Abstract
Objective
This study aimed to examine whether oxidized low-density lipoprotein (oxLDL) facilitates platelet aggregation, which is one cause for development of cardiovascular disease.
Methods
The susceptibility of platelets to aggregation was monitored by light transmittance aggregometry and a laser light scattering method using low-density lipoprotein (LDL) and oxLDL as agonists. β-thromboglobulin (β-TG) levels released from platelets were also measured after incubation with or without oxLDL.
Results
Platelet aggregation was suppressed by oxLDL as estimated by maximum light transmission. Additionally, adenosine diphosphate-induced further aggregation was slightly reduced by the presence of oxLDL. Aggregation levels of a low number of platelets, which was determined by the laser light scattering method, were lower upon addition of oxLDL compared with unoxidized LDL. After a short time of incubation, oxLDL increased secreted β-TG levels in platelet-rich plasma. However, further incubation with oxLDL caused relatively lower secreted β-TG levels compared with incubation with unoxidized LDL. This fluctuation was not due to β-TG degradation by oxLDL.
Conclusions
Levels of oxLDL in vitro weakly activate platelets at an early stage, but then inhibit platelet function, such as aggregation and β-TG secretion.
Keywords: Oxidized low-density lipoprotein, platelet, β-thromboglobulin, aggregometry, atherosclerosis, cardiovascular disease
Introduction
Elevated plasma levels of low-density lipoprotein (LDL) are a risk factor for development of atherosclerotic disease by causing production of oxidized LDL (oxLDL).1 oxLDL plays a central role in the initiation and progression of atherosclerosis because it mediates proinflammatory and procoagulatory effects on several vascular cells (e.g., neutrophils, monocytes/macrophages, smooth muscle cells, endothelial cells, and platelets).2,3 One inducer of LDL oxidation is catalytic activity of myeloperoxidase, which is derived from macrophages and neutrophils.4,5 However, platelet activation also induces initiation and progression of atherosclerosis by several mechanisms, including adhesion, subsequent monocyte recruitment to the endothelium, and induction of proinflammatory mediators.6–10 Many studies have shown that oxLDL is a causative substance in development of cardiovascular disease (CVD) by promoting activation and aggregation of platelets.2,11–13 However, low oxLDL levels attenuate platelet function and aggregation in vitro.14–16 These contradictions appear to stem from the heterogeneous nature of oxLDL, such as the oxidized domain and degree of oxidation.
To assess platelet aggregation, light transmittance aggregometry (LTA) is used in studies, such as clinical examinations. LTA is useful for assessing bleeding disorders because it determines whether aggregation of platelets is generated by strong agonists. However, LTA is not ideal for diagnosing thrombosis and determining the effects of antiplatelet drugs because of the complicated test process, including many types of agonists, a low sensitivity, and a lack of standardization.9
β-thromboglobulin (β-TG), which is a cysteine-X-cysteine (CXC) motif chemokine ligand 7 chemokine variant and is predominantly expressed in megakaryocytes and platelets, is released when platelets are activated.17,18 Through proteolytic truncation, β-TG is converted to neutrophil-activating peptide-2, which plays a role in early recruitment of neutrophils.9,11,19 β-TG may be the most available CXC chemokine marker of platelet activation followed by development of atherosclerosis in vivo. This is because β-TG is stored as a matured protein in α-granules and is rapidly released at an extremely high concentration compared with that in plasma.18,20
Although oxLDL affects platelet function, clarifying the actual effect of oxLDL on platelet activation by LTA using agonists, such as adenosine diphosphate (ADP), thrombin, and collagen, may be difficult. Therefore, this study aimed to examine whether oxLDL facilitates platelet aggregation. First, washed platelets were used for aggregation experiments to avoid the effects of many proteins in plasma, including lipoproteins. However, platelets are easily activated during purification. Therefore, in addition to investigation of the susceptibility of washed platelets by LTA using oxLDL as the agonist, β-TG levels released from platelets using platelet-rich plasma (PRP) were measured after incubation with or without oxLDL.
Materials and methods
Materials
Unless otherwise stated, all reagents were purchased from Nacalai Tesque (Tokyo, Japan), FUJIFILM Wako Pure Chemical (Osaka, Japan), and Sigma-Aldrich Japan (Tokyo, Japan).
