance has yet to be fully explored. Possible mechanisms underlying intolerance to EN feeding include gastrointestinal nerve and smooth muscle injuries, inflammation, surgery, opioid use, electrolyte disturbance, and hyperglycemia (8,12). However, studies on the relevant risk factors are still controversial (7,12). Arabi et al. (13) suggested that when EN is carried out for severe patients treated with vasopressors, attention must be paid to the occurrence of lactic acidosis, which may be an important indicator of parenteral ischemia.
Serum lactate can serve as a biomarker in the assessment of mortality risk in sepsis patients (14,15), due to the association between hyperlactic acidemia and organ failure (16). Lactate level was found to be significantly correlated with microcirculation perfusion in shock patients (17), and an elevated lactate level may be an indicator of intestinal perfusion in septic patients (18). However, no study to date has confirmed the relationship between serum lactate levels and EN feeding intolerance in patients with sepsis. Therefore, in the present study, we hypothesized that serum lactate levels in septic patients treated with vasopressors were associated with intolerance to EN. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6317).
Methods
Data collection and treatment
Patients
The institutional ethics committee of the General Hospital of the People’s Liberation Army approved this single-centre retrospective cohort study (S2017-055-02). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived. Consecutive septic patients aged ≥18 years old who were treated with vasopressors (noradrenaline, dopamine, or epinephrine) and were admitted to the hospital’s ICU between January 1, 2015, and May 1, 2018, were enrolled. The exclusion criteria were as follows: EN time ≤1 hour; existing intestinal obstruction; acute pancreatitis, peritonitis, or ischemic colitis; or incomplete or missing data.
Survey data
Patient demographic and clinical data
The following demographic and clinical data were collected for each patient from electronic medical record: sex and age; diagnosis; Acute Physiology and Chronic Health Evaluation (APACHE II) score; Sequential Organ Failure Assessment (SOFA) score; acute gastrointestinal injury (AGI) score; length of hospital stay, length of ICU stay, mechanical ventilation and bedside blood filtration; mean arterial pressure; and blood lactic acid and creatinine levels. For each patient, the gastrointestinal function, position of the nutrient canal, gastric residual volume, amount of vomitus, amount of feces, and frequency of defecation were also recorded. Clinical information and EN tolerance were collected by two assessment groups separately.
Definitions
The patients were divided into the tolerance group and the intolerance group on the basis of the assessment of EN tolerance. EN intolerance (19) is defined as a patient having gastric residue >250 mL, vomiting, positivity in abdominal plain film or abdominal computed tomography (CT) imaging, and intestinal ischemia, or perforation.
Statistical analysis
Statistical analyses were performed with EmpowerStats 2.17.8 (http://www.empowerstats.com/cn/) and R-project (version 3.3.2). Continuous variables were expressed as mean ± standard deviation (), and comparisons between groups were performed using the independent samples t-test. Categorical variables were analyzed using the odds ratio (OR) and chi-square (χ2) test. Multiple logistic regression analysis was performed with adjustment for possible baseline data imbalances. Curve fitting, an interaction test, and covariate screening were conducted, and the effects of each model were compared. In the stratified analysis, each continuous variable was divided into three groups: low, middle, and high. The receiver operating characteristic (ROC) curve was draw, and the area under the curve (AUC) was used to determine the optimal cutoff for serum lactate level in the prediction of feeding intolerance. A two-tailed P value of <0.05 was considered to be statistically significant. Intended sample size was not estimated for this retrospective cohort study.
Results
This study enrolled 132 patients with sepsis (Figure 1). The EN tolerance and EN intolerance groups comprised 97 and 35 patients, respectively. A general comparison of baseline data including the sex, age, and APACHE II, SOFA, and AGI scores of the patients revealed no statistical differences between the intolerance and tolerance groups. No significant difference was observed between the groups in the proportion of abdominal diseases or the proportion of feeding after pylorus. However, the mechanical ventilation ratio of the EN intolerance group was significantly higher than that of the tolerance group (P=0.018) (Table 1).
