Abstract
Objectives
Commensal bacteria in the nasal cavity may act as opportunistic pathogens that cause infections under certain conditions. Screening for commensal bacteria in the nasal cavity may aid in understanding their roles in microbiota balance and preventing potential infections.
Methods
Nasal samples were collected from healthy preclinical medical students and used to inoculate various bacterial culture media, by means of the WaspLab microbiology automated system. Bacterial colonies were then identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Antibiotic resistance phenotypes of Staphylococcus aureus were determined by antibiotic susceptibility tests.
Results
In total, 549 bacterial strains were isolated from 161 participants. These strains included the following genera: Staphylococcus, Streptococcus, Corynebacterium, Dolosigranulum, Bacillus, Micrococcus, Haemophilus, Neisseria, Moraxella, Pseudomonas, and members of Enterobacteriaceae (e.g., Escherichia, Klebsiella, Citrobacter, Enterobacter, and Serratia). Approximately 25.5% of students were carriers of S. aureus; most S. aureus isolates were resistant to penicillin, erythromycin, and clindamycin. The prevalence of methicillin-resistant S. aureus in nasal samples was 4.3%.
Conclusions
A diverse group of nasal commensal bacteria inhabited our population of healthy volunteers. These data can improve comprehension of the potential roles of these nasal commensal bacteria in regulating microbiota balance and promoting or mitigating potential future infections.
Keywords: Staphylococcus aureus, commensal bacteria, nasal vestibule, healthy population, nasal colonization screening, hospital infection control
Introduction
Commensal bacteria in the human nasal cavity are important members of the normal flora that contribute to maintenance of normal physiological and immune functions.1,2 These diverse commensal species possess immunoregulatory functions; they also inhibit or eliminate incoming pathogens. However, normal microbial flora may harbor opportunistic pathogens that cause infection in states of immune dysfunction or nasal microbial dysbiosis. Common bacterial infections are frequently associated with nasal colonization by a genetically homologous strain.3,4 Previous studies have suggested that some microbial interactions can affect human nasal colonization by opportunistic pathogens.1 However, the interactions that mediate pathogenicity involving nasal commensal bacteria remain poorly understood; the increasing resistance of these bacteria to most available antibiotics poses a substantial public health problem.3
Staphylococcus aureus is a Gram-positive facultative anaerobe with important roles in human health. It is both a commensal bacterium and a versatile opportunistic pathogen that primarily colonizes the human anterior nares (permanently or intermittently), with carrier rates of 20% to 30% in healthy populations.5–7 Following colonization, S. aureus translocates to multiple body sites and causes both hospital- and community-acquired infections with considerable morbidity and mortality.8 Many S. aureus strains are highly resistant to multiple antibiotics, which impedes treatment and leads to more severe illness and higher mortality rates.9 Indeed, the high prevalences of community- and hospital-acquired infections of methicillin-resistant S. aureus (MRSA) constitute serious public health concerns.10 Nasal colonization by S. aureus is considered a predisposing factor for nosocomial infections; its eradication from the nose is effective in reducing such infections.11
Medical students represent an important subset of healthy individuals in a given population, such that studies of their nasal bacteria might improve recognition of important commensal species that regulate microbiota balance. Furthermore, medical students represent an important group within the context of healthcare staff, who might spread bacteria to other community members or susceptible patients.12 Medical students could participate in greater awareness regarding hospital-acquired infections. Therefore, analyses of prevalence in medical students are needed to assess their carriage statuses before clinical rotations. This study evaluated the nasal carriage rates of cultivable commensal bacteria in healthy preclinical medical students in China, including S. aureus strains with antibiotic resistance. The results are expected to facilitate a broader understanding of the mechanisms that affect nasal colonization by opportunistic pathogens, as well as the potential risks of future infections.