Blood samples
Blood samples from healthy volunteers were drawn into a blood collection tube with EDTA-2Na for preparation of LDL or 3.2% sodium citrate (0.1 of the volume) for preparation of PRP. Informed consent was obtained from all volunteers. This study was approved by the ethics committee of the Faculty of Medicine, Shinshu University (No. 3284). Informed consent was obtained from all volunteers.
Isolation of LDL
LDL (density: 1.019–1.063 g/mL) was isolated from pooled plasma by ultracentrifugation as described previously21 and dialyzed against phosphate-buffered saline containing 0.1 mM EDTA-2Na.
LDL oxidation
LDL was oxidized using sodium hypochlorite solution as described previously.22 Briefly, LDL (approximately 3 mg protein/mL) was incubated with sodium hypochlorite solution (in phosphate-buffered saline with 0.1 mM EDTA-2Na) at a molar ratio of 1: 400 or the same volume of buffer (as control LDL) on ice for 15 minutes. The actual concentration of hypochlorite was spectrophotometrically determined using the molar absorption coefficient (350 L mol−1 cm−1) at 292 nm. After the reaction, the mixture was used for desalt gel-filtration chromatography (PD MiniTrap G-25; GE Healthcare Japan, Hino, Japan). Actually, oxLDL was eluted around the void volume and the excess hypochlorite was eluted later. We used chromatography as a quicker separation method of oxLDL from excess hypochlorite than dialysis. The degree of oxLDL was analyzed by agarose gel electrophoresis using a TITAN GEL Lipoprotein plate (HELENA, Saitama, Japan) with Fat Red 7B (Sigma-Aldrich Japan, Tokyo, Japan) staining.
Measurement of protein and lipid concentrations
Protein concentrations were determined by the Micro BCA Protein Assay Kit (Thermo Fisher Scientific, Tokyo, Japan). Concentrations of triglycerides (TG), total cholesterol (TC), LDL-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) were quantified using the following commercially available reagents: Quickauto Neo TGII (Shino-Test, Tokyo, Japan), Determiner L TC II (Hitachi Chemical Diagnostic Systems, Tokyo, Japan), Cholestest LDL, and Cholestest N HDL (Sekisui Medical, Tokyo, Japan), respectively.
Preparation of PRP and washed platelets
PRP was prepared by centrifugation of blood samples at 120 × g for 10 minutes with minimum acceleration and deceleration. To obtain washed platelets, PRP was mixed with three volumes of Buffer A (10 mM HEPES, pH 7.4, containing 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 11.9 mM NaHCO3, 0.42 mM NaH2PO4, 5.5 mM glucose, and 0.5% bovine serum albumin) with acid citrate dextrose solution A solution (9: 1) and centrifuged at 1000 × g for 10 minutes. Platelets were washed again with 2 mL of the same solution and finally resuspended in Buffer A. Washed platelet concentrations were measured by a blood cell analyzer (XN-9000; Sysmex, Kobe, Japan) and were diluted with Buffer A to adjust to 25 × 104 cells/µL. The diluted platelets were kept at room temperature and used within 4 hours after drawing blood.
Platelet aggregation assay
To evaluate platelet aggregation, an aggregometer, PA-200 (Kowa, Nagoya, Japan), was used because the PA-200 can detect a small amount of aggregation in several platelets. A total of 240 µL of washed platelets (25 × 104 cells/µL) and 30 µL of fibrinogen (5 mg/mL) were mixed in three special cuvettes for the PA-200 and pre-incubated at 37°C for 5 minutes. After setting these cuvettes in the aggregometer channels, monitoring of platelet aggregation by light transmission and laser light scattering commenced. After 1 minute, 30 µL of LDL or oxLDL, diluted to 1 mg protein/mL with Buffer A without glucose and bovine serum albumin (Buffer B), or Buffer B as the control was added to each cuvette. After 5 minutes of monitoring, 30 µL of ADP (44 µM) was added to the cuvettes and platelet aggregation was further monitored for 9 minutes.
