Abstract
Objective
This study was performed to describe the treatment of aortic arch pathologies with a physician-modified fenestration (PMF) technique in thoracic endovascular aortic repair (TEVAR).
Methods
From August 2015 to August 2017, 32 patients with aortic arch pathologies underwent TEVAR with the PMF technique. All patients’ clinical data were analyzed with GraphPad Prism 7.0.
Results
Thirty-four aortic stent-grafts were implanted in 32 patients. The mean proximal diameter of the stent-graft was 32.4 ± 3.4 cm, and the mean length was 170.0 ± 25.2 cm. Twenty-nine PMF procedures were performed to preserve the left subclavian artery (LSA) and three to preserve both the LSA and left common carotid artery. The mean distance between the pathology and LSA was 8.4 ± 4.0 mm. The mean procedure time (from first to last digital subtraction angiography) was 22.8 ± 20.8 min. The mean follow-up time was 8.3 ± 5.3 months. During follow-up, the all-cause survival rate was 83.3% and the patency rate of the branch artery after PMF was 96.0%.
Conclusion
The PMF technique is a relatively safe, feasible, and time-saving method to preserve the branch artery in TEVAR for aortic arch pathologies. The short- to middle-term result of this technique is satisfactory.
Keywords: Thoracic endovascular aortic repair (TEVAR), aortic arch pathologies, physician-modified fenestration, endovascular technique, stent-graft, left subclavian artery, left common carotid artery
Introduction
Endovascular treatment for aortic lesions has become more popular in recent years. Both physicians and patients often choose endovascular repair because of its minimal invasiveness and fewer complications than open surgery.1 The technical difficulty of thoracic endovascular aortic repair (TEVAR) has increased because of preservation of the carotid artery and/or left subclavian artery (LSA) with exclusion of the lesion in the aortic arch. The methods of maintaining patency of the aortic arch branches include in situ fenestration,2–5 custom-made stent-grafts,6 the chimney technique,7,8 and branched stent-grafts.8 The history of using these methods for revascularization of the supra-arch branch is relatively short. The obvious disadvantages of the above methods include their time-consuming nature, the need for sophisticated equipment, and the long learning curve for physicians. All of these disadvantages limit the use of these methods in TEVAR for aortic arch reconstruction. We herein report our early experience and technical aspects of the physician-modified (on-table) fenestration (PMF) technique as an alternative endovascular method for preserving the supra-arch branch. This method involves a simple maneuver with no need for special devices and less time to complete.
Methods
Patient population
This study included consecutive patients with various aortic arch pathologies who underwent TEVAR with the PMF technique to reconstruct the supra-aortic branches from August 2015 to August 2017. All patients underwent computed tomography angiography (CTA) and were treated by vascular surgeons. The indications for the PMF technique during TEVAR were as follows. First, a penetrating aortic ulcer (PAU) was located in the lesser aortic curvature, and the distance from the PAU to the LSA was <15 mm. Second, the primary intimal tear of the aortic dissection (AD) was located in the lesser aortic curvature, and the distance from the intimal tear to the LSA was <15 mm. Additionally, some patients’ condition was complicated by chronic AD, such as those with significant aortic dilatation (maximum thoracic aortic diameter of >5.5 cm), rapid aortic growth (>1 cm/year), unrelenting pain, uncontrollable hypertension, end-organ ischemia, or aortic rupture. Third, the neck length of the thoracic aortic aneurysm (TAA) or aortic pseudoaneurysm was <15 mm and the aneurysm diameter was >5.5 cm or rapid aortic growth had occurred (>1 cm/year). Fourth, a proximal endoleak of prior TEVAR was present, and the landing zone for the upcoming stent-graft was <15 mm. Finally, if the CTA and digital subtraction angiography (DSA) evaluations showed a left-dominant vertebral artery and the planned treatment was endovascular aortic repair (EVAR) for an abdominal aortic aneurysm in one stage with TEVAR (treatment was indicated for both thoracic and abdominal aortic lesions), fenestration of the LSA was more likely to be recommended if TEVAR was needed to cover the LSA. The exclusion criteria were as follows: the patient’s condition prevented the TEVAR procedure or anesthesia; the distance of the landing zone was adequate (>15 mm from the aortic lesion to the LSA); the landing zone was <15 mm, but the intimal tear of the AD was in the greater curvature of the aortic arch and the diameter was >10 mm; the proximal aneurysm neck was <15 mm, but the aneurysm was eccentric to the greater curvature of the aortic arch; CTA and DSA evaluations confirmed that the right vertebral artery was dominant or the left vertebral artery had an occlusion or congenital defect. The institutional ethics board of our hospital approved this retrospective study. Written informed consent was obtained prior to performing the operation and collecting the clinical data.