Figure 1.
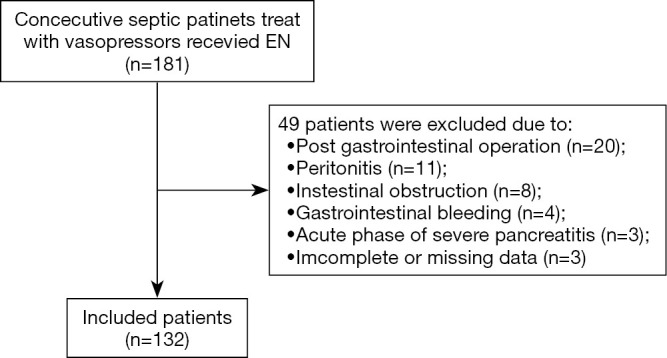
Flow diagram of participants including. EN, enteral nutrition.
Table 1. Baseline characteristics of participants in each group.
| Characteristics | Tolerance group | Intolerance group | P value |
|---|---|---|---|
| N | 97 | 35 | |
| Gender | 0.866 | ||
| Male | 68 (70.1%) | 24 (68.6%) | |
| Female | 29 (29.9%) | 11 (31.4%) | |
| Age | 61.1±18.3 | 59.1±17.8 | 0.593 |
| Hight | 168.0±7.4 | 170±8.9 | 0.208 |
| Weight | 64.9±11.4 | 67.8±11.2 | 0.202 |
| BMI | 23.4±4.2 | 23.6±2.3 | 0.788 |
| Comorbidities | |||
| Hypertension | 34 | 15 | 0.413 |
| CHD | 29 | 7 | 0.260 |
| Diabetes | 18 | 3 | 0.166 |
| CKD | 11 | 3 | 0.892 |
| Cirrhosis | 4 | 1 | 0.874 |
| COPD | 2 | 2 | 0.586 |
| Oncologic | 32 | 13 | 0.657 |
| Source of sepsis | |||
| Respiratory system | 21 | 9 | 0.623 |
| Abdominal | 28 | 13 | 0.364 |
| Urinary system | 4 | 1 | 0.857 |
| Skin and soft tissues | 27 | 8 | 0.567 |
| Bloodstream | 6 | 2 | 0.754 |
| Other | 11 | 2 | 0.531 |
| MV | 0.018* | ||
| No | 19 (19.6%) | 1 (2.9%) | |
| Yes | 78 (80.4%) | 34 (97.1%) | |
| Abdominal diseases | 0.935 | ||
| No | 63 (64.9%) | 23 (65.7%) | |
| Yes | 34 (35.1%) | 12 (34.3%) | |
| Feeding way | 0.681 | ||
| Jejunal tube | 19 (19.6%) | 8 (22.9%) | |
| Gastric tube | 78 (80.4%) | 27 (77.1%) | |
| EN total | 500.0 (300.0–700.0)# | 500.0 (200.0–500.0)# | 0.155 |
| EN speed | 41.8±36.3 | 36.7±21.1 | 0.435 |
| Lactate | 1.5±0.8 | 2.8±2.0 | <0.001* |
| Albumin | 31.4±3.9 | 32.2±7.0 | 0.400 |
| Hb | 106.5±27.3 | 89.5±13.4 | <0.001* |
| WBC | 11.2±6.7 | 10.5±4.8 | 0.553 |
| D-dimer | 5.7±4.7 | 5.0±3.8 | 0.464 |
| SOFA | 9.2±3.7 | 10.7±5.2 | 0.068 |
| APACHE II | 17.7±7.5 | 19.6±8.4 | 0.504 |
| AGI | 0.335 | ||
| 1 | 71 (73.2%) | 11 (31.4%) | |
| 2 | 18 (18.6%) | 13 (37.1%) | |
| 3 | 6 (6.2%) | 8 (22.9%) | |
| 4 | 2 (2.1%) | 3 (8.6%) |
*, P<0.05; #, median (Quartile1-Quartile). AGI, acute gastrointestinal abbreviated injury; APACHE, acute physical and chronic health assessment; BMI, body mass index; CHD, coronary heart disease; CKD, chronic kidney diseases; COPD, chronic obstructive pulmonary disease; EN, enteral nutrition; MV, mechanical ventilation; SOFA, sequential organ failure assessment.