β-TG assay
PRP was adjusted to 25 × 104 platelets/µL using saline with 3.2% sodium citrate (9: 1). Part of the diluted PRP was centrifuged at 1500 × g for 15 minutes and the supernatant was collected to measure levels of TC, TG, LDL-C, and HDL-C. The supernatant was also stored at −80°C for measuring β-TG levels, which were defined as the original level (before activation). To investigate the effect of LDL and oxLDL on activation of platelets, PRP was mixed with LDL (TC level: 1380 µg/mL, TG level: 139 µg/mL), oxLDL (TC: 1606 µg/mL, TG: 168 µg/mL), or Buffer B (as the control) at a ratio of 4: 1 and incubated at 37°C. At 2, 8, and 15 minutes, an aliquot was collected and centrifuged at 1500 × g for 15 minutes to obtain the supernatant, which was immediately stored at −80°C for measuring β-TG levels. Additionally, to examine the effect of LDL and oxLDL on degradation of β-TG, PRP was incubated with Buffer B for 15 minutes at 37°C to obtain a supernatant with a high level of β-TG, which was then incubated with LDL, oxLDL, or Buffer B (as control) again as described above. At 2, 8 and 15 minutes, an aliquot was collected and the supernatant was stored at −80°C for measuring β-TG levels. β-TG levels of all samples were measured by the EIA automatic analyzer AP960 (Hitachi Chemical Diagnostic Systems, Tokyo, Japan) using the assay kit Asserachrom β-TG (Fujirebio, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation. The data were analyzed by Dunnett’s test (IBM SPSS Statistics for Windows, version 25; IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
LDL oxidation
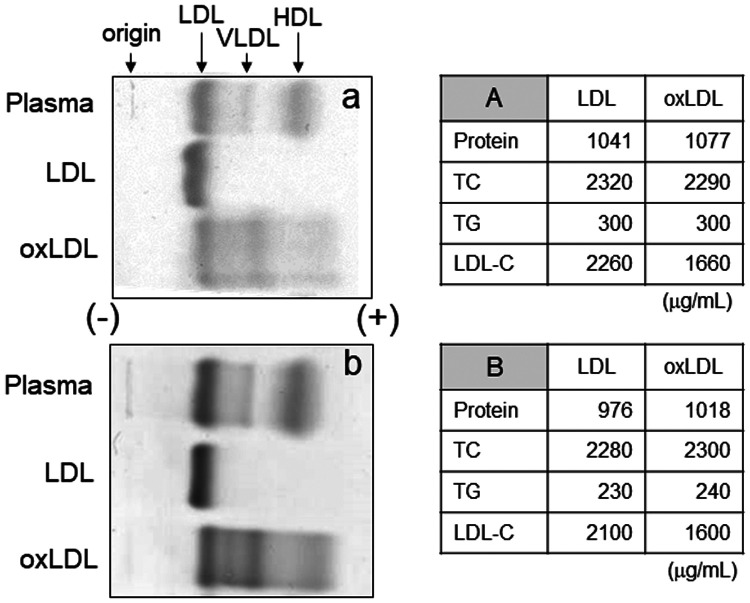
In this study, LDL was oxidized by hypochlorite, which is produced in vivo from hydrogen peroxide and chloride ions through the catalytic action of myeloperoxidase. The degree of oxidation of two batches of oxLDL, which were used for aggregation and the β-TG secretion experiments, were analyzed by agarose gel electrophoresis. At least four bands showed increased electrophoretic mobility in both oxLDL profiles in the anode side compared with the original mobility of LDL, which indicated successful production of oxLDL (Figure 1). Interestingly, one band was more negatively charged than that of HDL, and three bands were observed between LDL and very low-density lipoprotein. Although we observed no differences in the levels of protein, TC, and TG between LDL and oxLDL, LDL-C levels of oxLDL were lower than those of LDL (Figure 1). This finding indicated that direct measurement by a homogeneous assay kit for LDL-C partially failed to recognize oxLDL as intact LDL. Additionally, our results suggested that LDL was successfully oxidized by sodium hypochlorite.
Figure 1.
Oxidation of LDL by hypochlorite. Plasma, LDL, and oxLDL obtained from two batches of pooled plasma (a and b) were analyzed by agarose gel electrophoresis. The tables show protein and lipid concentrations of LDL and oxLDL. For the subsequent experiments of platelet aggregation and the β-TG assay, lot A and lot B were used, respectively
LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; HDL, high-density lipoprotein; oxLDL, oxidized low-density lipoprotein; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol.
