Abstract
Objective
To evaluate the efficacy and toxicity of intensity-modulated radiotherapy (IMRT) for the treatment of unresectable liver metastases.
Methods
Twenty-five patients with unresectable liver metastases treated with IMRT were enrolled from January 2003 to September 2016. The median longest diameter of the lesions was 3.3 cm (range, 1.6–16.7 cm). The fraction dose ranged from 2 to 5.2 Gy, with a median total dose of 50 Gy (range, 30–60 Gy).
Results
The median follow-up was 9.2 months (range, 2.1–48.8 months). The overall survival rates at 1 and 2 years were 46.4% and 27.4%, respectively. The 1-year local control rate was 69.8%. The 1-year progression-free survival rate was 26.3%. One patient had grade 4 liver dysfunction. One case of grade 4 leukopenia and one case of grade 3 leukopenia occurred, and one case of grade 3 leukopenia and thrombocytopenia was observed.
Conclusion
IMRT may be a promising and safe treatment for unresectable liver metastases and can be used as a treatment option.
Keywords: Liver metastasis, low-dose, intensity-modulated radiotherapy, oligometastases, survival, toxicity
Introduction
The liver is one of the most common sites of metastases. Lung, breast, and gastrointestinal cancers are frequent causes of liver metastases, and for some patients, the liver may be the only site of metastasis.1 The concept of an oligometastatic state was first proposed by Hellman and Weichselbaum in 1995 as a less advanced state of metastatic disease that is amenable to and potentially curable with local cancer treatments.2 Currently, the term “oligometastases” is most often used to describe five or fewer metastatic lesions and one to three organ sites with active primary lesions.3
Systemic chemotherapy remains the mainstay of treatment for patients with liver metastases, but long-term curative effects are unlikely unless systemic chemotherapy is combined with local therapy. For patients with oligometastases, it is possible that treatments, especially combined therapies, could result in prolonged disease-free periods with a possibility of a cure. Liver surgical resection remains the standard treatment for liver oligometastases.
Over half of the patients with colorectal cancer develop liver metastases. The liver is the only site of metastatic disease in approximately one-third of metastatic colorectal cancer patients, and surgical resection can achieve a 5-year survival rate of up to 30% in these patients. However, less than one-quarter of these patients are good candidates for surgical resection, so alternative interventions must be evaluated.4
Traditionally, radiation therapy is not considered to be a feasible treatment for liver metastases because the liver has a low radiation tolerance. However, recent advances in radiotherapy, such as the development of three-dimensional treatment planning, breathing-control techniques, and image guidance, have introduced the potential for high tumoricidal doses by stereotactic body radiotherapy (SBRT) or intensity-modulated radiotherapy (IMRT). Therefore, it should be possible to deliver a conformally high dose of radiation to the tumor while minimizing radiation exposure to normal liver tissue.
In this study, we reported a retrospective study of 25 patients with 38 liver oligometastases, regardless of the primary tumor location. We analyzed overall survival (OS), local control (LC), progression-free survival (PFS), and acute and late toxicity based on clinical and laboratory parameters.
Materials and methods
Patients
Data from adult patients with one to five hepatic metastases who met the study entry criteria were retrospectively analyzed. Patients in whom all lesions were liver metastases of various primary tumors that were considered to be technically or medically inoperable were screened for enrollment into this study. The other inclusion criteria were a performance status <3 (WHO scale), life expectancy of more than 3 months, more than 800 mL of uninvolved liver, Child–Pugh liver score of A or B, and serum liver enzymes less than three times the upper limit of normal values. No previous radiation therapy to the upper abdomen was allowed. Patients with ascites were excluded. The patients participating in the study provided written informed consent.