Materials and methods
Study Population
Samples were collected from healthy, third-year, preclinical medical students from the Clinical Medicine Science Department of the Fourth Military Medical University, with approval by the ethics committee of Xijing Hospital, Fourth Military Medical University (approval number: B670201422215). All participants provided written informed consent prior to participation in the study. All experiments were performed in accordance with relevant guidelines and regulations. Participants were excluded if they met any of the following criteria: systemic or local infectious disease within the past 1 month; administration of antibiotic drugs within the past 1 month; a history of travel history in the past 2 months; and/or a history of chronic respiratory disease (e.g., chronic obstructive pulmonary disease or bronchiectasis), immunocompromised state, autoimmune disease, chronic granulomatous disease, diabetes, or tumors.
Specimen collection and transportation
Nasal specimens were collected for bacterial isolation using the flocked eSwab transport system (Copan, Brescia, Italy). One moistened swab was introduced into both anterior nares approximately 2 cm into the nasal passage, gently rubbed across the nasal mucous membranes, and rotated five times (both clockwise and counterclockwise) to acquire squamous epithelial cells. All samples were immediately stored in eSwab Liquid Amies preservation medium and transported to the laboratory for processing, or stored at 4°C until further analysis.
Specimen processing and bacterial isolation
All swabs were loaded into the WASPLab laboratory automation systems (Copan) for plating on 5% sheep blood agar, chocolate agar, and MacConkey agar plates (Autobio, Zhengzhou, China). The inoculated plates were moved by a conveyor belt to a digital imager, where images were obtained at time point 0; they were then moved into the WASPLab incubator and incubated at 35°C with 5% CO2 for 16 hours, in accordance with the laboratory’s standard operating procedures. Upon reaching the end of incubation, a second image of plates was collected and used for manual assessment of bacterial growth. Plates with no bacterial growth after 16 hours of incubation were incubated for an additional 24 hours (40 hours total) and a third image was collected for manual assessment of bacterial growth. All isolates were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry.
Matrix-assisted laser desorption ionization-time of flight mass spectrometry identification
Plate preparation, mass spectrum generation, and processing were performed in accordance with previously described standards13 for the Vitek MS system (bioMérieux, Marcy l'Etoile, France). Briefly, a 1-mL loop was used to apply a portion of a single colony to a disposable polypropylene target slide (bioMérieux). One milliliter of a-cyano-4-hydroxycinnamic acid matrix solution (3.1% wt/vol; bioMérieux) was immediately applied to the isolate and allowed to dry at room temperature, prior to mass spectrometric analysis. All isolates were analyzed with the Vitek MS in linear positive-ion mode using the Vitek MS Acquisition Station software version 2.0 (bioMérieux). The similarities of samples with reference isolates in the MS-ID database (within Vitek MS Acquisition Station software) was then calculated and expressed as an optimized quantitative value (confidence value). Newly passaged isolates of Escherichia coli ATCC 8739, Enterococcus faecalis ATCC 19433, and Candida glabrata ATCC MYA2950 were used as control strains.
Antimicrobial susceptibility testing for S. aureus
Following identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry, S. aureus isolates were subjected to antimicrobial susceptibility testing to determine their antibiotic resistance phenotypes. Each bacterial colony was resuspended in 0.45% sodium chloride (bioMérieux) by using sterile swabs to prepare 0.5-McFarland suspensions, in accordance with the manufacturer’s recommendations. Subsequently, 280 µL of bacterial suspension was diluted in 3 mL of 0.45% sodium chloride and transferred into Vitek-2 AST-GP67 cards, which were then loaded into the Vitek-2 Compact system (bioMérieux) for automatic incubation and reporting. Raw minimum inhibitory concentrations (μg/mL) were converted to sensitive, intermediate, and resistant classifications, in accordance with the 2018 guidelines from the Clinical and Laboratory Standard Institute.14 S. aureus ATCC 25923 was used as a quality control for antimicrobial susceptibility testing.