Effect of LDL and oxLDL on washed platelet aggregation
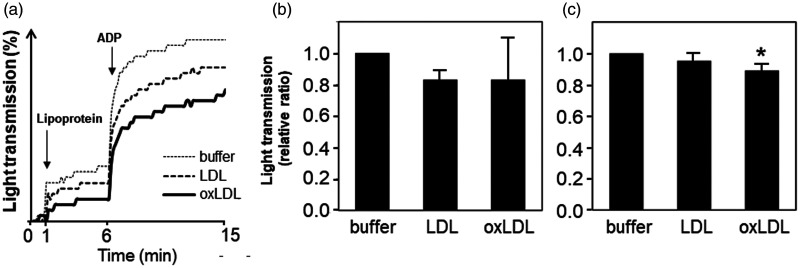
Aggregation of washed platelets with or without oxLDL was analyzed using an aggregometer. Representative aggregation profiles by light transmission are shown in Figure 2a. When Buffer B, LDL, or oxLDL was added to the washed platelets as the first trigger, light transmission slightly increased by each trigger. However, aggregation of washed platelets induced by adding Buffer B, LDL, or oxLDL was weak. The highest light transmission (at 6 minutes) was obtained by adding Buffer B (9.5% ± 2.1%), while LDL and oxLDL addition resulted in a lower transmission (8.2% ± 1.8% and 5.2% ± 1.2%, respectively). However, the apparent increase in light transmission after adding Buffer B only was induced according to a dilution of the mixture. These increases were relatively low because LDL and oxLDL absorb light at 555 nm, which is used by the PA-200 aggregometer. Consequently, the actual increase in light transmission induced by LDL and oxLDL (difference in light transmission between 1 minute and 20 s and 6 minutes) was not significantly different, although it tended to be relatively lower compared with that by Buffer B. When ADP, which is a weak platelet agonist, was added as the second trigger, each light transmission increased at a relatively large scale and tended to be largest upon addition of Buffer B, followed by LDL and oxLDL. These experiments were performed three times using different platelet preparations in triplicate. The relative increase in light transmission induced by the first (from 1 minute and 20 s to 6 minutes) and second triggers (from 6–15 minutes) is shown in Figure 2b and 2c, respectively. The relative ratio of increased light transmission after adding LDL or oxLDL was not significantly different, however, that of LDL appeared to be lower than that of Buffer B (Figure 2b). After addition of 4 µM ADP, the relative ratios of increased light transmission of LDL and oxLDL were lower than that of Buffer B, but significance was only observed between adding Buffer B and oxLDL (P < 0.05, Figure 2c).
Figure 2.
Monitoring of washed platelet aggregation by light transmission. The effect of oxLDL on platelet aggregation was analyzed by light transmission. Platelets obtained from different subjects were used in three experiments with triplicate assays. (a) Representative monitoring profiles of washed platelet aggregation are shown. Relative ratios of light transmission increased between 1 minute and 20 s and 6 minutes (b), and between 6 and 15 minutes (c) compared among Buffer B, LDL, and oxLDL (mean ± standard deviation, n = 3). The relative ratio of Buffer B was defined as 1. *P < 0.05 vs. Buffer B.
ADP, adenosine diphosphate; buffer, Buffer B; LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein.
