Abstract
The extracellular matrix is a critical component of every human tissue. ECM not only functions as a structural component but also regulates a variety of cellular processes such as cell migration, differentiation, proliferation, and cell death. In addition, current studies suggest that ECM is critical for the pathophysiology of various human diseases. ECM is composed of diverse components including several proteins and polysaccharide chains such as chondroitin sulfate, heparan sulfate, and hyaluronic acid. Each component of ECM exerts its own functions in cellular and pathophysiological processes. One of the interesting recent findings is that ECM is involved in inflammatory responses in various human tissues. In this review, we summarized the known functions of ECM in neuroinflammation after acute injury and chronic inflammatory diseases of the central nerve systems.
Keywords: DAMP, Extracellular matrix, Neurodegenerative disease and ECM, Neuroinflammation, Neuronal ECM
INTRODUCTION
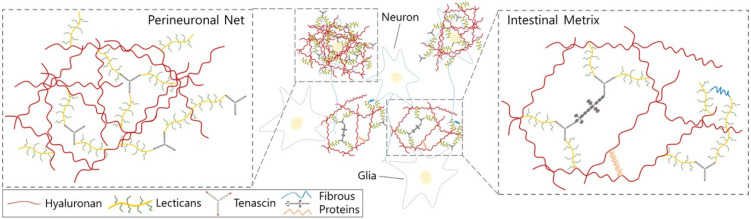
Neurons and glial cells in the central nervous system are tightly associated to each other, maintaining physiological homeostasis of the human body. In addition to the cell-cell interaction, the CNS is also composed of elaborated and complicated extracellular matrixes (ECMs). Neural ECMs are radically different from those of other tissues. The interstitial ECMs are mainly composed of a loose meshwork of hyaluronan, sulfated proteoglycans, and tenascins (1, 2). Perineuronal net (PNN) is a unique ECM structure surrounding the neuronal cell bodies and neurites to stabilize synaptic connections. PNN is also composed of hyalectans which are the meshwork of interconnected hyaluronans and lecticans (aggrecan, brevican, neurocan, and versican) (Fig. 1, 2) (3, 4). Neurons and glial cells are both responsible for the production and formation of neuronal ECMs (5). The expression of neuronal ECMs are actively regulated during CNS development. The functions of neuronal ECMs in development and synaptogenesis are extensively studied (6), but the correla-tions between neuronal ECMs and neuroinflammation are currently under intense research because they are not precisely understood. Upon the initial damage of CNS tissues by either traumatic injury or degenerative processes, the inflammatory responses in the CNS actively remodel the neuronal ECMs to prevent expansion of neuronal damage or to promote recovery of damaged tissue. These active changes in ECMs not only regulate transcription of genes involved in ECM production, but also modify existing ECM molecules by post translational modifications such as proteolytic cleavages, fragmentation, or release of GAG (glycosaminoglycan) residues from the core proteins. These modifications may either improve the recovery of neuronal damage or may aggravate the inflammatory cycles, leading to chronic inflammation in the CNS.
Fig. 1.
The extracellular matrices in central nervous systems. Interstitial ECM is loosely distributed in the CNS and neuronal cell bodies and are tightly associated and covered by PNN. Neural ECMs are mainly composed of proteoglycans and hyaluronic acid with a small amount of fibrous matrix.
Fig. 2.
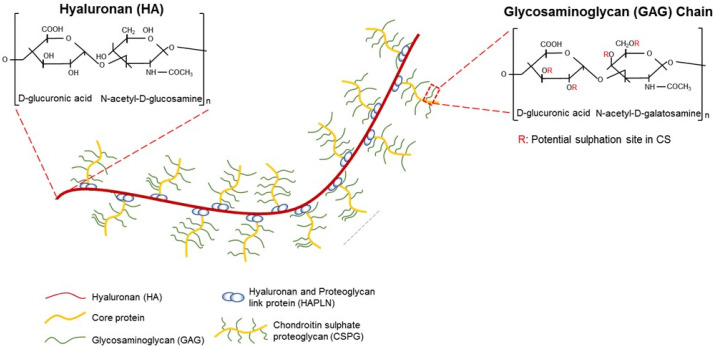
Hyalectan in neuronal ECMs. Neural ECMs are mainly composed of hyalectans which are the meshwork of interconnected hyaluronans and chondroitin sulfate proteoglycans such as aggrecan, brevican, neurocan, and versican.
In this review, we describe the structural composition of neural ECMs and the changes of neural ECMs initiated by damage to the CNS. Also, we summarize the inflammatory reactions regulated by the changes in neural ECMs.
EXTRACELLULAR MATRIXES IN CNS
Most of the ECMs in other tissues are mainly composed of fibrous proteins such as collagen, fibronectin, and laminin. In contrast, the major components of ECMs in the CNS are proteoglycans, hyaluronan, and tenascins meshwork which are interconnected to each other (1, 2). The ECM in the CNS takes about 20% of the total volume (7) and there are mainly two different types (Fig. 1).