Results
In total, 161 participants were enrolled in this study; the mean participant age was 20.36 ± 1.13 years and most participants were men (140, 87.0%). Table 1 shows the overall bacteriological profile of nasal specimens collected from participants in this study. Bacteriological analysis identified 549 cultivable bacterial strains from nasal samples, including the following genera: Staphylococcus, Streptococcus, Corynebacterium, Bacillus, Micrococcus, Haemophilus, Neisseria, and Moraxella, as well as the species Dolosigranulum pigrum and Pseudomonas aeruginosa. It also revealed the presence of Enterobacteriaceae members such as Klebsiella spp., Citrobacter spp., E. coli, Enterobacter cloacae, and Serratia marcescens. None of the nasal samples were devoid of bacteria, and no participants exhibited anaerobic or fungal pathogens. The results revealed that members of the Staphylococcus genus (207 strains) were the most prevalent bacterial isolates, followed by Corynebacterium (205 strains) and D. pigrum (38 strains). There was a high prevalence of coagulase-negative staphylococci (CoNS, 166 strains) among all nasal isolates of Staphylococcus; a small subset of these were S. aureus (41 strains).
Table 1.
Prevalences of cultivable bacteria in nasal specimens from medical students in China.
| Bacteriological classification | Number of isolates | Prevalence |
|---|---|---|
| Gram-positive | ||
| Staphylococcus aureus | 41 | 25.5% |
| Staphylococcus epidermidis | 150 | 93.1% |
| Staphylococcus haemolyticus | 8 | 5.0% |
| Staphylococcus cohnii | 4 | 2.5% |
| Staphylococcus capitis | 4 | 2.5% |
| Streptococcus pneumoniae | 1 | 0.6% |
| Streptococcus mitis | 4 | 2.5% |
| Corynebacterium accolens | 151 | 93.8% |
| Corynebacterium pseudodiphtheriticum | 54 | 33.5% |
| Dolosigranulum pigrum | 38 | 23.6% |
| Bacillus cereus | 32 | 19.9% |
| Bacillus brevis | 1 | 0.6% |
| Micrococcus luteus | 2 | 1.2% |
| Micrococcus lylae | 1 | 0.6% |
| Gram-negative | ||
| Escherichia coli | 2 | 1.2% |
| Klebsiella pneumoniae | 13 | 8.1% |
| Klebsiella aerogenes | 13 | 8.1% |
| Klebsiella oxytoca | 2 | 1.2% |
| Serratia marcescens | 3 | 1.9% |
| Enterobacter cloacae | 2 | 1.2% |
| Citrobacter koseri | 1 | 0.6% |
| Citrobacter freudii | 3 | 1.9% |
| Citrobacter werkmanii | 1 | 0.6% |
| Pseudomonas aeruginosa | 1 | 0.6% |
| Haemophilus influenzae | 2 | 1.2% |
| Haemophilus parainfluenzae | 1 | 0.6% |
| Moraxella nonliquefaciens | 8 | 5.0% |
| Moraxella lacunata | 1 | 0.6% |
| Moraxella osloensis | 3 | 1.9% |
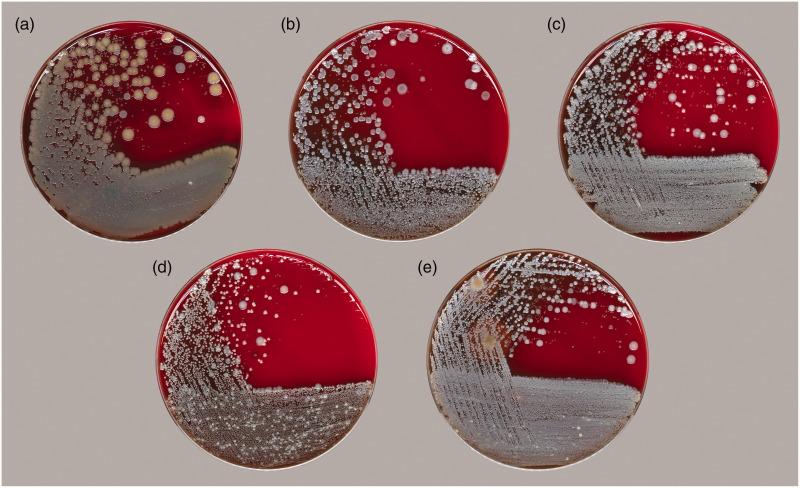
Overall, there were 72 co-occurrence patterns of commensal bacteria in nasal specimens cultured from the participants in this study; the number of bacterial species in a single sample varied between two and six. The compositions and prevalences of the top five co-occurrence patterns of cultivable nasal bacteria are listed in Table 2; representative photographs of these patterns of nasal commensal bacteria are shown in Figure 1. The dominant co-occurrence pattern of cultivable nasal bacteria consisted of S. aureus, Staphylococcus epidermidis, and Corynebacterium accolens, which was present in specimens from 13% of the participants.