Treatment
Concurrent chemotherapy included icotinib in one (4%) patient, sunitinib in one (4%) patient, capecitabine in one (4%) patient, paclitaxel + cisplatin in two (8%) patients, and pemetrexed disodium + carboplatin in one (4%) patient. The patients were immobilized in a vacuum pillow. A spiral CT scan with a slice thickness of 5 mm was performed, which included the localization system. Radiotherapy was initially planned as IMRT using Pinnacle (Philips Medical Systems, Fitchburg, Massachusetts, USA). A pre-therapeutic magnetic resonance imaging (MRI) scan or a positron emission tomography (PET)/(computed tomography) CT scan was performed, and the information was used to define the gross tumor volume (GTV). The GTV included contrast-enhanced disease that was visible on an exhale contrast-enhanced CT scan. The GTV was expanded by a 5- to 8-mm radial margin and a 10- to 12-mm craniocaudal margin to create the planning target volume (PTV). The radiation dose was prescribed to an isodose line that covered the PTV (90% to 95% isodose line). The total dose and dose per fraction were chosen based on normal tissue constraints (primarily liver, stomach, and duodenum) and the desire to minimize the total treatment duration to less than 6 weeks. Image guidance was used only for patients after 2009. Only 19 patients with liver metastases received image-guided radiation therapy (IGRT). Grayscale fusion and manual fusion were used for image fusion. The target fusion errors in the X, Y and Z axes were 0.25 ±0.20 cm, 0.25 ± 0.21 cm, and 0.18 ± 0.15 cm, respectively. IMRT was delivered using three to seven fields for a median total dose of 50 Gy (range, 30–60 Gy) by a linear accelerator with an energy of 6 MV. Dose constraints for the organs at risk (OARs) were implemented. The liver tissue received an average dose of less than 23 Gy. The percent of total kidney volume (sum of the left and right kidney volumes) that received 15 Gy was less than 35%. The maximum total dose to any point in the spinal cord and stomach/small intestine was limited to 40 Gy and 52 Gy, respectively.
Follow-up
The median follow-up period was 9.2 months (range, 2.1–49.8 months). The median follow-up period was 15.6 months (range, 2.1–26.7 months) for the surviving patients. The treatment results and side effects were prospectively evaluated by clinical examinations and CT or MRI scans 4 to 6 weeks after irradiation and repeated imaging was performed at 3-month intervals during the first year followed by 3- to 6-month intervals thereafter. Treatment response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines.5 Toxicity was evaluated with the Common Terminology for Adverse Events (CTCAE) v4.0 guidelines.6 The acute toxicities were assessed by physicians. The primary outcomes were OS, LC and PFS. The toxicities for further assessment included: nausea, liver pain, fatigue, leukopenia, thrombocytopenia, biochemical changes.
Statistical analysis
LC was calculated from the day of irradiation until either the day of local progression or death or until the last follow-up without local progression. PFS was calculated from the day of irradiation until either the day of relapse or death or until the last follow-up without relapse. OS was calculated from the day of irradiation until either the day of death or the day of the last follow-up. OS, LC, and PFS were calculated using Kaplan–Meier curves. All statistical tests were performed with SPSS version 19 (IBM Corp., Armonk, NY, USA).
Results
Patient demographics
Twenty-five patients with 38 hepatic metastases that were treated between January 2003 and September 2016 were identified and included in the analysis.
Only three patients had symptoms such as liver or shoulder pain before radiotherapy. All other patients were asymptomatic, and the tumors were discovered by imaging studies. Ten (40%) patients had additional metastases (lung, 4; bone, 5; abdominal aortic lymph nodes, 2; adrenal gland, 1; abdominal wall, 1), but there were five or fewer lesions. Only two liver metastases (8%) were histologically confirmed, and the remainder were diagnosed by imaging studies. Four patients (16%) received local treatment before radiotherapy, including one attempted surgical resection (unresectable, only for pathological diagnosis), two portal vein embolization procedures, and one radiofrequency ablation procedure. Seventeen patients had controlled extrahepatic lesions, and eight patients had extrahepatic lesions that were uncontrolled. Among the patients with liver metastases, three (12%) had hepatitis B infection and cirrhosis. The patients and tumor characteristics are presented in Table 1. The toxicities were evaluated by the attending physicians.
Table 1.
Patient characteristics.