Interstitial ECM is loosely distributed in the CNS and mainly composed of proteoglycans and hyaluronic acid with a small amount of fibrous matrix. The other specialized ECM in the brain is perineuronal net (PNN). Most neuronal cell bodies and neurites in the vicinity of the cell body are tightly associated and covered by PNN. PNN is believed to be more than mechanical support for neurons; rather, PNN regulates synaptic plasticity by stabilizing synaptic connections. PNN is also com-posed of a large amount of hyaluronic and proteoglycans, mainly sulfated proteoglycans and a small amount of fibrous proteins (3, 4). In this review we will summarize the structures and functions of sulfated proteoglycans and hyaluronic acid in neuronal ECM.
LECTICAN, THE SULFATED PROTEOGLYCAN IN NEURONAL ECMS
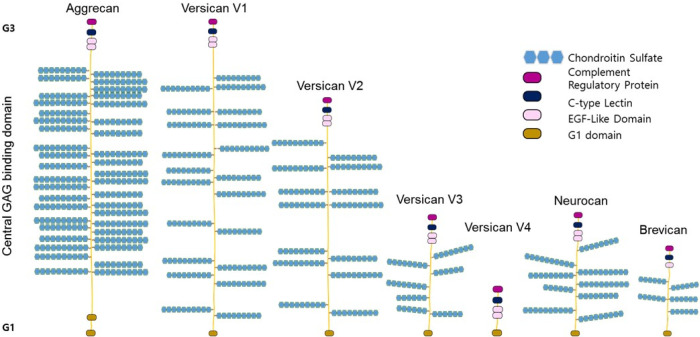
Lecticans are a class of chondroitin sulfate proteoglycan (CSPG) which share structural features and comprise major ECM net-works in the CNS. All lecticans have a conserved binding site to hyaluronan in their N-terminus G1-domain, the central domain which the GAG chain is attached to, and a lectin-containing G3 domain mediating the binding to glycoproteins such as tenascins (8, 9) (Fig. 3).
Fig. 3.
Lectican family of chondroitin sulfate proteoglycan. Lecticans are a class of chondroitin sulfate proteoglycan (CSPG) including Aggrecan, Versican, Neurocan, and Brevican. All lecticans have a G1-domain which is a conserved binding site to hyaluronan, the central GAG attachment domain, and a lectin-containing G3 domain mediating the binding to glycoproteins such as tenascins.
Although the entire lectican family of CSPGs shares structural similarity, lectican may exist as a variety of isoforms based on the chemical composition of GAG chains. The GAG chain is attached to the core proteins in the endoplasmic reticulum (ER) and Golgi compartment (10-13). The glycosylation of lectican proteoglycan is initiated by the addition of xylose residue to a serine in the central domain of lecticans. This reaction is mediated by xylotransferase in ER (10, 11, 13). Next, two additional galactoses are sequentially added to the xylose in the Golgi compartment prior to the addition of GAG chains (14). The GAG chains in CSPGs are repeating disaccharide units composed of N-acetyl-D-galactosamine (GalNAc) and D-glucuronic acid (GlcUA) (15). The length of a GAG chain is variable and more than 100 GalNAc-GlcUA disaccharide can be attached to the central domain of each lectican (15).
Beside the diversity of the length of GAG chains in lecticans, more complexity is due to the sulfation of chondroitin disaccharides. The modifications of chondroitin are mediated by sulfotransferase which adds sulfates on the specific carbons of either GalNAc or GlcUA (16). Based on the position of the sulfation, the CS chain is named CS-A (sulfation on C4 of GalNAc), CS-C (sulfation C6 of GalNAc), CS-D (sulfation on C6 of GalNAc and C2 of GlcUA), or CS-E (sulfation on C4 and C6 of GalNAc). CS-A and CS-C are known to be the most prevalent forms of chondroitin sulfate in the GAG chains of neuronal lecticans.
The functions of CSPG may be very different based on their number, sulfation, and the core proteins in CNS development and function. The diverse roles of CSPGs in normal brain development and functions are reviewed elsewhere.
AGGRECAN
Aggrecan is well-known as a major proteoglycan in cartilages (17). It functions to absorb pressure from mechanical loading. In the brain, aggrecan exerts different functions as it is predominantly observed in the perineuronal net (PNN) around the neuronal bodies and dendrites (18, 19). Neurons and astrocytes express aggrecan (20), which is thought to function on the maturation and stabilization of synaptic connections in the developing brain (19, 21).
As one of the major lecticans in the brain, aggrecan is connected to the hyaluronan via its N-terminus G1 domain, and its C-terminus binds to tenascin as a structural unit of neuronal ECMs (22). There are several suggested functions of aggrecan. Aggrecan secures high-rate synaptic transmission in PN-associated neurons (23), and protect neurons form the oxidative stress through scavenging redox-active cations (24).