PMF procedure
All procedures were performed in a hybrid operating theater with fluoroscopic and angiographic guidance. All patients underwent general anesthesia and tracheal intubation. Before TEVAR, the patients’ systolic blood pressure was controlled at around 100 mmHg and heart rate at around 60 beats/min. The patients’ left cubital fossa area and anterior cervical area were sterilized in the case of left brachial artery or/and left carotid artery puncture or incision.
One side of the common femoral artery was surgically exposed. A 5-Fr calibrated angiographic pigtail catheter was inserted for DSA with Ultravist 370 contrast agent (Bayer HealthCare, Leverkusen, Germany) to locate the aortic pathology and evaluate the bilateral vertebral arteries. The aortic lesions and aortic morphological features were measured on the CTA and DSA images. The diameter of the aortic stent-graft was oversized by 15% to 20% compared with the patients’ aortic diameter.
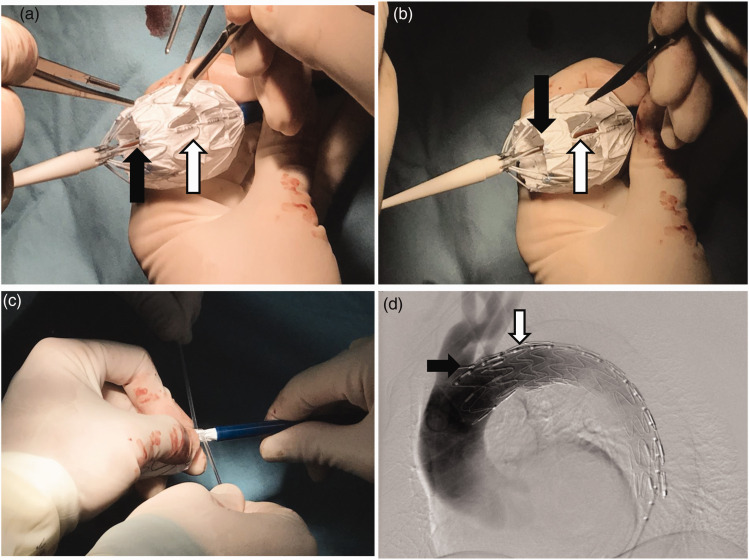
A Lunderquist extra-stiff guidewire (William Cook Europe, Bjaeverskov, Denmark) was advanced into the ascending aorta through the pigtail catheter after DSA. The patient was administered heparin (62.5 IU/kg) through the puncture sheath. All physicians used a modified aortic stent graft (Ankura; Lifetech Scientific Co., Ltd., Shenzhen, China). This kind of stent-graft is broadly used throughout China. It has a strut (longitudinal metal axis in one side to provide more support in the great curvature) and a radiopaque marker that divides the bare stent part and the membrane part. The marker shows the symbol “∞” when it is vertical to the x-ray beam and “一” when it is parallel with the x-ray beam. When delivery is in the correct position, the strut will show in the outermost part of the stent-graft under screening. In the present study, the outer sheath of the aortic stent-graft was moved backward 3 to 4 cm, letting the proximal part of the stent-graft come out (Figure 1(a)). The fenestration site was located under the help of the “∞” radiopaque marker and strut. A sharp knife and scissors were used to remove the membrane of the intend fenestration area. The fenestration location and size were determined by the size and number of branch arteries needed. The strut of the stent graft was located in the longitudinal middle line of the fenestration (Figure 1(b)). After completion of the fenestration, the assistant used a belt to constrain the stent-graft appropriately, and the outer sheath was moved forward until it reached the original site (Figure 1(c)). All stent-grafts were reloaded into the sheath. The stent-graft was thereafter inserted along the extra-stiff guidewire to the aortic lesion. By recognizing the X-ray plaque mark and strut at the location of the fenestration, the stent-graft could be adjusted to fit the branch orifice under fluoroscopic screening (Figure 1(d)). Self-rotation of the stent-graft was observed by the strut formation under fluoroscopic screening, and rotation of the whole delivery system could adjust the rotation. Fusion imaging played an important role when the fenestration was fitted to the branch. After the location was determined to be satisfactory, the sheath of the stent-graft was retreated and the