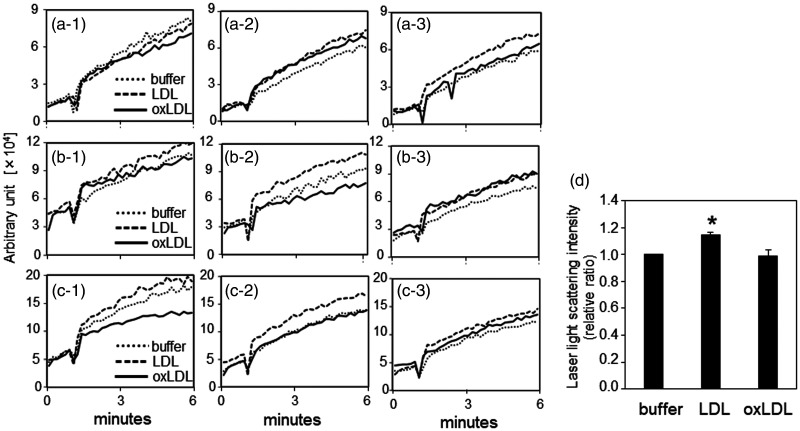
Production of small platelet aggregates induced by adding Buffer B, LDL, or oxLDL in three experiments was determined (Figure 3a, 3b, and 3c). Dispersion was observed in the profiles in three experiments and in triplicate assays within each experiment. However, the relative ratio of the maximum laser light scattering values (at 6 minutes) was highest with LDL (P < 0.05 versus Buffer B), while the ratios with Buffer B and oxLDL were similar (Figure 3d).
Figure 3.
Monitoring of washed platelet aggregation by laser light scattering. The effect of oxLDL on platelet aggregation was also assessed by laser light scattering in the same experimental conditions as indicated in Figure 2, except that monitoring was only performed for the initial 6 minutes. Washed platelets were obtained from three different subjects (a, b, and c) and aggregation profiles are shown in triplicate (–1, –2 and –3) for each platelet sample. (d) To evaluate the results of the three experiments, the relative ratios of the mean laser light scattering intensity of triplicate data at 6 minutes (data for Buffer B were defined as 1) were used as the result of each experiment. The results are shown as mean ± standard deviation. *P < 0.05 vs. buffer.
buffer, Buffer B; LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein.
Effect of LDL and oxLDL on β-TG levels in PRP
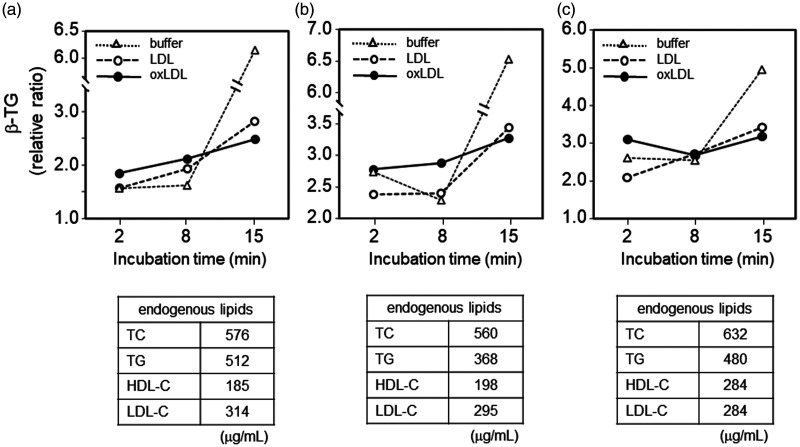
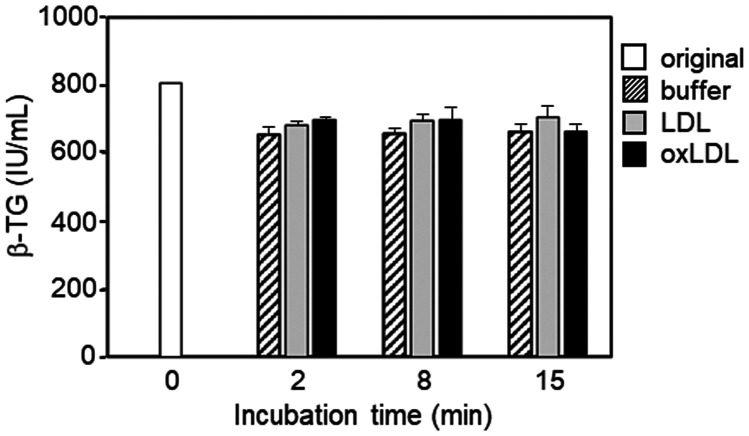
To confirm activation of platelets, β-TG levels in supernatants obtained before and after incubation of PRP (three lots) with Buffer B, LDL, or oxLDL were measured and expressed relative to the baseline level (before incubation), which was defined as 1 (Figure 4). In this experiment, we used PRP to minimize activation of platelets during preparation. In the relatively short incubation time (2 and 8 minutes), oxLDL tended to induce the highest β-TG level in the supernatant. However, in contrast to the mild increase or steady β-TG levels after incubation with oxLDL for 8 and 15 minutes, LDL and Buffer B induced a larger relative increase in β-TG levels. Finally, β-TG levels in supernatant after incubation with Buffer B (without LDL or oxLDL) for 15 minutes were extremely high compared with those after addition of LDL or oxLDL. Furthermore, after 15 minutes of incubation with oxLDL, supernatant obtained from PRP showed the lowest β-TG level.