No statistical difference was observed in the length of hospital stay, length of ICU stay, or duration of mechanical ventilation between the two groups. The intolerance group had a significantly higher 28-day mortality rate than the tolerance group (Table 2).
Table 2. The comparison of intolerance and outcomes between two groups.
| Outcomes | Tolerance group | Intolerance group | P value |
|---|---|---|---|
| N | 97 | 35 | |
| ICU stay (d) | 25.0±24.7 | 23.7±14.7 | 0.756 |
| Duration of MV | 16.2±21.3 | 17.5±13.5 | 0.395 |
| 28-days mortality | 0.042 | ||
| Non-survival | 15 (15.5%) | 11 (31.4%) | |
| Survival | 82 (84.5%) | 24 (68.6%) |
MV, mechanical ventilation.
The univariable analysis revealed feeding intolerance to be significantly associated with serum lactate levels, mechanical ventilation (MV), and AGI score (Table 1). The stratified analysis indicated that age was a significant effect modifier for the relationship between serum lactate levels and intolerance (P=0.0261) (Table 3). Covariate screening selected MV and AGI.
Table 3. Stratified analysis of characteristics and interaction modifiers.
| Characteristics | N | Intolerance [OR (95% CI)] | P value | P value of interaction |
|---|---|---|---|---|
| Age | 0.0216* | |||
| <65 | 69 | 2.0 (1.2, 3.3) | 0.0053* | |
| ≥65 | 63 | 8.8 (2.5, 31.0) | 0.0007* | |
| APACHE II tertile | 0.0716 | |||
| Low | 41 | 5.1 (1.3, 20.2) | 0.0197* | |
| Middle | 46 | 4.7 (1.7, 13.0) | 0.0029* | |
| High | 45 | 1.5 (0.8, 2.8) | 0.2399 | |
| SOFA tertile | 0.5888 | |||
| Low | 44 | 4.3 (1.4, 13.9) | 0.0135* | |
| Middle | 32 | 2.7 (0.8, 9.8) | 0.1190 | |
| High | 56 | 2.2 | 0.0078* | |
| MV | 0.8573 | |||
| Non-MV | 20 | 1.7 (0.0, 141.7) | 0.8252 | |
| MV | 112 | 2.5 (1.5, 4.1) | 0.0003* | |
| Feeding way | 0.9345 | |||
| Jejunal tube | 27 | 2.6 (0.7, 9.3) | 0.1328 | |
| Gastric tube | 105 | 2.8 (1.6, 4.8) | 0.0003* | |
| EN total tertile | 0.8595 | |||
| Low | 44 | 2.6 (1.1, 6.3) | 0.0313* | |
| Middle | 1 | 0.0 | 0.9929 | |
| High | 87 | 2.9 (1.5, 5.5) | 0.0010* | |
| Abdomen disease | 0.7677 | |||
| No | 86 | 2.6 (1.5, 4.6) | 0.0008* | |
| Yes | 46 | 3.1 (1.2, 8.0) | 0.0206* | |
| EN speed tertile | 0.5475 | |||
| Low | 30 | 2.6 (1.0, 7.0) | 0.0483* | |
| Middle | 55 | 4.0 (1.6, 10.2) | 0.0036* | |
| High | 47 | 2.1 (1.1, 4.1) | 0.0306* |
*, P<0.05. Continuous variables were divided in to three grade groups as low, middle and high. MV, mechanical ventilation; EN, enteral nutrition.