rear release was unlocked, allowing for complete delivery of the stent-graft. A pigtail catheter was inserted again and DSA was performed to ensure that the intended preserving branch was patent. The patients were discharged from the hospital, and a CTA recheck was performed 1 month after the procedure. Anti-hypertension drugs and beta-blockers were used for continuous control of the blood pressure and heart rate. Follow-up was performed by outpatient CTA or telephone follow-up at 3, 6, and 12 months after discharge.
Figure 1.
(a) Location of the fenestration site using the “∞” marker (black arrow) and strengthening strut (white arrow). A sharp knife and scissors were used to create the fenestration in the membrane of the stent-graft. (b) The fenestration is finished, and the strut (white arrow) is in the middle longitudinal line of the rectangular fenestration. (c) The assistant uses a belt to constrain the stent-graft and push it back into the sheath appropriately without twisting the stent-graft. (d) Under fluoroscopy screening, after the delivery, the “∞” mark (black arrow) and strengthening strut (white arrow) are in the correct position of the larger curvature of the aorta. Digital subtraction angiography shows that the left subclavian artery is patent (black arrow shows the “∞” marker and white arrow show the strengthening strut).
Statistical analysis
Continuous data are presented as mean ± standard deviation, and categorical data are presented as count (percentage). Kaplan–Meier analysis was used to generate estimates for survival, freedom from endoleakage, and freedom from all adverse events. The estimates are presented with 95% confidence intervals (CIs). Analyses were conducted using GraphPad 7.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Thirty-two consecutive patients (25 male, 7 female) were included in this study. The patients’ mean age was 58.2 ± 11.2 years (range, 32–76 years). The spectrum of pathologies included a PAU in 11 (34.4%) patients (5 of the 11 patients had a PAU combined with an abdominal aortic aneurysm), acute AD in 15 (46.9%), TAA in 4 (12.5%), aortic pseudoaneurysm in 1 (3.1%), and type Ia endoleakage after prior TEVAR in 1 (3.1%). Table 1 shows the characteristics and comorbidities of all patients. Although the patients’ aortic pathology and clinical situation were not absolute contraindications for open surgery, all patients refused open surgery with thoracotomy and aortic arch replacement. The perioperative complications among all 32 patients were as follows: stroke, n = 0; paraplegia, n = 0; blood transfusion, n = 0; access artery complications, n = 0; LSA occlusion, n = 1 (3.1%); and type I endoleakage, n = 1 (3.1%).
Table 1.
Characteristics of the 32 patients in the study.
| Age, y | 58.2 ± 11.2 |
| Male | 25 |
| Indications for TEVAR | |
| Aortic dissection | 15 (46.9) |
| Penetrating aortic ulceration | 11 (34.4) |
| Thoracic aortic aneurysm | 4 (12.5) |
| Aortic pseudoaneurysm | 1 (3.1) |
| Type I endoleak after prior TEVAR | 1 (3.1) |
| Comorbidities | |
| Hypertension | 28 (87.5) |
| Pneumonia | 7 (21.9) |
| Chronic kidney disease | 2 (6.3) |
| Diabetes | 1 (3.1) |
| Cholangitis | 1 (3.1) |
| Malignant tumor | 1 (3.1) |
TEVAR, thoracic endovascular aortic repair.