VERSICAN
Versican is the second most dominant neuronal ECM. It is expressed by neurons, astrocytes, and oligodendrocytes (25-27). As with other lecticans, the structure of versican includes an N-terminus G1 domain, C-terminus G3 domain, and central GAG attachment sites. One of the unique properties of versican is that it is expressed as four splicing variants by alternative splicing (28). The central GAG attachment domain is a major splicing site; versican-V0 has the largest GAG attachment sites while versican-V1 and V2 have shorter central domains. The central GAG glycosylation region is spliced out in Versican-V3. The isoforms of versican are expressed in various tissues and Versican-V2 is predominantly expressed in the brain (29). Versican is involved in various cellular functions such as cell migration and inflammation. Especially, during an inflammatory response, versican regulate leukocytes migration and infiltration by integrating with CD44 and Toll-like receptors (30).
BREVICAN
Brevican is one of the CNS specific lecticans and is expressed in broad regions of the brain (31, 32). Both neurons and astrocytes express it (26). In addition to the secreted ECM components, brevican is also expressed in an alternative spliced form which gives rise to the GPI linked form of proteoglycan. As a structural component of PNN, brevican seems to regulate neuronal plasticity. Brevican was suggested to be involved in CNS injury and Alzheimer's disease (33).
NEUROCAN
Neurocan is also a CNS specific lectican and is predominantly found in PNN (34, 35). Although the exact function of neurocan in brain function and development is still under research, a genetic association study has suggested that the neurocan gene is associated with human bipolar disorder and schizophrenia (36, 37).
LECTICANS AND NEUROINFLAMMATION
The functions of CSPGs in neuroinflammation are most well studied in acute injury and inflammatory responses of the CNS.
The immediate responses to an acute injury of the CNS is the activation of astrocytes (38). Activated astrocytes migrate to the injury site, secrete inflammatory factors, and remodel the neuronal ECMs to prevent the expansion of neuronal damage (39, 40). One of the major outcomes of the inflammatory reaction mediated by activated astrocytes is to construct glial scars comprised of CSPGs. The glial scar functions as a barrier and isolates the damaged region from other tissues (41). This barrier protects neuronal tissues from further blood-driven inflammatory factors such as fibrinogen TNF-α and IL-1β (42). The common response of activated astrocytes and infiltrated leucocytes upon neuronal damage is increased expression of several CSPGs such as neurocan, versican, and brevican around the injury (43). In contrast, aggrecan expression was reported to be downregulated. The major signaling molecules modulating CSPG expression is thought to be TGF-β molecules. Once TGF-β molecules leak out from the blood stream, they activate SMAD2 phosphorylation. SMAD2 is known as a critical signal for proliferation and activation of astrocytes. In addition, activated SMAD2 induces CSPG expression from the astrocytes (43). Recent study showed that CSPG expression is differentially regulated by SMAD2 or SMAD3 such that neurocan expression requires both Smad2 and Smad3, whereas phosphacan and chondroitin synthase 1 expression is regulated by Smad2 but not Smad3 (44). Other research, however, reported that TGF-β induces expression of three different CSPGs by PI3K-Akt-mTOR signaling pathway, not by the canonical SMAD signaling pathways (45).
Although the CSPG-mediated changes in ECMs protect the expansion of inflammatory signals from the injury site, increased expression of CSPGs inhibits the axonal regeneration by inducing growth cone collapse (46). This inhibitory function of CSPGs suppresses the recovery from CNS damages. Furthermore, CSPGs inhibit NSPCs neural progenitor cell migration while, facilitating differentiation of neural progenitor cells into astrocytes (47).
The length and sulfation pattern of GAG seem to have differential effects on the neuroinflammation. For example, CS-A and CS-B GAGs are more potent in promoting the release of inflammatory factors such as IL-1β, IL-2, and IL-12 (48-50). In contrast to the proinflammatory function, aggrecan derived 6-sulfated CS possesses protective functions from the neuro-inflammation by suppressing of NF-κB nuclear translocation which inhibits the infiltration of T-cells and activation of microglial cells (51, 52). Also, Chondroitin 6-O-sulfate was reported to ameliorates CNS damages in experimental autoimmune encephalomyelitis model (53). However, other research showed that liberated CS may increase proinflammatory cytokines.
CSPGs also modulate chronic neuroinflammatory diseases such as Alzheimer’s disease (AD) and multiple sclerosis (MS) (54). CS-GAG was shown to promote the aggregation of Aβ1–42 fibrils in vitro (55). Among CSPGs, chondroitin 4-sulfate, chondroitin 6-sulfate, and unsulfated chondroitin were immunostained in senile plaques and neurofibrillary tangles in AD (56). One of the brain specific CSPGs, brevican, is shown to be found in smaller CS side chains (57). In addition, brevican binds to Aβ1–42 fibrils (58).
MS is an inflammatory auto-immune disease characterized by gradual loss of myelination in the CNS. Several lecticans such as neurocan, aggrecan, and versican are known to be upregulated around MS lesions (59, 60). CSPGs in the multiple sclerosis are known to facilitate the activity and migration of leucocytes the central nervous system (61). In addition, the polymorphism of the ChGN1 gene, encoding a critical glycosyl transferase for CS production, is shown to be associated with MS progression (62).