Figure 4.
Effect of LDL and oxLDL on β-thromboglobulin secretion. Three experiments (a, b, and c) were performed using separately prepared platelet-rich plasma. β-TG values are expressed as the ratio to the baseline level of β-TG (corresponding to 0 minutes of incubation and defined as 1), which was calculated by multiplying 4/5 (for volume correction) by the β-TG level of the supernatant obtained from used platelet-rich plasma (25 × 104 cells/µL platelets) in each experiment. The table under each graph indicates the calculated lipid concentrations derived from platelet-rich plasma in the mixture.
β-TG, β-thromboglobulin; buffer, Buffer B; LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
We then determined whether the relatively low β-TG levels in supernatant after 15 minutes of incubation of PRP with oxLDL or LDL were caused by β-TG degradation. Supernatant with high β-TG levels, which was prepared by incubating PRP with Buffer B, was incubated with oxLDL or LDL for 2, 8, and 15 minutes. We found that LDL and oxLDL did not affect β-TG levels (Figure 5).
Figure 5.
Effect of LDL and oxLDL on β-TG. The results are shown as the mean ± standard deviation of triplicate assays.
β-TG, β-thromboglobulin; buffer, Buffer B; LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein.
Discussion
Atherosclerosis, which is a main cause of CVD, develops by unregulated uptake of excess cholesterol derived from oxLDL in macrophages and subsequent formation of foam cells that accumulate in the intima.23 CVD is also induced by oxLDL by promoting activation and aggregation of platelets.2,11–13 However, some conflicting results have been reported as follows.14–16 Using various concentrations (40–120 µg/mL) of oxLDL, low oxLDL concentrations were found to inhibit platelet aggregation.14 Additionally, at a degree of oxidation of < 15%, oxLDL promoted platelet aggregation, but at > 30% oxidation, oxLDL attenuated platelet aggregation.15 These discrepancies between studies may be due to using different methods to oxidize LDL and to evaluate activation and aggregation of platelets, including the type of oxidant, LTA, and using platelet agonists. Therefore, in the present study, we measured β-TG levels to evaluate platelet activation in addition to LTA, using oxLDL as an agonist.
In our study, LDL treated with sodium hypochlorite was analyzed by agarose gel electrophoresis to confirm oxLDL generation. As expected, the electrophoretic mobility of oxLDL was increased compared with non-treated LDL. However, the electrophoretic profile in our study is completely different from that of LDL oxidized by CuSO4.24 Taking into account induction of four types of oxLDL by sodium hypochlorite, each particle type might have different effects on activation and aggregation of platelets. Actually L5, which is the most electronegative subfraction of LDL, induces platelet activation.25 In our study, there was no significant difference in protein and lipid composition between LDL and oxLDL, except for LDL-C concentrations. This finding indicated that LDL was successfully oxidized and not correctly recognized as normal LDL by the homogeneous method of the LDL-C assay. Although we obtained fresh plasma from healthy volunteers to isolate LDL, we could not obtain modification-free LDL because of oxidation during preparation. We also need to consider the difference in oxidization between in vitro and in vivo conditions.
In the platelet aggregation experiment, oxLDL, even naturally oxidized LDL, slightly inhibited platelet aggregation and possibly inhibited enhanced platelet aggregation induced by ADP stimulation. Actually, Coleman et al.12 reported that high oxLDL levels with low ADP levels were less effective at stimulating aggregation than low oxLDL levels. Akkerman26 also reported that excess oxidation renders oxLDL an aggregation inhibitor. The reason for this finding is that the fibrinogen receptor, integrin αIIbβ3, is constitutively associated with CD36, and binding of oxLDL to CD36 interferes with the binding of fibrinogen to αIIbβ3.