In multivariate regression analysis, the dependent variables were mechanical ventilation and EN tolerance, and the independent variables were serum lactate levels, MV, and AGI score. The results indicated that an elevated lactate level was an independent risk factor for EN intolerance [OR: 2.7; 95% confidence interval (CI): 1.6–4.4; P<0.001]. Additionally, smooth curve fitting was conducted after adjustment for all variables together (Figure 2).
Figure 2.
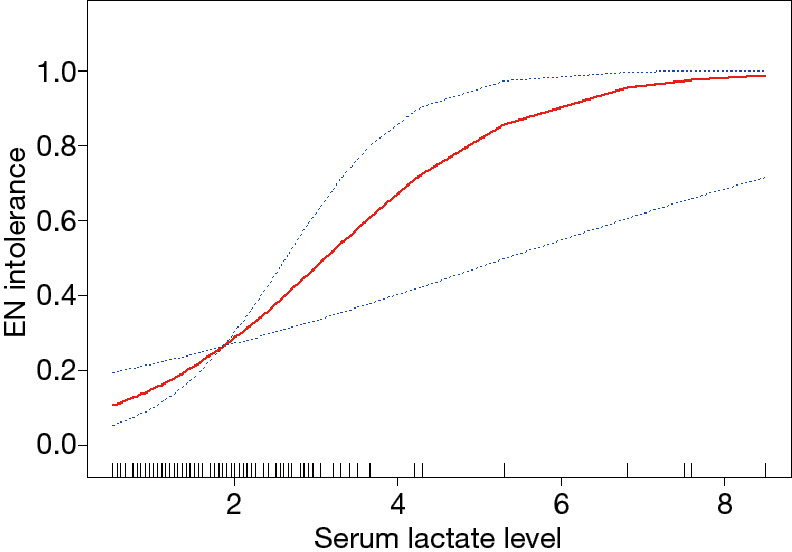
Smooth curve fitting of serum lactate levels and tolerance to enteral nutrition in patients with sepsis. Adjustment variables: age, sex, MV, and AGI. MV, mechanical ventilation; AGI, acute gastrointestinal injury.
Figure 3 shows the ROC curve of serum lactate levels in predicting EN intolerance. The AUC for serum lactate was 0.764 (95% CI: 0.664–0.864), with a cutoff value of 2.125 mmol/L. With age as an effect modifier, analysis was performed with the patients stratified into two groups by age: the ≥65 age group and the <65 age group. In the ≥65 age group and the <65 age group, the OR values of association between serum lactate levels and feeding intolerance were 9.5 (95% CI, 2.1–42.4; P=0.003) and 1.8 (95% CI: 1.0–3.1; P=0.053) when adjusted for MV and AGI, respectively (Figure 4). The AUC for serum lactate in predicting EN intolerance in patients aged ≥65 was 0.831 (95% CI: 0.667–0.985) (Figure 5).
Figure 3.
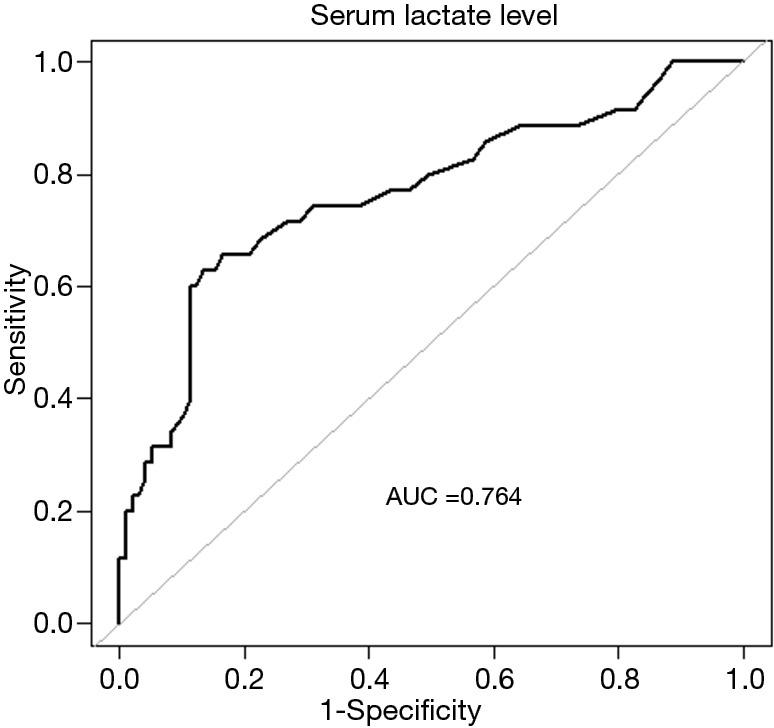
Receiver operating characteristic curve of the relationship between serum lactate levels and tolerance to enteral nutrition in patients with sepsis. AUC, area under the curve.