Table 2.
Top five major co-occurrence patterns of cultivable bacteria in nasal specimens from medical students in China.
|
Co-occurrence patterns of cultivable nasal bacteria |
Number of carriers | Prevalence | |||||
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Staphylococcus epidermidis | Corynebacterium accolens | Corynebacterium pseudodiphtheriticum | Dolosigranulum pigrum | Bacillus cereus | ||
| √ | √ | √ | 21 | 13.0% | |||
| √ | √ | 19 | 11.8% | ||||
| √ | √ | √ | 9 | 5.6% | |||
| √ | √ | √ | √ | 9 | 5.6% | ||
| √ | √ | √ | 8 | 5.0% | |||
Figure 1.
Representative photographs of the top five co-occurrence patterns of commensal bacteria in nasal specimens collected from medical students in China, grown on appropriate culture medium. (a) Staphylococcus aureus, Staphylococcus epidermidis, and Corynebacterium accolens; (b) S. epidermidis and C. accolens; (c) S. epidermidis, C. accolens, and Corynebacterium pseudodiphtheriticum; (d) S. epidermidis, C. accolens, C. pseudodiphtheriticum, and Dolosigranulum pigrum; and (e) S. epidermidis, C. accolens, and Bacillus cereus.
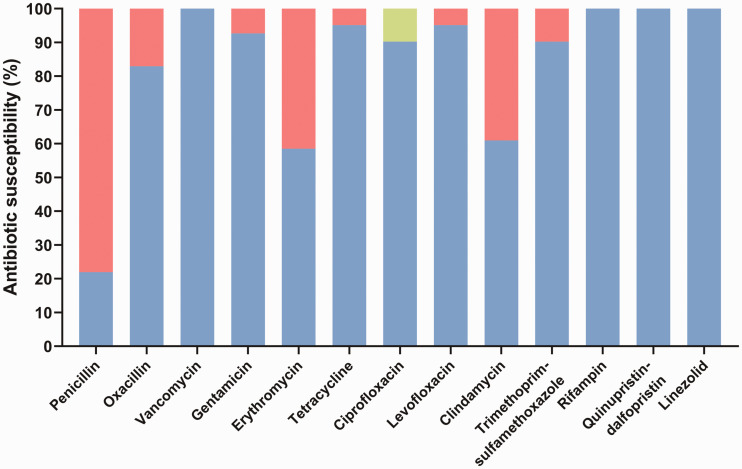
The susceptibility patterns of S. aureus to various antibiotics are shown in Figure 2. Among the 41 S. aureus isolates, 32 (78.04%) were highly resistant to penicillin. Erythromycin and clindamycin resistances were observed in 17 (41.46%) and 16 (39.02%) isolates, respectively. Gentamicin (7.32%), levofloxacin (4.88%), and trimethoprim/sulfamethoxazole (9.76%) all exhibited resistance rates of <10%. Four (9.76%) isolates exhibited intermediate resistance to ciprofloxacin. All isolates were susceptible to vancomycin, rifampin, quinupristin/dalfopristin, and linezolid. Of the 41 S. aureus isolates, seven (17.07%) were resistant to oxacillin and classified as MRSA, while 34 (82.93%) were classified as methicillin-sensitive S. aureus. The overall prevalences of S. aureus and MRSA in nasal samples were 25.5% and 4.3%, respectively.
