Abstract
Hereditary haemorrhagic telangiectasia (HHT; Osler–Weber–Rendu disease) is an autosomal dominant vascular disease characterized by nosebleeds, mucocutaneous telangiectases, visceral arteriovenous malformations (AVM) and a first-degree relative with HHT. Diagnosis is definite if three or four criteria are present. This case report describes a 19-year-old male with incidentally detected polycythaemia and an associated soft-tissue opacity over the left lower lobe on his frontal chest radiogram. He had experienced dyspnoea on exertion since infancy and clubbing at physical examination. Polycythaemia vera, chronic obstructive pulmonary disease, sleep apnoea and cyanotic congenital heart disease were excluded. Chest computed tomography (CT) was initially refused by the patient, but 3 years later he presented with severe epistaxis. Considering the unvarying soft tissue mass and erythrocytosis, an HHT-associated pulmonary AVM (PAVM) was eventually confirmed by chest CT. A pathogenic family-specific ENG c.817-2 A>C mutation was detected in the patient. The large PAVM was successfully treated using AMPLATZER™ vascular plug embolization. A combination of the multisystemic nature of his symptoms, the age-related penetrance of HHT symptoms and insufficient patient compliance delayed the diagnosis of HHT in this current case.
Keywords: Hereditary haemorrhagic telangiectasia, prevalence, penetrance, pulmonary arteriovenous malformation, polycythaemia, compliance
Introduction
Hereditary haemorrhagic telangiectasia (HHT; Osler–Weber–Rendu disease) is an autosomal dominant vascular disease described by the four Curacao criteria: (i) spontaneous and recurrent nosebleeds (with approximate frequency of 90–95%; mean age at onset of 12 years); (ii) multiple telangiectases at characteristic sites such as the lips, oral cavity, fingers and nose (90%; 30 years); (iii) visceral lesions as gastrointestinal telangiectasia (20–80%) and pulmonary (30–50%), hepatic (32–48%) and cerebral or spinal (23%) arteriovenous malformations (AVM); and (iv) family history with a first-degree relative with HHT.1 Diagnosis is definite if three or four criteria are present, possible if two criteria are present and unlikely if fewer than two criteria are present.1 The worldwide prevalence of HHT is estimated to be 1:5000–1:10 000.2 Approximately 85% of HHT cases have heterozygous family-specific mutations either in the ENG (encodes endoglin) or the ACVRL1 (encodes activin receptor-like kinase 1) genes, causing HHT type 1 and 2, respectively.3 These genes encode proteins belonging to the transforming growth factor-beta (TGF)-β superfamily.2 While AVMs are considered to be congenital, HHT shows age-related penetrance regarding epistaxis and telangiectases.2 As a consequence, the sensitivity of the Curacao criteria might be insufficient in children and young adults, emphasizing the significance of genetic testing in these age groups.4
Case report
In March 2011, a 19-year-old non-smoking male with a prior medical history of traumatic left rib 8 and 9 fractures was referred to the Department of Otorhinolaryngology, Ferenc Markhot County Hospital, Eger, Hungary with polycythaemia (haemoglobin 20.5 g/dl), which was detected as part of a routine complete blood count test preceding a blood donation. He reported dyspnoea on exertion since his infancy and a negative family history. On physical examination, clubbing was observed. At resting (supine) pulse oximetry, SaO2 was 91%. Cyanotic congenital heart disease was excluded by transthoracic echocardiography, while transoesophageal echocardiography was refused by the patient. Frontal chest radiography showed a 3 × 4 cm oval soft-tissue opacity projected over the left lower lobe of the lung. A pulmonologist excluded both tuberculosis (by an interferon gamma release assay) and chronic obstructive pulmonary disease (COPD); and referred the patient for computed tomography (CT), but he did not attend for the scheduled examination.
Although polycythaemia vera was excluded by the World Health Organization 2008 diagnostic criteria valid at that time (with no JAK2 V617F and exon 12 mutations; elevated erythropoietin level),5 antiplatelet therapy (100 mg aspirin administered orally, daily in the subsequent 6 months) was initiated and the patient underwent a series of venesections. Despite all of these interventions, the polycythaemia persisted (with haemoglobin 18.4–20.0 g/dl) and in November 2012, the patient was lost to follow-up.