| Patients | No. | % |
|---|---|---|
| Sex | ||
| Female | 5 | 20 |
| Male | 20 | 80 |
| Age, years | ||
| Median | 58 | |
| Range | 35–82 | |
| Primary sites | ||
| Nasopharynx | 7 | 28 |
| Colorectal | 5 | 20 |
| Lung | 5 | 20 |
| Esophageal | 4 | 16 |
| Kidney | 1 | 4 |
| Liver | 1 | 4 |
| Breast | 1 | 4 |
| Endometrium | 1 | 4 |
| Time since primary tumor diagnosis, months | ||
| Median | 15.9 | |
| Range | 0–126.1 | |
| Time since diagnosis of liver metastases, months | ||
| Median | 5.1 | |
| Range | 0–20.3 | |
| No. of liver lesions | ||
| 1 | 17 | 68 |
| 2 | 5 | 20 |
| 3 | 2 | 8 |
| 5 | 1 | 4 |
| Timing of Metastases, months | ||
| At initial diagnostic/synchronous | 8 | 32 |
| Metachronous | 17 | 68 |
| Previous local therapy | ||
| Yes | 4 | 16 |
| No | 21 | 84 |
| Previous chemotherapy | ||
| Yes | 19 | 76 |
| No | 6 | 24 |
| Maximum lesion diameter, cm | ||
| Median | 3.3 | |
| Range | 1.6–16.7 | |
| >3 | 13 | 52 |
| ≤3 | 12 | 48 |
| No. of previous chemotherapy regimens | ||
| 0 | 6 | 24 |
| 1 | 4 | 16 |
| 2–3 | 4 | 16 |
| ≥4 | 11 | 44 |
| Presence of active extrahepatic disease | ||
| Yes | 11 | 44 |
| No | 14 | 56 |
| Lesion volume, mL | ||
| Median | 15.39 | |
| Range | 3.15–2214.75 | |
| Total dose, Gy | ||
| Median | 50 | |
| Range | 30-60 | |
| Dose/fraction, Gy | ||
| Median | 3 | |
| Range | 2–5.2 | |
| BED, Gy | ||
| Median | 67.2 | |
| Range | 39–79.04 | |
| No. of fractions | ||
| Median | 18 | |
| Range | 7–30 | |
| Liver average dose, Gy | ||
| Median | 15.56 | |
| Range | 4.30–22.91 | |
GTV, gross tumor volume; Gy, grays; BED, biologically effective dose.
Survival outcomes
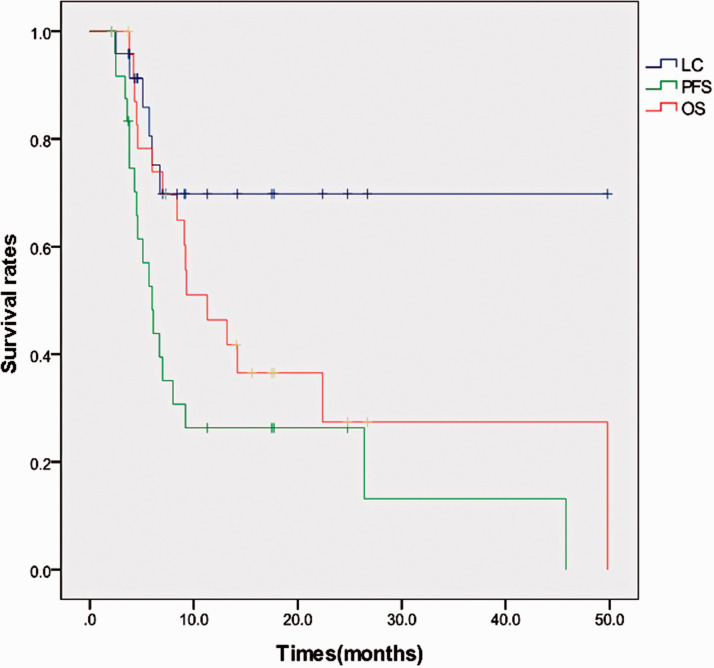
At the time of the analysis, 16 (64%) patients were deceased, and nine (36%) patients were alive. Among the deceased patients, 13 died of tumor recurrence and metastasis, one died of pulmonary infection, one died of gastrointestinal bleeding (esophageal cancer), and one died of diffuse intravascular coagulation (DIC). The median actuarial OS was 9.2 months (95% confidence interval [CI], 6.0–14.2 months). The 1-year and 2-year OS rates were 46.4% (95% CI, 25.6%–67.2%) and 27.4% (95% CI, 5.7%– 48.1%), respectively. The survival curves are shown in Figure 1.
Figure 1.
Survival curves for overall survival (OS), local control (LC), and progression-free survival (PFS).
The treatment responses included partial response (PR) in 11 patients and stable disease (SD) in 14 patients after radiotherapy. The median LC was 7.0 months (95% CI, 4.6–11.3 months). The actuarial LC rate at 1 year was 69.8% (95% CI, 49.4%–90.2%). Six patients had local failures, which occurred 3, 4, 5, 6, 6, and 7 months after treatment. The longest duration of LC was recorded for the patient followed up for 49.8 months. Most patients died because of systemic metastases or progression of the primary tumor (13/16) with locally controlled irradiated lesions.