Figure 2.
Antimicrobial susceptibilities of Staphylococcus aureus isolates (n = 41) in nasal specimens collected from medical students in China. Red indicates resistant phenotype; green indicates intermediate phenotype; blue indicates sensitive phenotype.
Discussion
Early studies of the human microbiota indicated that commensal bacteria in the healthy respiratory tract comprise species from the following genera (including some potential pathogens): Staphylococcus, Streptococcus, Corynebacterium, Moraxella, Neisseria, and Haemophilus.1,6,15 The Human Microbiome Project has revealed that the genera Corynebacterium, Moraxella, Propionibacterium, and Staphylococcus are predominant in the anterior nares.16 In our study, members of the Staphylococcus (S. epidermidis and S. aureus), Corynebacterium (C. accolens and Corynebacterium pseudodiphtheriticum), and Dolosigranulum (D. pigrum) genera were the most prevalent bacterial species in nasal specimens collected from of healthy preclinical medical students; there were also low prevalences of Enterobacteriaceae members and P. aeruginosa, indicating a normal nasal microbiota with considerable diversity.
Most nasal commensal bacteria function as mutualists, such that they alter the local host microenvironment in a manner that can help to protect against colonization by (and proliferation of) pathobionts, such as S. aureus, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1 Notably, S. pneumoniae, H. influenzae, and M. catarrhalis are the most common causative bacterial pathogens associated with community-acquired respiratory tract infections (e.g., pneumonia, bronchitis, pharyngitis, otitis media, and paranasal sinusitis).17 These pathogens can also cause life-threatening invasive infections such as meningitis, epiglottitis, periorbital cellulitis, and bacteremia. Colonization by S. pneumoniae, H. influenzae, and M. catarrhalis has been associated with lower levels of microbial diversity in the upper respiratory tract.18 Furthermore, children who exhibited low levels of nasal colonization by Streptococcus and Haemophilus spp. had greater relative abundances of Corynebacterium and Dolosigranulum spp.18,19 Furthermore, C. accolens has been reported to inhibit pneumococcal growth during in vitro cocultivation on medium that mimics the nasal environment.20 These findings in prior studies demonstrated that nasal commensal bacteria may be protective against pneumococcal infections.
Although commensal bacteria have been shown to exhibit beneficial effects, there is evidence that these bacteria can also serve as opportunistic pathogens. S. aureus is typically regarded as a bacterial pathogen because it can cause multiple types of invasive infections. Nevertheless, S. aureus asymptomatically colonizes the nares in approximately 30% of the human population, and can therefore be considered a commensal species.3,4,6–9 Individual carriers of S. aureus who are hospitalized and immunocompromised are at a substantially greater risk of S. aureus-associated infections, presumably caused by a commensal nasal strain, compared with non-carriers.4,11 Therefore, at-risk individuals in many countries are screened for the presence of MRSA in the nose; to aid in decolonization, MRSA nasal carriers are isolated and treated with the antibiotic mupirocin as a preventive measure.11 In our study, approximately 25.5% of the participants were nasal carriers of S. aureus, while 4.3% were nasal carriers of MRSA. Our results are similar to those of another study conducted on a population of preclinical medical students, which demonstrated prevalences of 25.5% for S. aureus and 9.4% for MRSA in nasal specimens.21 These colonized S. aureus strains displayed robust antimicrobial resistances to penicillin, erythromycin, and clindamycin. Furthermore, staphylococcal species are the most frequently detected microbiota on the human body surface. Most of these species are CoNS, which constitute opportunistic pathogens that cause nosocomial infections in immunocompromised individuals during long-term hospitalization and medical device usage.22
Notably, most catheter-related, device-related, and prosthetic joint infections are caused by CoNS, especially by S. epidermidis and Staphylococcus haemolyticus, which are widely distributed in skin and mucosa and readily colonize indwelling artificial medical devices.22 Because of the rapid development and spread of antibiotic resistance, CoNS may serve as hidden reservoirs for antibiotic-resistant isolates; these often reduce therapeutic efficacy and pose substantial health and economic challenges.22 C. accolens is a common inhabitant of the upper respiratory tract including eyes, ears, nose, and oropharynx. It has been implicated in endocarditis of native aortic and mitral valves, as well as in sepsis, otitis media, keratoconjunctivitis, sinusitis maxillaris, breast