Continuous data are presented as mean ± deviation, and categorical data are presented as count (percentage).
PMF procedure
TEVAR was successfully performed in all patients. Thirty-four thoracic stent-grafts were implanted in 32 patients. The mean proximal diameter of the stent-grafts was 32.4 ± 3.4 cm, and the mean length was 170.0 ± 25.2 cm. Twenty-nine PMF procedures were performed to preserve the LSA. The fenestration size was 1.5 × 1.5 cm for LSA-only fenestration. Three procedures were performed to preserve both the LSA and left common carotid artery (LCCA). The fenestration size for both the LCCA and LSA was 2.5–3.0 × 1.5 cm. Two Bard Fluency® Plus covered stent-grafts of 6 × 60 mm diameter (Angiomed GmbH & Co., Karlsruhe, Germany) were implanted using the chimney technique in the LSA after the fenestration in two patients as bailout for revascularization of the LSA.
The mean distance from the pathology to the LSA was 8.4 ± 4.0 mm. When the distance between the LCCA and LSA was <10 mm (n = 3 patients, 9.4%), the PMF was made larger for both the LCCA and LSA (Figure 2).
Figure 2.
Fenestration for both the left common carotid artery (LCCA) and left subclavian artery (LSA) is large because the distance between these two branches is <10 mm. The black arrow shows the “∞” marker. Distal to the “∞” marker, the white arrow shows the large fenestration for both the LCCA and LSA. Digital subtraction angiography shows that both the LCCA and LSA are patent.
The TEVAR technique success rate was 100%. The technical success rate of fenestration was 93.8%. The success evaluation standard was as follows: (1) the lesion was completely excluded, and no endoleakage occurred immediately after TEVAR; (2) the target artery (i.e., the artery that we intended to preserve) was patent immediately after PMF-TEVAR; and (3) the patient’s vital signs were stable when the procedure was finished. The mean procedure time (from first to last DSA) was 22.8 ± 20.8 minutes (range, 13–132 min). The longest procedure time was when TEVAR and EVAR were performed in one stage. The mean length of hospital stay was 10.8 ± 4.3 days, and the mean intensive care unit (ICU) stay was 2.3 ± 1.4 days (only 4 patients were transferred to the ICU after TEVAR). The 30-day perioperative mortality rate was 0%. The 30-day patency rate of the destination branch artery of PMF was 96.7% (29/30). The 30-day rate of type I endoleakage was 6.25% (2/32).
Follow-up results
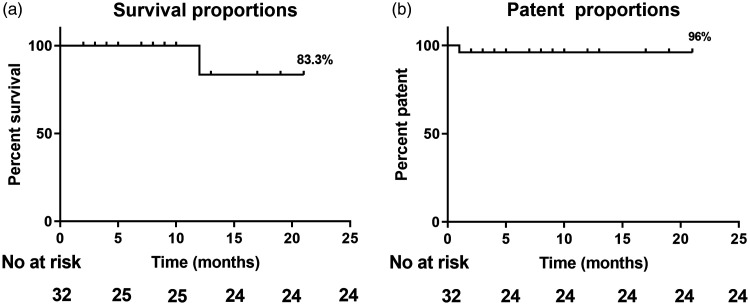
Follow-up was performed on an outpatient and telephone basis. Twenty-five patients were successfully followed up. The follow-up rate was 78.1%. The mean follow-up time was 8.3 ± 5.3 months among these patients, and the longest follow-up time was 21 months. The all-cause survival rate was 83.3% (Figure 3(a)). Only one patient died of stent-graft infection 12 months after TEVAR. The rate of branch artery patency after PMF was 96.0% (Figure 3(b)).
Figure 3.
(a) All-cause survival rate after the physician-modified fenestration technique in thoracic endovascular aortic repair was 83.3%. (b) The patency rate of the target branch artery to be preserved was 96.0%.