Figure 4.
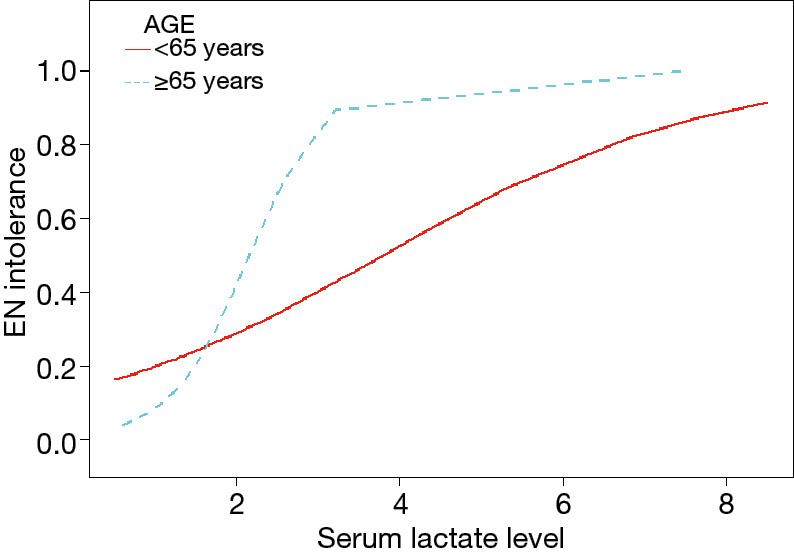
Subgroup smooth curve fitting of serum lactate level and EN tolerance in septic patients stratified by age. Adjustment variables: age, gender, MV, and AGI. EN, enteral nutrition; MV, mechanical ventilation; AGI, acute gastrointestinal injury.
Figure 5.
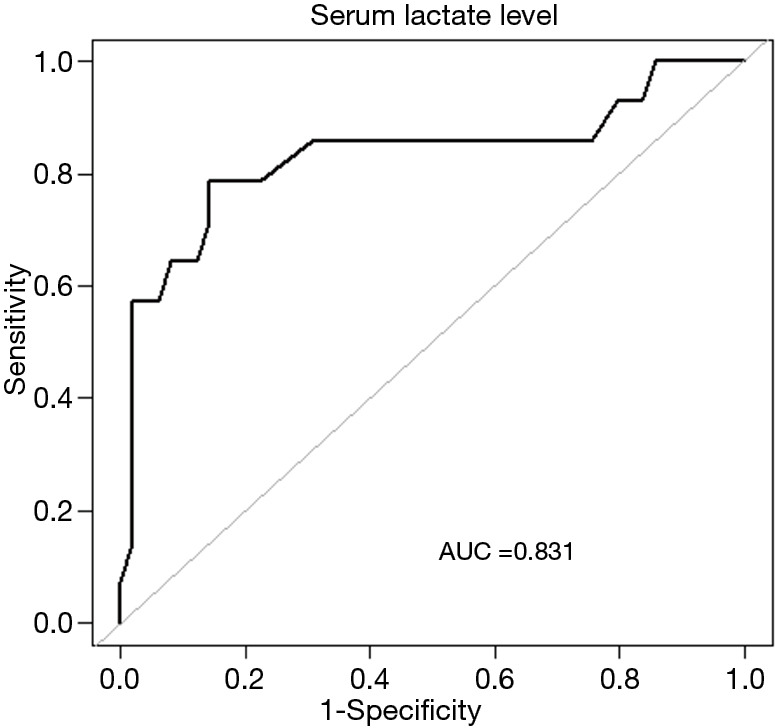
Receiver operating characteristic curve of the relationship between serum lactate level and tolerance to enteral nutrition in septic patients aged ≥65. AUC, area under the curve.