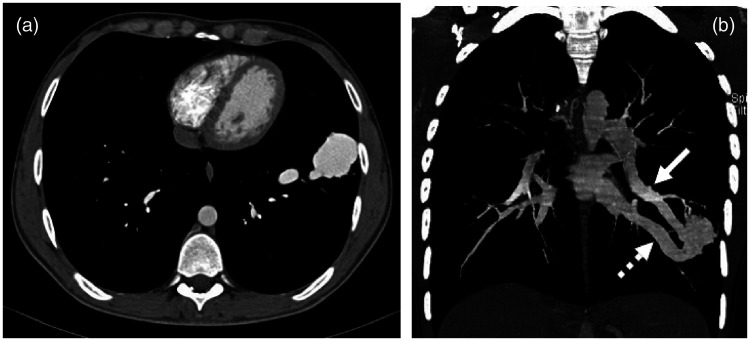
In April 2014, the patient presented again to the Department of Otorhinolaryngology, Ferenc Markhot County Hospital, Eger, Hungary with episodic epistaxis in the preceding 5 days, requiring both unilateral anterior and posterior nasal packings. He had never suffered from nosebleeds before. On frontal chest radiogram, the aforementioned soft-tissue opacity was detected in an unvarying size and location (Figure 1) as compared with that observed 3 years previously. A pulmonary AVM (PAVM) resulting in a right-to-left shunt was suspected as a cause for secondary erythrocytosis, and eventually, the patient underwent the recommended chest CT. After the administration of intravenous iodinated contrast medium, a 52 × 42 × 36 mm mass showing an homogenous enhancement in the early arterial phase was detected (Figure 2a), with clearly identifiable feeding and draining vessels in the coronal plane images (Figure 2b).
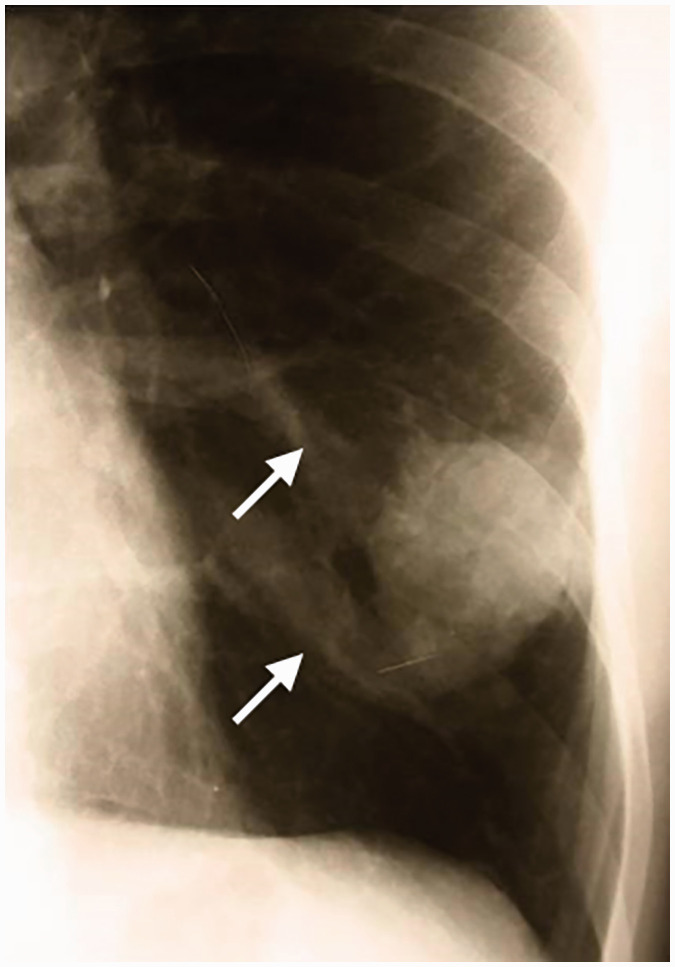
Figure 1.
Frontal chest radiogram in April 2014 of a 19-year-old male with incidentally detected polycythaemia shows a 3 x 4 cm oval soft-tissue opacity projected over the left lower lobe. Reviewing the film, there were two undocumented prominences (arrows) medial to the mass.
Figure 2.
(a) Axial contrast-enhanced computed tomography image on a mediastinal window setting of a 19-year-old male with incidentally detected polycythaemia demonstrated a 52 × 42 × 36 mm mass in the anterior basal segment of the left lung showing homogenous enhancement in the early arterial phase. (b) A contrast-enhanced coronal plane image shows an identifiable feeding vessel (arrow) originating from the lower lobe branch of the left pulmonary artery and a draining vessel (dotted arrow) joining the inferior pulmonary vein.