Despite conflicting observations on the functions of CSPGs, targeting CSPGs is a promising strategy to enhance neuronal regeneration around the lesion. One of the strategies to control CSPGs in a CNS lesion is to use chondroitinase-ABC (Ch-ABC), which is a bacterial enzyme that liberates CS-GAG from the CSPG core proteins. Treating Ch-ABC in a CNS lesion efficiently reduces the accumulation of CSPGs in the glial scar (63, 64). Furthermore, Ch-ABC treatment promoted axonal regeneration in the CNS lesion and resulted in better recovery in mouse models of spinal cord injury (64, 65). Recent studies showed that intrathecal injection of ChABC increases IL-10 and reduces proinflammatory IL-12 (66). Ch-ABC was also effectively used to ameliorate AD symptoms in animal models (58).
Another strategy to modulate CSPGs after CNS injury is to inhibit CSPG synthesis by treating β-d-xylopyranosides, an inhibitor for xylose attachment to the serine residue of lecticans. Treating β-d-xylopyranosides two days after spinal cord injury significantly improved recovery (67). Interestingly, immediate treatment of xyloside after injury inhibited the recovery, indicating that the timing for targeting CSPG is critical for better recovery (67). In addition to the use of xyloside to treat acute lesions, xyloside treated lysolecithin‐demyelinated mice increased the number of oligodendrocytes in lesions and improved remyelination (68).
In addition to changing the expression of CSPGs, neuronal injury also triggers fragmentation of CSPGs. Liberated fragments of CSPGs may directly influence the inflammatory responses (69). Fragmented CSPG is known to function as damage associated-molecular pattern (DAMP) and directly binds to pattern recognition receptors (PRR) such as Toll-like receptors (TLR) (70). Indeed, for an example, versican can directly bind to the TLR-2 and activate macrophages (71). Theses fragmented-ECMs and their pathogenic roles as DAMP molecules are well recognized in other ECM-related disorders such as arthritis (72). The pathogenic function of fragmented CSPGs certainly needs to be studied further in neuroinflammation.
HYALURONAN (HYALURONIC ACID; HA)
Hyaluronic acid (HA) is the most abundant GAG in CNS ECMs. Compared to the CSPGs, HA is not sulfated and is not attached to the core proteins (Fig. 2). In normal conditions, HA exist as high-molecular weight polymers of repeating disaccharide, D-glucuronic acid, and N-acetyl-D-glucosamine, in neuronal ECM. The average molecular weight of HA is above 1000 kDa in normal conditions.
HA polymer is synthesized by HA synthase (HAS1, HAS2, HAS3) (73) and HASs are mainly expressed in the astrocytes in the brain (74). The HA chains serve as scaffolds for other CSPGs to bind and form meshwork of ECMs.
HYALURONAN AND NEUROINFLAMMATION
Beside the structural functions as a major ECM component, HA plays various roles in regulation of neuroinflammation. In normal conditions, high molecular weight HA (HMW-HA) binds to CD44 and reduces TLR mediated inflammatory signals in microglia (75). However, HA functions as a proinflammatory signal in inflammatory situations (76). During the inflammatory responses after injury, HMW-HA is fragmented into low molecular weight-HA (LMW-HA) and this fragmented LMW-HA is released from the damaged ECM (76).
The LMW-HA functions a universal DAMP to induce innate immunity and proinflammatory factors. LMW-HA exerts a proinflammatory role by binding to the CD44 on microglia and astrocytes upon neuronal injury. Furthermore, LMW-HA induces NF-κB signaling and increases the expression of TNF-α and IL-1β in cultured neurons (77). Recent studies also suggested that HA concentration in cerebrospinal fluid is corelated to increased blood-brain barrier permeability (78).
TENASCINS
There are six family members of tenascins in mammals: tenascin-C, tenascin-R, tenascin-W. tenascin-X, tenascin-Y, and tenascin-N. Tenascin-R is exclusively expressed in the CNS while the others are expressed in a variety of tissues (79).
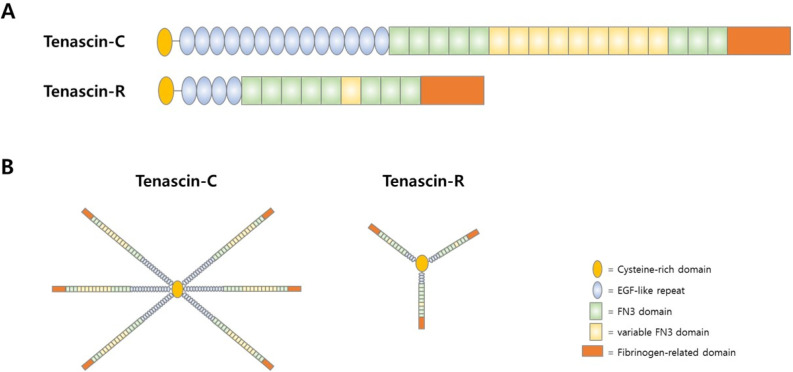
The structures of tenascins are very similar among the family members. Each tenascin member possesses a cysteine-rich N-terminus domain which mediates the oligomerization of tenas-cins to functions as a structural component of ECMs (80). Tenascins form oligomers by disulfide bond via this N-terminus oligomerization domain (Fig. 4). The central region of tenascin is composed of EGF like repeats and the C-terminus region is composed of fibrinogen III (FNIII) repeats. The C-terminus FNIII repeat domains are known to mediate bindings to other ECMs such as CSPGs.