Discussion
This retrospective cohort study found that serum lactate levels are associated with intolerance to enteral feeding among elderly patients with sepsis who undergo treatment with vasopressors. Saturation analysis showed a correlation of the serum lactate levels with EN intolerance when the serum lactate levels are below 2.5 mmol/L. In the present study, 26.5% patients who received EN suffered intolerance, which is similar to the rate reported in a previous study (20). In patients with sepsis, the intestinal blood flow is decreased, the intestinal villi are damaged, the intestinal flora is displaced, and the intestinal tract releases pro-inflammatory response media, resulting in damage to the intestinal mucosa and the deterioration of intestinal function. Various intolerances frequently occur during the course of EN. Patients with sepsis still have persistent hypotension after fluid resuscitation, and vasopressors are required to maintain the mean arterial pressure ≥65 mmHg (21). Moreover, the mortality rate is remarkably increased in patients with a serum lactate level >2.0 mmol/L. The definition of Sepsis 3.0 was validated in a cohort study using a large electronic medical record data set (22), wherein the serum lactate levels >2.0 mmol/L was an indicator of septic shock. This study shows that a higher serum lactate level is an independent predictor for EN intolerance in septic patients.
Elevated serum lactate levels indicate the presence of hypoxia in tissues and gastrointestinal dysfunction in patients with hemodynamic instability, which further affects their tolerance to EN. In shock patients with mechanical ventilation, no significant differences were found to exist between the serum lactate levels of EN-tolerant and intolerant patients (19); however, the proportion of patients with elevated blood lactate levels was reported to be lower in the EN tolerance group than in the EN intolerance group. Our study showed that the serum lactate levels in the EN intolerance group were higher than those in the EN tolerance group, and multifactor regression analysis revealed that an increase in blood lactic acid was correlated with and was an independent risk factor for EN intolerance. Increased serum lactic acid levels indicate ischemia and hypoxia in tissues, and possibly in the gastrointestinal tract, thereby ischemia and hypoxia may lead to EN intolerance. Shock patients with increased serum lactate may have an increased risk of developing intolerance to EN.
Septic patients may suffer from gastrointestinal dysfunction and EN intolerance due to hemodynamic instability and the application of vasopressors. Therefore, the optimal time for starting EN has not been established. The current guidelines (10) recommend that EN should be delayed for patients with hemodynamic instability until adequate resuscitation or hemodynamic stability has been achieved. Patients who are administered a reduced dosage of vasopressors can carefully start or restart EN. The tolerance rate of shock patients was reported to reach 75% when the equivalent dose of noradrenaline was 12.5 µg·min−1 (23). Merchan et al. (19) showed that the EN tolerance of patients treated with methylepinephrine was 62% when the equivalent dosage was <0.14 µg·kg−1·min−1. However, their study did not further analyse the factors related to the high occurrence rate of EN intolerance in shock patients.
EN-intolerant patients have a higher mortality rate. This study showed that the blood lactic acid levels and the mortality rate of the EN intolerance group were higher than those of the EN tolerance group. These results may be related to the disease severity of patients, as EN intolerance can also manifest as gastrointestinal failure. Furthermore, tissue hypoxia worsened the condition of the patients. Further studies are needed to determine whether EN intolerance is an independent risk factor for mortality in septic patients.