The median actuarial PFS was 5.7 months (95% CI, 3.8–8.0 months). The actuarial 1-year PFS rate was 26.3% (95% CI, 8.3%–44.3%). The main pattern of progression was distant progression, with 15 patients developing systemic progression, 12 patients developing additional liver metastases, and five patients developing extrahepatic distant metastases. Among the newly developed extrahepatic distant metastases, four were in the lung, and one was in the brain.
Toxicities
Overall, the treatment was well tolerated, with no observed radiation-induced liver disease (RILD). Toxicities occurring within 3 months of IMRT were considered to be acute, and toxicities occurring after 3 months were considered to be late. Thirteen (52%) patients developed acute toxicity, and all hematological toxicities occurred in patients who received either chemotherapy or chemoradiotherapy before IMRT (Table 2).
Table 2.
Biochemical changes and acute toxicity of liver IMRT.
|
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| CTCAE Toxicity | No. | % | No. | % | No. | % | No. | % |
| Nausea | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 |
| Liver pain | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukopenia | 2 | 8 | 5 | 20 | 2 | 8 | 1 | 4 |
| Thrombocytopenia | 2 | 8 | 2 | 8 | 1 | 4 | 0 | 0 |
| Biochemical changes | ||||||||
| Bilirubin | ||||||||
| Baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Worst grade | 1 | 4 | 0 | 0 | 0 | 0 | 1 | 4 |
| Liver enzymes | ||||||||
| Baseline | 7 | 28 | 2 | 8 | 0 | 0 | 0 | 0 |
| Worst grade | 9 | 36 | 4 | 16 | 0 | 0 | 1 | 4 |
| Albumin | ||||||||
| Baseline | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Worst grade | 2 | 8 | 3 | 12 | 0 | 0 | 0 | 0 |
IMRT, intensity-modulated radiotherapy; CTCAE, Common Terminology Criteria for Adverse Events.
One patient with hepatitis B and cirrhosis developed grade 4 elevated liver enzymes and bilirubin levels 1.5 months after radiotherapy. After symptomatic and supportive treatment, the liver enzymes and bilirubin levels returned to normal. The average radiation dose of the liver was 1543.9 cGy. V30 for the liver minus gross the tumor volume was 14.71%, the normal liver volume was 1070.91 mL, the liver volume receiving more than 15 Gy was limited to 352.95 mL, and the total dose was 52 Gy in 13 fractions.
One patient who developed late toxicity had grade 4 elevated bilirubin levels 5 months after radiotherapy, which was associated with direct tumor obstruction of the common bile duct.
Several patients received co-treatment with other therapies. One patient with grade 4 leukemia received five cycles of paclitaxel + cisplatin before radiotherapy and two cycles concurrently. One patient with grade 3 leukopenia and thrombocytopenia was treated concurrently with sunitinib, and one patient with grade 3 leukopenia received one cycle of cisplatin + fluorouracil chemotherapy before radiotherapy.
Discussion
The present study suggests that IMRT is a safe and effective treatment for liver metastases. Patients with liver metastases in this study received a total dose of 30 to 60 Gy (median, 50 Gy). The biologically effective dose (BED) was 39 to 79.04 Gy (median, 67.2 Gy). The actuarial LC rate at 1 year was 69.8%, which is similar to that from previous studies.7–9 However, the LC was lower than that reported by Rusthoven et al.,10 Wulf et al.,11 Fumagalli et al.,12 Rule et al.,13 and Goodman et al.14 In these studies, the median BED was 79.2 to 180 Gy, and the LC rate at 1 year was 84.5% to 100%. This finding may be a result of the small lesion size and the high BED in these studies. The OS and PFS in the present study were similar to those that were previously reported, although the LC rate was somewhat higher than that reported in several previous studies.