abscess, meningitis, and pelvic osteomyelitis.23–25 C. pseudodiphtheriticum is a member of the oropharyngeal microbiota, which is known to cause pneumonia in various patient populations (e.g., patients with pre-existing chronic respiratory infections, endocarditis, keratitis, urinary tract infections, or skin wound infections).26 D. pigrum has been associated with nosocomial pneumonia and septicemia,27 synovitis,28 acute cholecystitis accompanied by acute pancreatitis,29 and biomaterial-associated arthritis.30 Because of the increasing prevalences of multidrug-resistant S. aureus and other nasal commensal bacteria, as well as their propensities for community spread, there is a need for emphasis of standard practices to minimize their transmission during clinical training, which can help to reduce the risks of community- or hospital-acquired infections.
An important limitation of this study was that it relied upon a traditional culture method, rather than metagenomic sequencing; thus, the results could not demonstrate overall microbial species diversity, because the nasal cavity harbors a diverse population of uncultivable commensal microbiota.2 Another important limitation was the small sample size, because eligible individuals were limited to a predefined student population. Further investigations are necessary to gain insight into the mechanisms involved in interspecies interactions that regulate microbiota balance. Moreover, additional data are needed to develop effective microbiota-based approaches for control of bacterial pathogens.
In conclusion, we demonstrated the prevalences and compositions of commensal microbiota in nasal samples from preclinical medical students; our results indicated considerable diversity in the healthy nasal commensal community in this population of healthy individuals. These findings can improve general knowledge concerning the potential roles of these nasal commensal bacteria in regulation of microbiota balance, as well as the risks of future infections that involve these species. The results highlight the need for enhanced awareness among medical students regarding their roles in the transmission of pathogens and prevention of relevant infections.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from Disciplinary Research Startup Program of Xijing Hospital in Fourth Military Medical University (XJZT19ML23 and XJZT18ML54).
ORCID iD: Fei Sun https://orcid.org/0000-0003-2378-2896
References
- 1.Brugger SD, Bomar L, Lemon KP. Commensal-pathogen interactions along the human nasal passages. PLoS Pathog 2016; 12: e1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wos-Oxley ML, Chaves-Moreno D, Jauregui R, et al. Exploring the bacterial assemblages along the human nasal passage. Environ Microbiol 2016; 18: 2259–2271. [DOI] [PubMed] [Google Scholar]
- 3.Yang ES, Tan J, Eells S, et al. Body site colonization in patients with community-associated methicillin-resistant Staphylo-coccus aureus and other types of S. aureus skin infections. Clin Microbiol Infect 2010; 16: 425–431. [DOI] [PubMed] [Google Scholar]
- 4.Von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344: 11–16. [DOI] [PubMed] [Google Scholar]
- 5.Young BC, Wu CH, Gordon NC, et al. Severe infections emerge from commensal bacteria by adaptive evolution. eLife 2017; 6: e30637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krismer B, Weidenmaier C, Zipperer A, et al. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol 2017; 15: 675–687. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins A, Diep BA, Mai TT, et al. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 2015; 6: e02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn J, Stelzner K, Rudel T, et al. Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol 2018; 308: 607–624. [DOI] [PubMed] [Google Scholar]
- 9.Lee GC, Dallas SD, Wang Y, et al. Emerging multidrug resistance in community-associated Staphylococcus aureus involved in skin and soft tissue infections and nasal colonization. J Antimicrob Chemother 2017; 72: 2461–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BY, Singh A, David MZ, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 2013; 19: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362: 9–17. [DOI] [PubMed] [Google Scholar]
- 12.Collazos Marín LF, Estupiñan Arciniegas G and Chavez Vivas M. Characterization of Staphylococcus aureus isolates that colonize medical students in a hospital of the city of Cali, Colombia. Int J Microbiol 2015; 2015: 358489. [DOI] [PMC free article] [PubMed]
- 13.Dubois D, Grare M, Prere MF, et al. Performances of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid identification of bacteria in routine clinical microbiology. J Clin Microbiol 2012; 50: 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, Twenty-eight Informational Supplement. Document M100–S28, 2018. Wayne, PA: CLSI.