The findings of the present study suggested that age (≥65) was the significant interaction modifier for the relationship between serum lactate levels and EN intolerance in septic patients. Previous studies have shown that elderly patients have a higher likelihood of experiencing gastric emptying delay combined with increased gastric residue than younger patients, old age is an independent risk factor influencing the prognosis (24). Park et al. (25) reported high mortality and poor prognosis among patients with ischemic bowel disease without surgical intestinal resection and nonobstructive ischemic bowel disease. Halm et al. (26) showed that advanced age (age >80 years old) is an independent risk factor for the occurrence of EN-related complications in cardiac surgery patients. However, Rai et al. (7) believed that despite delayed gastric emptying, early EN should also be started on patients with hemodynamic instability. Age has no effect on the prognosis of gastrointestinal diseases and EN complications. Barone et al. (27) showed that age is not an influencing factor of EN complications, although it is relevant to the rate of comorbidities. Regardless of the high incidence of comorbidities in elderly patients, age is not an indicator that influences the recovery of gastrointestinal function or the prognosis of patients with acute upper gastrointestinal haemorrhage who are treated with endoscopy (25). However, the present study suggested that serum lactate levels were closely correlated with EN intolerance in elderly patients (age ≥65 years), while no such association was observed in the other patients (age <65 years). Therefore, an elevated serum lactate level may be an early predictor of EN intolerance in elderly patients with sepsis who receive treatment with vasopressors. For elderly septic patients who have been treated with vasopressors, serum lactate levels could be a valuable biomarker for informing treatment decision-making.
There are several limitations to this study. Firstly, as a retrospective cohort study, bias could not be avoided. The intolerance group had a higher AGI score and MV rate, which might be confounders. However, the results were confirmed by multiple analysis. Second, the study’s single-center nature and small sample size may affect the generalizability of the results. Larger studies using prospective methods are needed in the future.
In conclusion, serum lactate levels were associated with EN intolerance in patients with sepsis, especially in elderly patients. These results indicate that serum lactate levels may be an early biomarker for predicting EN intolerance in septic patients in eldely patients. However, our findings need to be confirmed in further studies.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by Special Research of Military Health Fare (No. 17BJZ30), as well as grants from Special Research of Military Medical Innovation (No. 18CXZ026) and Innovation Research of Chinese PLA general hospital (No. CX10010), WU JIEPING MEDICAL FOUNDATION (No. 320.6750.18383), and Fostering Fund of Chinese PLA General Hospital for National Distinguished Young Scholar Science Fund (No. 2019-JQPY-002).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional ethics committee of the General Hospital of the People’s Liberation Army approved this single-centre retrospective cohort study (No. S2017-055-02). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6317
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6317
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6317). The authors have no conflicts of interest to declare.
(English Language Editor: J. Reynolds)
References
- 1.Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005;31:12-23. 10.1007/s00134-004-2511-2 [DOI] [PubMed] [Google Scholar]
- 2.Auiwattanakul S, Chittawatanarat K, Chaiwat O, et al. Effects of nutrition factors on mortality and sepsis occurrence in a multicenter university-based surgical intensive care unit in Thailand (THAI-SICU study). Nutrition 2019;58:94-9. 10.1016/j.nut.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 3.Mao Z, Yu Q, Liu C, et al. The impact of daily use of an enteral feeding checklist on clinical outcomes in shock patients: a retrospective cohort study. Asia Pac J Clin Nutr 2019;28:230-7. [DOI] [PubMed] [Google Scholar]