In this study, we were unable to statistically analyze the relevant factors for LC because of the small number of cases. Many factors affect the LC rate, and radiation dose is the most important factor. In a similar study, Chang et al.9 evaluated LC rates and found that the total dose (P = 0.0015), dose/fraction (P = 0.003), and BED (P = 0.004) were all correlated with LC of the lesion. Wulf et al.11 found that radiation dosage was the only significant factor for predicting LC (P = 0.0089). However, tumor size is also an important factor that affects the LC rate. In a similar multivariate study by Rusthoven et al.,10 the 2-year LC was 100% for lesions with a maximum diameter of 3 cm but was only 77% for lesions greater than 3 cm (P = 0.015). Similarly, Lee et al.8 used a univariate analysis and found that LC was improved in small-volume tumors compared with large-volume tumors (<75.2 mL; P = 0.001) and the small tumors had a higher delivered dose compared with the large tumors (P = 0.01). LC was evaluated by Mahadevan et al.15 in 430 liver metastases from 324 patients; the 2-year LC rates were better with a BED ≥100 Gy (77.2% vs. 59.6%), and the median LC was better for tumors <40 cm3 (52 vs. 39 months). The effect of primary tumor types on LC the rate is controversial. Habermehl et al.16 found that liver metastases from breast tumors had improved LC rates compared with those from colorectal tumors. However, other studies have found that the primary tumor site has no effect on the LC rate of liver metastases.
The dose per fraction in this study was significantly lower compared with that of similar studies. Because of the current lack of large prospective clinical studies, the most appropriate dose and fractionation scheme has not yet been determined. In the literature, the total dose for liver metastases was more than 70 Gy (BED), and the fraction dose was more than 10 Gy.16 The associated toxicity of high-dose radiotherapy is not well accepted in many patients, especially gastrointestinal toxicity, which compromises patient quality of life. There was no significant correlation between the radiotherapy dose and OS in the literature. In these studies, conservative treatments with a small dose per fraction and a relatively low total dose were used, which may explain the gap in LC.
Acute toxicities exceeding CTC grade 2 were reported in the literature include gastrointestinal toxicities such as stomach ulcers, gastritis, esophagitis, and nausea; hematologic toxicities such as leukemia and thrombocytopenia; and elevated liver enzymes. Rusthoven et al.10 reported radiodermatitis and soft tissue toxicities, but no RILD was reported. In our study, toxicities exceeding CTC grade 2 included hematologic toxicity and elevated liver enzymes, but we did not observe gastrointestinal toxicity. It is possible that the dose per fraction used in this study was lower than that in other studies, which reduced gastrointestinal toxicity. Three patients that developed hematologic toxicity were treated with chemotherapy or targeted therapy before radiotherapy or concurrent chemoradiotherapy. The hematologic toxicities reported in this study may be more likely in patients receiving co-treatments compared with patients receiving monotherapy. Therefore, in this study, we demonstrated that low-dose IMRT has fewer side effects compared with high-dose IMRT, with no observable gastrointestinal toxicity or RILD. Our study has, therefore, provided useful information regarding the benefits of low-dose IMRT for liver metastases, and low-dose IMRT can be considered to be an option for radiotherapy in appropriate patient populations.
We found that there was limited hepatoxicity in this study. One patient developed grade 4 elevated liver enzymes approximately 1.5 months after IMRT, but this elevation may have been related to the patient’s hepatitis B carrier status and cirrhosis, which are associated with a higher risk of radiotherapy-induced liver injury. Although liver metastases has a higher tolerable dose than hepatocellular carcinoma, grade 4 elevated liver enzymes occasionally develop and may be related in part to a long-term drinking history. Hepatitis B virus infection is common in China. Patients should, therefore, receive appropriate serum testing and prophylactic antiretroviral therapy before initiating radiotherapy to avoid radiation-induced liver injury. Stringent dose constraints for normal liver tissue are also essential.
This study evaluated liver metastases in patients who were either medically inoperable or who refused surgery. Therefore, most patients were diagnosed using imaging studies, and only two patients were diagnosed by surgery or puncture cytology. Without a pathological diagnosis, the tumor origin cannot be confirmed, so some tumors may have been primary hepatocellular carcinoma or cholangiocarcinoma lesions. For cholangiocarcinoma, the clinical target volume (CTV) of radiotherapy generally needs to include the lymphatic drainage area. If primary liver tumors are misdiagnosed as secondary metastases, the CTV for radiotherapy may not be sufficient, which can result in treatment failure. This problem is present in most studies of liver metastases. All patients in this study (except for one case of hepatocellular carcinoma) had normal alpha fetoprotein (AFP) levels, which suggests that the liver tumors were not primary tumors. Although measuring the AFP level reduces the risk of misdiagnosis, pathological evaluations of lesions are essential to confirm that the lesions are metastatic carcinomas.