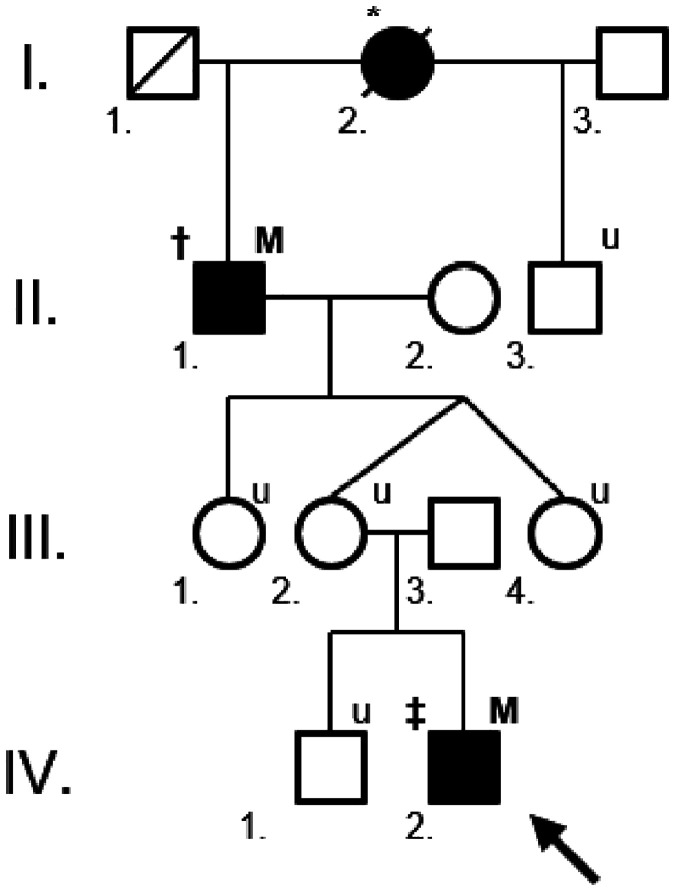
Epistaxis and PAVM suggested HHT, although epistaxis was not recurrent and the patient had no telangiectases at the characteristic sites. Hepatic AVMs were excluded by the simultaneous upper abdominal CT scan. The patient was offered the opportunity to investigate the possibility of cerebral AVMs by undertaking magnetic resonance imaging, but again, he refused the examination. Since the systematic screening of HHT (including physical examination, AVM screening and genetic test) was already under way at that time in the primary care clinic of our setting, enquiries based on the surnames of individuals in the patient’s family identified his maternal grandfather as a known definite HHT patient (Figure 3). The patient’s mother refused the HHT screening (she has no nosebleeds and telangiectases according to information provided by the grandfather and the patient). Thus, adhering strictly to the diagnostic criteria (his epistaxis was not recurrent), the patient was diagnosed with possible HHT (PAVM + family history), confirmed by the detection of the family-specific pathogenic ENG c.817-2 A>C splice-site mutation.6
Figure 3.
The proximate branch of a large hereditary haemorrhagic telangiectasia (HHT) family with an ENG c.817-2 A>C mutation. The current patient, a 19-year-old male with incidentally detected polycythaemia, is marked with an arrow (IV.2.). Filled individuals are affected, either by carrying the mutation and/or having definite HHT. In the upper left index † = definite HHT, ‡ = possible HHT, * = deceased or unavailable patient with epistaxis and/or telangiectases by hearsay. In the upper right index M = family-specific ENG mutation, u = patient unavailable for family screening. The patient’s 66-year-old maternal grandfather (II.1.) was diagnosed by the systematic screening of HHT patients in the primary care area of our setting. The patient’s 44-year-old mother (III.2.) refused the HHT screening, but, according to the HHT inheritance, she must be a mutation carrier.
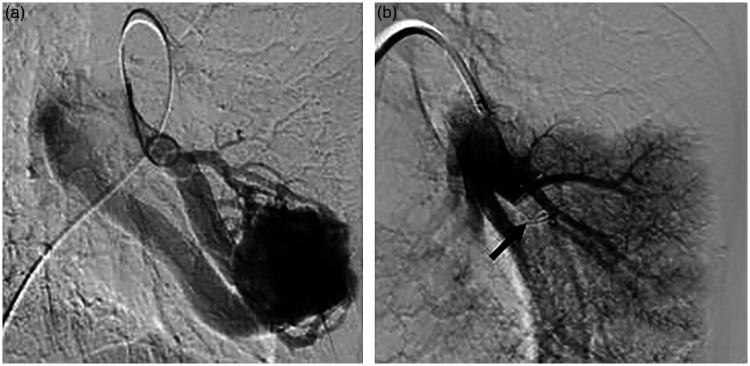
Finally, following subsequent compliance problems, the large pulmonary AVM was successfully treated using AMPLATZER™ vascular plug (Abbott Laboratories, Abbott Park, IL, USA) embolization in October 2016 (Figure 4). The patient has not been observed since his post-embolization CT, but according to his family practitioner, he is presently asymptomatic.