Fig. 4.
Structure of Tenascins. (A) The domain structure of major tenascin, Tenascin-C and Tenascin-R. (B) Tenascins form oligomers and the N-terminus of tenascin meditates oligomerization.
Beside the structural functions, tenascins influence neuronal differentiation and regulate axonal guidance and neurite outgrowth during brain development (81, 82). Furthermore, tenascins are known to regulate voltage-gated sodium channels and synaptic plasticity (83).
TENASCINS AND NEUROINFLAMMATION
Tenascin-C and tenascin-R have been reported to be upregulated after brain injury or spinal cord injury. Reactive astrocytes, along with oligodendrocyte and neurons, are major sources of tenascins expression upon CNS injury (84).
The molecular mechanism by which tenascins regulate immune systems and inflammatory responses are well-studied in non-neural tissues. Tenascin-C expression is increased in response to the inflammatory cytokines such as INF-γ, IL1-β, and TNF-α in various tissues (85, 86). The pattern recognition receptor TLR-4 is a major interaction partner of tenascin-C (87) and the expression of TLR-4 is increased after brain injury. The interaction of tenascin-C with TLR-4 promotes the expression of proinflammatory cytokines (88). Consistent with these observations, tenascin-C mutant mice displayed better recovery after experimental brain injury partly by suppressed apoptosis and TLR-mediated proinflammatory responses (89).
In addition to the acute inflammatory responses, tenascin-C was shown to be involve in chronic inflammatory neurodegeneration such as multiple sclerosis (MS) and Alzheimer’s disease (AD). MS is an autoimmune disorder in CNS developed by progressive loss of myelination. One of the direct causes of demyelination in MS is the integrinα9β1-mediated autoimmune response. The c-terminus FNIII domain of tenascin-C contains integrin-α9β binding motif AEIDGIEL (90), and promotes integrin-α9β1-mediated cytokine expression in experimental autoimmune encephalomyelitis (EAE) models (91). EAE is a widely used experimental model for MS study. Furthermore, an integrinα9β1 specific tenascin-C mutant promoted proinflammatory cytokines and this induction was abolished by treating integrin-α9β1 neutralizing antibody (91).
Chronic inflammation may also be responsible for AD and recent studies showed significant correlation in tenascin-C staining to Aβ plaques in human AD brain samples.
Compared to tenascin-C, tenascin-R function in neuroinflammation is not well understood. However, it was reported that tenascin-R is also upregulated after CNS injury. Further study needs to be performed to elucidate tenascin-R function in neuroinflammation.
CONCLUSION
The functions of ECM in inflammation have long been studied in human disorders such as fibrosis, cancers, and rheumatoid arthritis. Recent studies provide a substantial amount of experimental and clinical data indicating that ECM is critically involved in both acute and chronic inflammatory responses in the CNS. However, the complexity of ECM composition and diverse modifications in disease conditions hinder clear understanding of the molecular functions of neuronal ECM in neuroinflammation. Also, the functions of ECM in neuroinflamma-tion change dramatically upon its modification such as frag-mentation and alternative splicing. However, several experimental data targeting to modulate ECM to promote CNS injury suggested that neuronal ECM is a promising target to develop therapeutics. Among the most immediate challenges is to elucidate the molecular mechanism of each ECM component and its variants regulating neuroinflammation in CNS. The molecular interactions among ECM and inflammatory signals are under intensive investigation currently. Especially, the clinical data on the efficacy of ECM modifying enzymes and chemicals treating neuroinflammation must be accumulated.