In contrast, small platelet aggregation, as estimated by laser light scattering, clearly occurred upon addition of Buffer B, LDL, or oxLDL. In particular, LDL induced a relatively higher level of small platelet aggregation compared with Buffer B and oxLDL. These data suggest that prepared LDL may be slightly oxidized during preparation.27 This reaction weakly stimulates aggregation formed by a small number of platelets and oxLDL inhibits this reaction. Weakly oxidized LDL, such as LDL used in this experiment, is present in the blood circulation, suggesting that weakly oxidized LDL stimulates platelets in vivo.
Our results of β-TG levels in PRP suggested that oxLDL caused an initial weak activation of platelets, and subsequently inhibited platelet function of secreting β-TG (at 15 minutes). Furthermore, these results are consistent with those of aggregation experiments using LTA. These results might be consistent with a previous report that β-TG was not associated with the risk of CVD.28 However, endogenous lipid levels in PRP, such as TC, TG, HDL-C, and LDL-C, were different in each experiment. Although, we cannot rule out that these differences affect secretion of β-TG from platelets in PRP, the profile of β-TG levels may reflect the effect of oxLDL. This is because the amount of oxLDL (as the cholesterol level) added was almost equal to the amount of endogenous LDL in PRP and similar profiles were obtained from these three experiments. The slight differences in profiles may have been induced by a difference in the platelets’ origin or HDL-C levels. This is because similar profiles of β-TG levels were obtained using PRP derived from the same subject at different times (Figure 4a and 4b), while the profile shown in Figure 4c was obtained from a different subject. Although the functional difference in platelets obtained from two subjects was unclear, HDL-C levels in PRP were obviously different between these subjects. HDL interferes with platelet activation induced by oxLDL.29,30 Additionally, we confirmed that these profiles of β-TG levels were not induced by β-TG degradation during incubation with oxLDL.
The predominant limitation of this study is that although the same batch of oxLDL was used in several experiments, the platelets were prepared from plasma obtained from different subjects. In fact, the results shown in Figure 4a and 4b, which were obtained using platelets derived from the same subject, showed similar profiles. However, the results shown in Figure 4c, which were obtained using platelets derived from a different subject, showed a slightly different profile. This difference may have been caused by a difference in the platelets’ origin. Therefore, we need to consider the heterogeneity of platelets in addition to the effect of preparation of platelets on their function.
Conclusions
oxLDL in vitro weakly activates platelets at an early stage, but then inhibits platelet function, such as aggregation and β-TG secretion. Therefore, platelets in vivo may be activated and facilitate aggregation by transient contact with oxLDL.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 17H00666).
ORCID iD: Takeshi Uehara https://orcid.org/0000-0002-7694-9015
References
- 1.Hartley A, Haskard D, Khamis R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis - Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc Med 2019; 29: 22–26. [DOI] [PubMed] [Google Scholar]
- 2.Obermayer G, Afonyushkin T, Binder CJ. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost 2018; 16: 418–428. [DOI] [PubMed] [Google Scholar]
- 3.Tsimikas S, Miller YI. Oxidative modification of lipoproteins: mechanism, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des 2011; 17: 27–37. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty A, Dunn JL, Rateri DL, et al. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 1994; 94: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Samad G, Bazzi S, Karam M, et al. Effect of myeloperoxidase modified LDL on bovine and human aortic endothelial cells. Exp Ther Med 2019; 18: 4567–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flierl U, Bauersachs J, Schäfer A. Modulation of platelet and monocyte function by the chemokine fractalkine (CX3 CL1) in cardiovascular disease. Eur J Clin Invest 2015; 45: 624–633. [DOI] [PubMed] [Google Scholar]
- 7.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015; 126: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 2003; 9: 61–67. [DOI] [PubMed] [Google Scholar]