- 4.Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016;44:390-438. 10.1097/CCM.0000000000001525 [DOI] [PubMed] [Google Scholar]
- 5.Singer P, Berger MM, Van den Berghe G, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr 2009;28:387-400. 10.1016/j.clnu.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 6.Pu H, Doig GS, Heighes PT, et al. Early Enteral Nutrition Reduces Mortality and Improves Other Key Outcomes in Patients With Major Burn Injury: A Meta-Analysis of Randomized Controlled Trials. Crit Care Med 2018;46:2036-42. 10.1097/CCM.0000000000003445 [DOI] [PubMed] [Google Scholar]
- 7.Rai SS, O'Connor SN, Lange K, et al. Enteral nutrition for patients in septic shock: a retrospective cohort study. Crit Care Resusc 2010;12:177-81. [PubMed] [Google Scholar]
- 8.Lavrentieva A, Kontakiotis T, Bitzani M. Enteral nutrition intolerance in critically ill septic burn patients. J Burn Care Res 2014;35:313-8. 10.1097/BCR.0b013e3182a22403 [DOI] [PubMed] [Google Scholar]
- 9.Reignier J, Boisrame-Helms J, Brisard L, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133-43. 10.1016/S0140-6736(17)32146-3 [DOI] [PubMed] [Google Scholar]
- 10.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. 10.1177/0148607115621863 [DOI] [PubMed] [Google Scholar]
- 11.Wischmeyer PE. Nutrition Therapy in Sepsis. Crit Care Clin 2018;34:107-25. 10.1016/j.ccc.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Wang T, Chen T, et al. Identification of risk factors for enteral feeding intolerance screening in critically ill patients. Saudi Med J 2017;38:816-25. 10.15537/smj.2017.8.20393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arabi YM, McClave SA. Enteral Nutrition Should Not Be Given to Patients on Vasopressor Agents. Crit Care Med 2020;48:119-21. 10.1097/CCM.0000000000003362 [DOI] [PubMed] [Google Scholar]
- 14.Moran JL, Santamaria J. Reconsidering lactate as a sepsis risk biomarker. PLoS One 2017;12:e0185320. 10.1371/journal.pone.0185320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrelli G, Koch E, Sterk E, et al. Early recognition of sepsis through emergency medical services pre-hospital screening. Am J Emerg Med 2019;37:1428-32. 10.1016/j.ajem.2018.10.036 [DOI] [PubMed] [Google Scholar]
- 16.Jansen TC, van Bommel J, Woodward R, et al. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med 2009;37:2369-74. 10.1097/CCM.0b013e3181a0f919 [DOI] [PubMed] [Google Scholar]
- 17.Yeh YC, Wang MJ, Chao A, et al. Correlation between early sublingual small vessel density and late blood lactate level in critically ill surgical patients. J Surg Res 2013;180:317-21. 10.1016/j.jss.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 18.Jacquet-Lagreze M, Bonnet-Garin JM, Allaouchiche B, et al. A new device for continuous assessment of gut perfusion: proof of concept on a porcine model of septic shock. Crit Care 2014;18:R153. 10.1186/cc13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchan C, Altshuler D, Aberle C, et al. Tolerability of Enteral Nutrition in Mechanically Ventilated Patients With Septic Shock Who Require Vasopressors. J Intensive Care Med 2017;32:540-6. 10.1177/0885066616656799 [DOI] [PubMed] [Google Scholar]
- 20.Lee ZY, Ibrahim NA, Mohd-Yusof BN. Prevalence and duration of reasons for enteral nutrition feeding interruption in a tertiary intensive care unit. Nutrition 2018;53:26-33. 10.1016/j.nut.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancl EE, Muzevich KM. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. JPEN J Parenter Enteral Nutr 2013;37:641-51. 10.1177/0148607112470460 [DOI] [PubMed] [Google Scholar]
- 24.Gungabissoon U, Hacquoil K, Bains C, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr 2015;39:441-8. 10.1177/0148607114526450 [DOI] [PubMed] [Google Scholar]
- 25.Park WM, Gloviczki P, Cherry KJ, Jr, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg 2002;35:445-52. 10.1067/mva.2002.120373 [DOI] [PubMed] [Google Scholar]
- 26.Halm MA. Acute gastrointestinal complications after cardiac surgery. Am J Crit Care 1996;5:109-18; quiz 19-20. 10.4037/ajcc1996.5.2.109 [DOI] [PubMed] [Google Scholar]
- 27.Barone M, Viggiani MT, Amoruso A, et al. Influence of age and type of underlying disease on complications related to home enteral nutrition: a single Italian center experience. JPEN J Parenter Enteral Nutr 2014;38:991-5. 10.1177/0148607113498422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