- 15.Hull MW, Chow AW. Indigenous microflora and innate immunity of the head and neck. Infect Dis Clin North Am 2007; 21: 265–282. [DOI] [PubMed] [Google Scholar]
- 16.Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 2012; 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang F, Wang H, et al. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated from community-acquired respiratory tract infections in China: results from the CARTIPS Antimicrobial Surveillance Program. J Glob Antimicrob Resist 2016; 5: 36–41. [DOI] [PubMed] [Google Scholar]
- 18.Pettigrew MM, Laufer AS, Gent JF, et al. Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl Environ Microbiol 2012; 78: 6262–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufer AS, Metlay JP, Gent JF, et al. Microbial communities of the upper respiratory tract and otitis media in children. mBio 2011; 2: e00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomar L, Brugger SD, Yost BH, et al. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. mBio 2016; 7: e01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma XX, Sun DD, Wang S, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus among preclinical medical students: epidemiologic and molecular characteristics of methicillin-resistant S. aureus clones. Diagn Microbiol Infect Dis 2011; 70: 22–30. [DOI] [PubMed] [Google Scholar]
- 22.Heilmann C, Ziebuhr W, Becker K. Are coagulase-negative staphylococci virulent?. Clin Microbiol Infect 2019; 25: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 23.Ang LM, Brown H. Corynebacterium accolens isolated from breast abscess: possible association with granulomatous mastitis. J Clin Microbiol 2007; 45: 1666–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claeys G, Vanhouteghem H, Riegel P, et al. Endocarditis of native aortic and mitral valves due to Corynebacterium accolens: report of a case and application of phenotypic and genotypic techniques for identification. J Clin Microbiol 1996; 34: 1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong JS, Seaward LM, Ho CP, et al. Corynebacterium accolens-associated pelvic osteomyelitis. J Clin Microbiol 2010; 48: 654–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkovski A. Corynebacterium pseudodiphtheriticum: Putative probiotic, opportunistic infector, emerging pathogen. Virulence 2015; 6: 673–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecuyer H, Audibert J, Bobigny A, et al. Dolosigranulum pigrum causing nosocomial pneumonia and septicemia. J Clin Microbiol 2007; 45: 3474–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall GS, Gordon S, Schroeder S, et al. Case of synovitis potentially caused by Dolosigranulum pigrum. J Clin Microbiol 2001; 39: 1202–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JC, Hou SJ, Huang LU, et al. Acute cholecystitis accompanied by acute pancreatitis potentially caused by Dolosigranulum pigrum. J Clin Microbiol 2006; 44: 2298–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnsen BO, Ronning EJ, Onken A, et al. Dolosigranulum pigrum causing biomaterial-associated arthritis. APMIS 2011; 119: 85–87. [DOI] [PubMed] [Google Scholar]