Figure 4.
(a) Pre-embolization left pulmonary catheter arteriography from femoral venous access demonstrating a simple arteriovenous malformation (AVM), the enlargement of feeding vessels and early venous return. (b) Post-embolization pulmonary arteriogram shows complete occlusion of the lesion, without additional feeders and unsuspected new or remaining pulmonary AVMs. The photo was made between the opening and the detachment of the 14 mm AMPLATZER™ vascular plug (arrow).
The HHT investigation protocol was approved by the Institutional Research Ethics Board of the Department of Otorhinolaryngology, Ferenc Markhot County Hospital, Eger, Hungary (no. IKEB/HHT-FOG Osztály/2013/2). Written informed consent for patient information and images to be published was provided by the patient.
Discussion
In this current case, erythrocytosis was considered to be a secondary, hypoxaemia-driven form, which might have been long-standing, based upon the dyspnoea that had been apparent on exertion since infancy and the signs of clubbing. After the exclusion of COPD, sleep apnoea and cyanotic congenital heart disease, the co-existence of hypoxaemia, erythrocytosis and the unknown pulmonary mass should have suggested a PAVM as the cause of right-to-left vascular shunting.7 Reviewing the frontal chest radiogram, there were two undocumented prominences medial to the mass, corresponding to vascular markings (Figure 1). The radiologist was presumably not aware of the detailed medical history, and furthermore, more common diseases (including malignant neoplasms) exist with a similar plain film appearance.8 Since CT, the reference standard investigation for diagnosing PAVMs, was eventually performed (Figure 2), the diagnosis of the mass as a PAVM was unequivocal.
Pulmonary AVMs are abnormal dilated vessels that provide direct communication between the pulmonary arteries and veins, resulting in a right-to-left shunt and bypassing the pulmonary capillary bed.8,9 Their prevalence is 1:2600, with a mild female predominance (1:1.5–1.8).10 Upon radiological features, they are classified as simple (an aneurysmal sac with a single feeder artery and one or more draining veins, like in this current case) or complex (vascularized by more than one artery and multiple veins). Both forms might be single or multiple, with a predilection in the lower lobes. Diffuse forms involving one or more segments or even an entire lobe and telangiectatic forms are extremely rare. A variety of vascular (true and false aneurysms, pulmonary varices, pulmonary artery collaterals) and non-vascular (bronchocoeles, primary or metastatic tumours) might mimic PAVMs.8
Although the majority of PAVMs are asymptomatic and diagnosed by incidental imaging, they are associated with serious acute and chronic morbidity and mortality, related to right-to-left shunting (cyanosis, dyspnoea), bypassing the pulmonary capillary filtration (paradoxical embolic stroke, cerebral abscesses, transient cerebral ischaemic attacks, myocardial infarction, infections) and haemorrhage (haemoptysis, haemothorax, maternal death in pregnancy).11 Beyond some rare forms (sporadic, surgical cavo-pulmonary shunts, trauma), approximately 70% of PAVM cases are associated with HHT, and conversely, 15–50% of HHT patients (with HHT1 predominance) will develop PAVM.12 Solid organ AVMs are usually congenital in HHT, starting out small, at-near capillary-like dimensions.3,13 Detectable PAVMs are rare in the first decade of life and usually complete development by early adulthood, although they might enlarge in number and size later on in pregnancy or following alterations in pulmonary circulation (including embolization of existing PAVMs).9 In this current case, the first chest radiogram was made at the age of 19, though the mild complaints in the medical history suggest an earlier onset at infancy.