In this study, 76% of the patients received previous chemotherapy, and 44% received more than four previous rounds. Previous chemotherapy may have killed the most sensitive tumor cells, resulting in poor radiosensitivity in the remaining tumors and poor prognoses. Fumagalli et al.12 found that a history of previous chemotherapy was a major risk factor for recurrence outside of the treatment volume after radiotherapy (hazard ratio = 4.51, 95% CI, 1.10–18.47, P = 0.007). Furthermore, patients with large tumors may have received chemotherapy before the initiation of radiotherapy, and this cohort of patients may have poor prognoses. A large randomized clinical study is needed to determine the relative value of chemotherapy and radiotherapy in the treatment of liver metastases.
In conclusion, IMRT may be a promising and safe treatment modality for unresectable liver metastases. A relatively low total dose and fractionated dose was also found to be effective. In patients with a history of hepatitis B virus infection or cirrhosis, prophylactic antiretroviral therapy before radiotherapy is necessary, and strict limitations for the radiation dose to the liver should be followed. This study may provide the basis for future clinical trials to evaluate the effects of low-dose IMRT for liver metastasis.
Supplemental Material
Supplemental material, IMR892382 Supplemental Material for A retrospective cohort study of low-dose intensity-modulated radiotherapy for unresectable liver metastases by Su Pei, Kaihua Chen, Yunli Yang, Long Chen and Xiaodong Zhu in Journal of International Medical Research
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics statement
This retrospective study was approved by the Ethics Committee of the Guangxi Medical University Cancer Hospital (No. LLW2018052) and it was conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from the participating patients. All patients’ information was anonymous.
Funding
This work was supported by a grant from the Key R&D Program Project of Guangxi Province (Grant No. Guike AB18221007).
ORCID iD
Xiaodong Zhu https://orcid.org/0000-0002-7997-8268
Supplemental material
Supplemental material for this article is available online.
References
- 1.Hess KR, Varadhachary GR, Taylor SHet al. Metastatic patterns in adenocarcinoma. Cancer 2006; 106: 1624–1633. 2006/03/07. DOI: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13: 8–10. 1995/01/01. DOI: 10.1200/jco.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Milano MT, Katz AW, Zhang Het al. Oligometastases treated with stereotactic body radiotherapy: Long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012; 83: 878–886. 2011/12/17. DOI: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Fong Y, Cohen AM, Fortner JGet al. Liver resection for colorectal metastases. J Clin Oncol 1997; 15: 938–946. 1997/03/01. DOI: 10.1200/jco.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts Jet al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 6.Common Terminology Criteria for Adverse Events v4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 7.Katz AW, Carey-Sampson M, Muhs AGet al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 2007; 67: 793–798. 2007/01/02. DOI: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Lee MT, Kim JJ, Dinniwell Ret al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009; 27: 1585–1591. 2009/03/04. DOI: 10.1200/jco.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 9.Chang DT, Swaminath A, Kozak Met al. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer 2011; 117: 4060–4069. 2011/03/25. DOI: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 10.Rusthoven KE, Kavanagh BD, Cardenes Het al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009; 27: 1572–1578. 2009/03/04. DOI: 10.1200/jco.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 11.Wulf J, Guckenberger M, Haedinger Uet al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006; 45: 838–847. 2006/09/20. DOI: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 12.Fumagalli I, Bibault JE, Dewas Set al. A single-institution study of stereotactic body radiotherapy for patients with unresectable visceral pulmonary or hepatic oligometastases. Radiat Oncol 2012; 7: 164. 2012/09/28. DOI: 10.1186/1748-717x-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rule W, Timmerman R, Tong Let al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011; 18: 1081–1087. 2010/11/04. DOI: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 14.Goodman BD, Mannina EM, Althouse SKet al. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract Radiat Oncol 2016; 6: 86–95. 2016/01/05. DOI: 10.1016/j.prro.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Mahadevan A, Blanck O, Lanciano Ret al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) patient registry. Radiat Oncol 2018; 13: 26. 2018/02/15. DOI: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habermehl D, Herfarth KK, Bermejo JLet al. Single-dose radiosurgical treatment for hepatic metastases–therapeutic outcome of 138 treated lesions from a single institution. Radiat Oncol 2013; 8: 175. 2013/07/11. DOI: 10.1186/1748-717x-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, IMR892382 Supplemental Material for A retrospective cohort study of low-dose intensity-modulated radiotherapy for unresectable liver metastases by Su Pei, Kaihua Chen, Yunli Yang, Long Chen and Xiaodong Zhu in Journal of International Medical Research