ACKNOWLEDGEMENTS
This study was supported by National Research Foundation grant funded by the Korea Ministry of Science and ICT (2018R1A2B2007545, 2019R1I1A1A01060664, and IBS-R022-D1).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Novak U, Kaye AH. Extracellular matrix and the brain: components and function. J Clin Neurosci. 2000;7:280– 290. doi: 10.1054/jocn.1999.0212. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- 3.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/S0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 4.Deepa SS, Carulli D, Galtrey C, et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 5.Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018;136:101–108. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Lu P, Takai K, Weaver VM, Werb Z. Extra-cellular matrix degradation and remodeling in develop-ment and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bignami A, Hosley M, Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat Embryol (Berl) 1993;(Berl):419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspberg A, Miura R, Bourdoulous S, et al. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci U S A. 1997;94:10116–10121. doi: 10.1073/pnas.94.19.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearns AE, Campbell SC, Westley J, Schwartz NB. Initiation of chondroitin sulfate biosynthesis: a kinetic analysis of UDP-D-xylose: core protein beta-D-xylosyltransferase. Biochemistry. 1991;30:7477–7483. doi: 10.1021/bi00244a016. [DOI] [PubMed] [Google Scholar]
- 11.Vertel BM, Walters LM, Flay N, Kearns AE, Schwartz NB. Xylosylation is an endoplasmic reticulum to Golgi event. J Biol Chem. 1993;268:11105–11112. [PubMed] [Google Scholar]
- 12.Jonsson M, Eklund E, Fransson LA, Oldberg A. Initiation of the decorin glycosaminoglycan chain in the endoplasmic reticulum-Golgi intermediate compartment. J Biol Chem. 2003;278:21415–21420. doi: 10.1074/jbc.M210977200. [DOI] [PubMed] [Google Scholar]
- 13.Nuwayhid N, Glaser JH, Johnson JC, Conrad HE, Hauser SC, Hirschberg CB. Xylosylation and glucuro-nosylation reactions in rat liver Golgi apparatus and endo-plasmic reticulum. J Biol Chem. 1986;261:12936–12941. [PubMed] [Google Scholar]
- 14.Moses J, Oldberg A, Fransson LA. Initiation of galactosaminoglycan biosynthesis. Separate galactosylation and dephosphorylation pathways for phosphoxylosylated decorin protein and exogenous xyloside. Eur J Biochem. 1999;260:879–884. doi: 10.1046/j.1432-1327.1999.00228.x. [DOI] [PubMed] [Google Scholar]
- 15.Glowacki A, Kozma EM, Olczyk K. [Biosyn-thesis of keratan sulfate, chondroitin sulfate and dermatan sulfate proteoglycans] Postepy Biochem. 2004;50:170–181. [PubMed] [Google Scholar]
- 16.Gama CI, Tully SE, Sotogaku N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 17.Bobinski R, Olczyk K, Krzyzowska-Bobinska E. [Hyaluronan aggregating proteoglycans] Postepy Biochem. 1998;44:245–251. [PubMed] [Google Scholar]
- 18.Ueno H, Fujii K, Suemitsu S, et al. Expression of aggrecan components in perineuronal nets in the mouse cerebral cortex. IBRO Rep. 2018;4:22–37. doi: 10.1016/j.ibror.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia. 2010;58:857–869. doi: 10.1002/glia.20970. [DOI] [PubMed] [Google Scholar]
- 21.Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Lundell A, Olin AI, Morgelin M, al-Karadaghi S, Aspberg A, Logan DT. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure. 2004;12:1495–1506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Bruckner G, Brauer K, Hartig W, et al. Perineuronal nets provide a polyanionic, glia-associated form of micro-environment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 24.Morawski M, Bruckner MK, Riederer P, Bruckner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–315. doi: 10.1016/j.expneurol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Hamel MG, Mayer J, Gottschall PE. Altered production and proteolytic processing of brevican by transforming growth factor beta in cultured astrocytes. J Neurochem. 2005;93:1533–1541. doi: 10.1111/j.1471-4159.2005.03144.x. [DOI] [PubMed] [Google Scholar]
- 26.John N, Krugel H, Frischknecht R, et al. Brevican-containing perineuronal nets of extracellular matrix in dis-sociated hippocampal primary cultures. Mol Cell Neurosci. 2006;31:774–784. doi: 10.1016/j.mcn.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Yamagata M, Sanes JR. Versican in the develo-ping brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J Neurosci. 2005;25:8457–8467. doi: 10.1523/JNEUROSCI.1976-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naso MF, Zimmermann DR, Iozzo RV. Charac-terization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994;269:32999–33008. [PubMed] [Google Scholar]
- 29.Schmalfeldt M, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Versican V2 is a major extra-ellular matrix component of the mature bovine brain. J Biol Chem. 1998;273:15758–15764. doi: 10.1074/jbc.273.25.15758. [DOI] [PubMed] [Google Scholar]
- 30.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Molecular cloning of brevican, a novel brain pro-teoglycan of the aggrecan/versican family. J Biol Chem. 1994;269:10119–10126. [PubMed] [Google Scholar]
- 32.Seidenbecher CI, Richter K, Rauch U, Fassler R, Garner CC, Gundelfinger ED. Brevican, a chondroitin sulfate proteoglycan of rat brain, occurs as secreted and cell surface glycosylphosphatidylinositol-anchored isoforms. J Biol Chem. 1995;270:27206–27212. doi: 10.1074/jbc.270.45.27206. [DOI] [PubMed] [Google Scholar]