Epistaxis, the earliest and most common clinical symptom of HHT, has an overall penetrance of 50% by 10 years and 80–95% by 21 years.12 Mucosal and dermal telangiectases usually appear in the third decade of life.14 This current patient showed none of these symptoms at his first admission, and furthermore, he was not aware of his family history of HHT. At that time, only a promptly diagnosed PAVM and a carefully recorded family history might have been suggestive of the underlying HHT. This should have been confirmed by the detection of the pathogenic family-specific mutation in the patient. Although not a diagnostic criterion, the significance of genetic screening in each available at-risk individual in HHT families must be emphasized.4
In patients with PAVMs of a size amenable to treatment (feeding artery diameter above 2–3 mm), embolization with vascular plugs is the first-line therapy, irrespective of symptoms.10 Surgical resection is reserved for selected cases (e.g. a proportion of complex, diffuse or multiple AVMs, symptomatic lesions with feeders too small for embolization, persistent neurological symptoms despite embolization). Long-term follow-up is needed either by CT or on a clinical basis to reduce radiation exposure.4,11 Explanations for the recurrence of PAVM might be persistent small PAVMs that would grow due to the altered blood flow or continuous defects in the signalling pathway of the TGF-β/bone morphogenic protein superfamily leading to angiodysgenesis.15 Patients with diagnosed PAVMs need judicious dental hygiene and antibiotic prophylaxis prior to dental and surgical procedures to reduce the rate of cerebral abscesses.9 As secondary erythrocytosis is an adaptive response to hypoxaemia in PAVM, venesection is not recommended, unless the patient shows hyperviscosity symptoms.11
This current case has been presented to draw attention to the potential pitfalls of delaying the diagnosis of HHT: (i) HHT is a rare disease; (ii) it has an age-related penetrance regarding the most common symptoms of epistaxis and telangiectases; (iii) congenital internal AVMs are most often asymptomatic, until diagnosed incidentally or by their life-threatening complications; (iv) especially young asymptomatic or mildly symptomatic individuals might therefore be noncompliant; (v) each discipline (haematology, pulmonology, cardiology, radiology and otorhinolaryngology in this current case) might identify the symptoms of a multisystemic disease one by one, but without realising that they are linked by the underlying HHT syndrome.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
The genetic test involved in the study was funded by grants from the Hungarian National Research Fund (no. OTKA K116228) and by the Ministry of National Economy, Hungary (no. GINOP-2.3.2-15-2016-00039).
References
- 1.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91: 66–67. [DOI] [PubMed] [Google Scholar]
- 2.Sharathkumar AA, Shapiro A. Hereditary haemorrhagic telangiectasia. Haemophilia 2008; 14: 1269–1280. [DOI] [PubMed] [Google Scholar]
- 3.McDonald J, Damjanovich K, Millson A, et al. Molecular diagnosis in hereditary hemorrhagic telangiectasia: findings in a series tested simultaneously by sequencing and deletion/duplication analysis. Clin Genet 2011; 79: 335–344. [DOI] [PubMed] [Google Scholar]
- 4.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011; 48: 73–87. [DOI] [PubMed] [Google Scholar]
- 5.Thiele J, Kvasnicka HM. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis. Curr Hematol Malig Rep 2009; 4: 33–40. [DOI] [PubMed] [Google Scholar]
- 6.Major T, Gindele R, Szabo Z, et al. The stratified population screening of hereditary haemorrhagic telangiectasia. Pathol Oncol Res 2019. doi: 10.1007/s12253-019-00602-7. [Epub ahead of print] [DOI] [PubMed]
- 7.McMullin MF. The classification and diagnosis of erythrocytosis. Int J Lab Hematol 2008; 30: 447–459. [DOI] [PubMed] [Google Scholar]
- 8.Gill SS, Roddie ME, Shovlin CL, et al. Pulmonary arteriovenous malformations and their mimics. Clin Radiol 2015; 70: 96–110. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis-Girod S, Cottin V, Shovlin CL. The lung in hereditary hemorrhagic telangiectasia. Respiration 2017; 94: 315–330. [DOI] [PubMed] [Google Scholar]
- 10.Rauh N, Gurley J, Saha S. Contemporary management of pulmonary arteriovenous malformations. Int J Angiol 2017; 26: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med 2014; 190: 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayrak-Toydemir P, McDonald J, Markewitz B, et al. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet 2006; 140: 463–470. [DOI] [PubMed] [Google Scholar]
- 13.McMullan DM, Riemer RK. Embryology and anatomy of intrapulmonary shunts. Echocardiography 2015; 32: 190–194. [DOI] [PubMed] [Google Scholar]
- 14.Plauchu H, de Chadarévian JP, Bideau A, et al. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32: 291–297. [DOI] [PubMed] [Google Scholar]
- 15.Saji N, Kawarai T, Miyamoto R, et al. Exome sequencing identifies a novel intronic mutation in ENG that causes recurrence of pulmonary arteriovenous malformations. J Neurol Sci 2015; 352: 29–33. [DOI] [PubMed] [Google Scholar]