- 9.Hayward CPM, Moffat KA, Brunet J, et al. Update on diagnostic testing for platelet function disorders: What is practical and useful? Int J Lab Hematol 2019; 41 Suppl 1: 26–32 doi: 10.1111/ijlh.12995. [DOI] [PubMed] [Google Scholar]
- 10.Nording H, Baron L, Langer HF. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020: S0021-9150(20)30292-6. [DOI] [PubMed] [Google Scholar]
- 11.Brandt E, Ludwig A, Petersen F, et al. Platelet-derived CXC chemokines: old players in new games. Immunol Rev 2000; 177: 204–216. [DOI] [PubMed] [Google Scholar]
- 12.Coleman LG, Jr, Polanowska-Grabowska RK, Marcinkiewicz M, et al. LDL oxidized by hypochlorous acid causes irreversible platelet aggregation when combined with low levels of ADP, thrombin, epinephrine, or macrophage-derived chemokine (CCL22). Blood 2004; 104: 380–389. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZT, Wang Z, Hu YW. Possible roles of platelet-derived microparticles in atherosclerosis. Atherosclerosis 2016; 248: 10–16. [DOI] [PubMed] [Google Scholar]
- 14.Chou DS, Chan CH, Hsiao G, et al. Inhibitory mechanisms of low concentrations of oxidized low-density lipoprotein on platelet aggregation. J Biomed Sci 2006; 13: 333–343. [DOI] [PubMed] [Google Scholar]
- 15.Korporaal SJ, Gorter G, Van Rijn HJ, et al. Effect of oxidation on the platelet-activating properties of low-density lipoprotein. Arterioscler Thromb Vasc Biol 2005; 5: 867–872. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen TB, Nielsen MH, Handberg A. In vitro incubation of platelets with oxLDL does not induce microvesicle release when measured by sensitive flow cytometry. Front Cardiovasc Med 2015; 2: 37. doi: 10.3389/fcvm.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resmi KR, Krishnan LK. Protease action and generation of beta-thromboglobulin-like protein followed by platelet activation. Thromb Res 2002; 107: 23–29. [DOI] [PubMed] [Google Scholar]
- 18.Brandt E, Petersen F, Ludwig A, et al. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol 2000; 67: 471–478. [DOI] [PubMed] [Google Scholar]
- 19.Ehlert JE, Gerdes J, Flad HD, et al. Novel C-terminally truncated isoforms of the CXC chemokine beta-thromboglobulin and their impact on neutrophil functions. J Immunol 1998; 161: 4975–4982. [PubMed] [Google Scholar]
- 20.Files JC, Malpass TW, Yee EK, et al. Studies of human plate alpha-granule release in vivo. Blood 1981; 58: 607–618. [PubMed] [Google Scholar]
- 21.Yano K, Ohkawa R, Sato M, et al. Cholesterol Efflux Capacity of Apolipoprotein A-I Varies with the Extent of Differentiation and Foam Cell Formation of THP-1 Cells. J Lipids 2016; 2016: 9891316. Epub 2016 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazell LJ, Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J 1993; 290: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbrecher UP, Parthasarathy S, Leake DS, et al. Modification of low-density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low-density lipoprotein phospholipids. Proc Natl Acad Sci USA 1984; 81: 3883–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rota S, McWilliam NA, Baglin TP, et al. Atherogenic Lipoproteins Support Assembly of the Prothrombinase Complex and Thrombin Generation: Modulation by Oxidation and Vitamin E. Blood 1998; 91: 508–515. [PubMed] [Google Scholar]
- 25.Shen MY, Chen FY, Hsu JF, et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016; 127: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akkerman JW. From low-density lipoprotein to platelet activation. Int J Biochem Cell Biol 2008; 40: 2374–2378. [DOI] [PubMed] [Google Scholar]
- 27.Carbonneau MA, Cartron E, Leger CL, et al. New insight on the relationship between LDL composition, associated proteins, oxidative resistance and preparation procedure. Free Radic Res 2002; 36: 127–142. [DOI] [PubMed] [Google Scholar]
- 28.Kubota Y, Alonso A, Folsom AR. b-Thromboglobulin and incident cardiovascular disease risk: The Atherosclerosis Risk in Communities study. Thromb Res 2017; 155: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badrnya S, Assinger A, Volf I. Native high density lipoproteins (HDL) interfere with platelet activation induced by oxidized low density lipoproteins (OxLDL). Int J Mol Sci 2013; 14: 10107–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Stoep M, Korporaal SJ, Van Eck M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc Res 2014; 103: 362–371. [DOI] [PubMed] [Google Scholar]