- 33.Frischknecht R, Seidenbecher CI. Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol. 2012;44:1051–1054. doi: 10.1016/j.biocel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 34.McKeon RJ, Jurynec MJ, Buck CR. The chond-roitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauch U, Karthikeyan L, Maurel P, Margolis RU, Margolis RK. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992;267:19536–19547. [PubMed] [Google Scholar]
- 36.Muhleisen TW, Mattheisen M, Strohmaier J, et al. Association between schizophrenia and common varia-tion in neurocan (NCAN), a genetic risk factor for bipolar disorder. Schizophr Res. 2012;138:69–73. doi: 10.1016/j.schres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Oruc L, Kapur-Pojskic L, Ramic J, Pojskic N, Bajrovic K. Assessment of relatedness between neurocan gene as bipolar disorder susceptibility locus and schizoph-renia. Bosn J Basic Med Sci. 2012;12:245–248. doi: 10.17305/bjbms.2012.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun D, Jakobs TC. Structural remodeling of astrocytes in the injured CNS. Neuroscientist. 2012;18:567–588. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 40.Colombo E, Farina C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 42.Schachtrup C, Ryu JK, Helmrick MJ, et al. Fibrino-gen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiller M, Javelaud D, Mauviel A. TGF-beta-in-duced SMAD signaling and gene regulation: consequen-ces for extracellular matrix remodeling and wound hea-ling. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-beta-mediated induction of chond-roitin sulfate proteoglycans. J Neurochem. 2011;119:868–878. doi: 10.1111/j.1471-4159.2011.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahan N, Hannila SS. Transforming growth factor beta-induced expression of chondroitin sulfate proteogly-cans is mediated through non-Smad signaling pathways. Exp Neurol. 2015;263:372–384. doi: 10.1016/j.expneurol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/S1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 47.Zhong J, Lan C, Zhang C, et al. Chondroitin sulfate proteoglycan represses neural stem/progenitor cells migra-tion via PTPsigma/alpha-actinin4 signaling pathway. J Cell Biochem. 2019;120:11008–11021. doi: 10.1002/jcb.28379. [DOI] [PubMed] [Google Scholar]
- 48.Rachmilewitz J, Tykocinski ML. Differential effects of chondroitin sulfates A and B on monocyte and B-cell activation: evidence for B-cell activation via a CD44-dependent pathway. Blood. 1998;92:223–229. doi: 10.1182/blood.V92.1.223.413k15_223_229. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Sun F, Niu H, Wang Q, Duan J. Mechanistic insights into cellular immunity of chondroitin sulfate A and its zwitterionic N-deacetylated derivatives. Carbohydr Polym. 2015;123:331–338. doi: 10.1016/j.carbpol.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 50.Yang R, Yan Z, Chen F, Hansson GK, Kiessling R. Hyaluronic acid and chondroitin sulphate A rapidly promote differentiation of immature DC with upregulation of costimulatory and antigen-presenting molecules, and enhancement of NF-kappaB and protein kinase activity. Scand J Immunol. 2002;55:2–13. doi: 10.1046/j.0300-9475.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 51.Tan GK, Tabata Y. Chondroitin-6-sulfate attenu-ates inflammatory responses in murine macrophages via suppression of NF-kappaB nuclear translocation. Acta Biomater. 2014;10:2684–2692. doi: 10.1016/j.actbio.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Rolls A, Cahalon L, Bakalash S, Avidan H, Lider O, Schwartz M. A sulfated disaccharide derived from chondroitin sulfate proteoglycan protects against inflammation-associated neurodegeneration. FASEB J. 2006;20:547–549. doi: 10.1096/fj.05-4540fje. [DOI] [PubMed] [Google Scholar]
- 53.Miyamoto K, Tanaka N, Moriguchi K, et al. Chon-droitin 6-O-sulfate ameliorates experimental autoimmune encephalomyelitis. Glycobiology. 2014;24:469–475. doi: 10.1093/glycob/cwu014. [DOI] [PubMed] [Google Scholar]
- 54.Heindryckx F, Li JP. Role of proteoglycans in neuro-inflammation and central nervous system fibrosis. Matrix Biol. 2018;68:589–601. doi: 10.1016/j.matbio.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Castillo GM, Cummings JA, Yang W, et al. Sulfate content and specific glycosaminoglycan backbone of perlecan are critical for perlecan's enhancement of islet amyloid polypeptide (amylin) fibril formation. Diabetes. 1998;47:612–620. doi: 10.2337/diabetes.47.4.612. [DOI] [PubMed] [Google Scholar]
- 56.DeWitt DA, Silver J, Canning DR, Perry G. Chondroitin sulfate proteoglycans are associated with the lesions of Alzheimer's disease. Exp Neurol. 1993;121:149–152. doi: 10.1006/exnr.1993.1081. [DOI] [PubMed] [Google Scholar]
- 57.Ajmo JM, Bailey LA, Howell MD, et al. Abnormal post-translational and extracellular processing of brevican in plaque-bearing mice over-expressing APPsw. J Neurochem. 2010;113:784–795. doi: 10.1111/j.1471-4159.2010.06647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howell MD, Bailey LA, Cozart MA, Gannon BM, Gottschall PE. Hippocampal administration of chondroitinase ABC increases plaque-adjacent synaptic marker and diminishes amyloid burden in aged APPswe/PS1dE9 mice. Acta Neuropathol Commun. 2015;3:54. doi: 10.1186/s40478-015-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobel RA, Ahmed AS. White matter extracel-lular matrix chondroitin sulfate/dermatan sulfate proteo-glycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–1207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- 60.Kamermans A, Planting KE, Jalink K, van Horssen J, de Vries HE. Reactive astrocytes in multiple sclerosis impair neuronal outgrowth through TRPM7-mediated chondroitin sulfate proteoglycan production. Glia. 2019;67:68–77. doi: 10.1002/glia.23526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephenson EL, Mishra MK, Moussienko D, et al. Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain. 2018;141:1094–1110. doi: 10.1093/brain/awy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saigoh K, Yoshimura S, Izumikawa T, et al. Chond-roitin sulfate beta-1,4-N-acetylgalactosaminyltransferase-1 (ChGn-1) polymorphism: Association with progression of multiple sclerosis. Neurosci Res. 2016;108:55–59. doi: 10.1016/j.neures.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mecha-nism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci. 2008;28:14107–14120. doi: 10.1523/JNEUROSCI.2217-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nori S, Khazaei M, Ahuja CS, et al. Human Oligo-dendrogenic Neural Progenitor Cells Delivered with Chondroitinase ABC Facilitate Functional Repair of Chronic Spinal Cord Injury. Stem Cell Reports. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barritt AW, Davies M, Marchand F, et al. Chondro-itinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ. Regulation of IL-10 by chondroitinase ABC pro-motes a distinct immune response following spinal cord injury. J Neurosci. 2014;34:16424–16432. doi: 10.1523/JNEUROSCI.2927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolls A, Shechter R, London A, et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 2008;5:e171. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau LW, Keough MB, Haylock-Jacobs S, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72:419–432. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- 69.Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol. 2014;258:24–34. doi: 10.1016/j.expneurol.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 71.Kim S, Takahashi H, Lin WW, et al. Carcinoma-pro-duced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and inter-pretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 74.Fowke TM, Karunasinghe RN, Bai JZ, Jordan S, Gunn AJ, Dean JM. Hyaluronan synthesis by developing cortical neurons in vitro. Sci Rep. 2017;7:44135. doi: 10.1038/srep44135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Austin JW, Gilchrist C, Fehlings MG. High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J Neurochem. 2012;122:344–355. doi: 10.1111/j.1471-4159.2012.07789.x. [DOI] [PubMed] [Google Scholar]
- 76.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/S0945-053X(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 77.Wang MJ, Kuo JS, Lee WW, Huang HY, Chen WF, Lin SZ. Translational event mediates differential production of tumor necrosis factor-alpha in hyaluronan-stimulated microglia and macrophages. J Neurochem. 2006;97:857–871. doi: 10.1111/j.1471-4159.2006.03776.x. [DOI] [PubMed] [Google Scholar]
- 78.Ventorp F, Barzilay R, Erhardt S, et al. The CD44 ligand hyaluronic acid is elevated in the cerebrospinal fluid of suicide attempters and is associated with increased blood-brain barrier permeability. J Affect Disord. 2016;193:349– 354. doi: 10.1016/j.jad.2015.12.069. [DOI] [PubMed] [Google Scholar]
- 79.Jakovcevski I, Miljkovic D, Schachner M, Andjus PR. Tenascins and inflammation in disorders of the nervous system. Amino Acids. 2013;44:1115–1127. doi: 10.1007/s00726-012-1446-0. [DOI] [PubMed] [Google Scholar]
- 80.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 81.Lochter A, Schachner M. Tenascin and extra-cellular matrix glycoproteins: from promotion to polariza-tion of neurite growth in vitro. J Neurosci. 1993;13:3986–4000. doi: 10.1523/JNEUROSCI.13-09-03986.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Husmann K, Faissner A, Schachner M. Tenascin promotes cerebellar granule cell migration and neurite outgrowth by different domains in the fibronectin type III repeats. J Cell Biol. 1992;116:1475–1486. doi: 10.1083/jcb.116.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao ZC, Ragsdale DS, Malhotra JD, et al. Tenascin-R is a functional modulator of sodium channel beta sub-units. J Biol Chem. 1999;274:26511–26517. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- 84.Laywell ED, Dorries U, Bartsch U, Faissner A, Schachner M, Steindler DA. Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc Natl Acad Sci U S A. 1992;89:2634–2638. doi: 10.1073/pnas.89.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiovaro F, Chiquet-Ehrismann R, Chiquet M. Transcriptional regulation of tenascin genes. Cell Adh Migr. 2015;9:34–47. doi: 10.1080/19336918.2015.1008333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol. 2010;184:2655–2662. doi: 10.4049/jimmunol.0903359. [DOI] [PubMed] [Google Scholar]
- 87.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 88.Claycomb KI, Winokur PN, Johnson KM, et al. Aber-rant production of tenascin-C in globoid cell leukody-strophy alters psychosine-induced microglial functions. J Neuropathol Exp Neurol. 2014;73:964–974. doi: 10.1097/NEN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Momcilovic M, Stamenkovic V, Jovanovic M, et al. Tenascin-C deficiency protects mice from experimental autoimmune encephalomyelitis. J Neuroimmunol. 2017;302:1–6. doi: 10.1016/j.jneuroim.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Yokosaki Y, Palmer EL, Prieto AL, et al. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- 91.Ito K, Morimoto J, Kihara A, et al. Integrin alpha9 on lymphatic endothelial cells regulates lymphocyte egress. Proc Natl Acad Sci U S A. 2014;111:3080–3085. doi: 10.1073/pnas.1311022111. [DOI] [PMC free article] [PubMed] [Google Scholar]