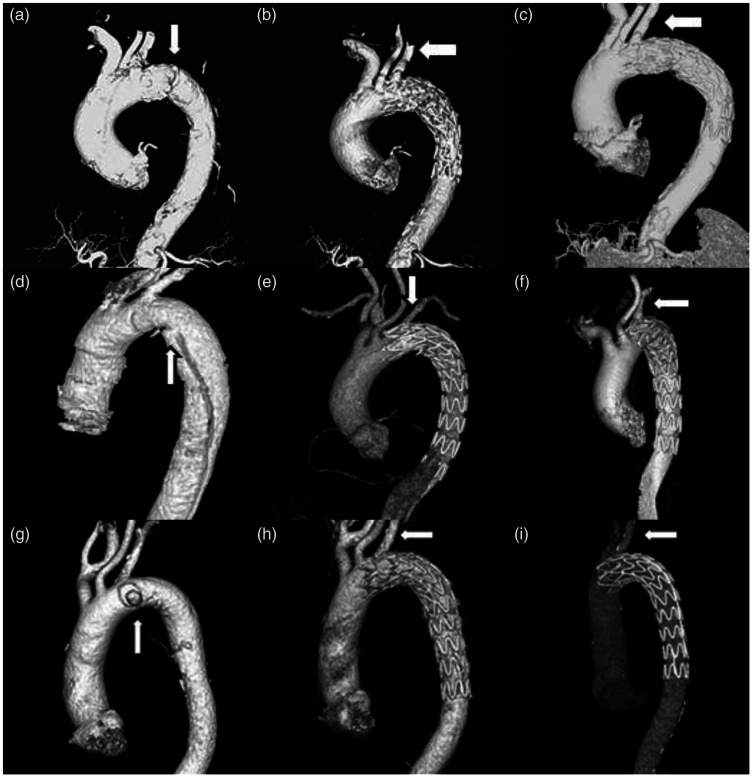
The more important follow-up results were the imaging findings. The patients were instructed to return to the hospital for CTA at 3, 6, and 12 months postoperatively and annually thereafter. The stent-graft location, presence of endoleakage, patency of the fenestrated branch artery, and screening for a distal false lumen were assessed by CTA. Particular attention was given to the patency of the fenestrated branch artery. In all pathologies [PAU (Figure 4(a)–(c)), AD (Figure 4(d)–(f)), and TAA (Figure 4(g)–(i))], the fenestrated artery showed satisfactory results with respect to patency of the branch artery.
Figure 4.
Computed tomography angiography follow-up results of different aortic pathologies after the physician-modified fenestration technique in thoracic endovascular aortic repair (PMF-TEVAR). (a) Multiple penetrating ulcers near the left subclavian artery (LSA). PMF-TEVAR is performed. (b) One-month follow-up and (c) 3-month follow-up after PMF-TEVAR. (d) Aortic dissection. The intima tear is in the lesser aortic curvature (white arrow). (e) One-month follow-up and (f) 3-month follow-up. White arrows show that the fenestrated LSA is patent. (g) Pseudoaneurysm of the aortic arch (white arrows) (h) 1-month and (i) 3-month follow-up. White arrows show that the fenestrated LSA is patent
Discussion
Aortic pathologies involving the supra-arch branch arteries usually occur during endovascular treatment of the aorta. In the first decade of TEVAR, the LSA was always covered by a stent-graft when the proximal landing zone was not long enough. However, some studies9–11 have shown that the LSA provides important circulation to the spinal cord, brain, and left upper extremity; therefore, covering the LSA with a stent-graft might not be inconsequential. Stroke, paraplegia, and coronary ischemia in the setting of a left internal mammary artery bypass as well as left upper extremity ischemia have been described in various reports. The left carotid artery and innominate artery require revascularization during the aortic arch endovascular treatment. The endo-revascularization methods of aortic arch branches include in situ fenestration,2–5 custom-made stent-grafts,6 the chimney technique,7,8 and branched stent-grafts.9 Although these techniques can preserve the blood flow to the branches, they still have some shortcomings such as a long operation time, long physician learning curve, high risk of endoleakage, and low equipment availability. In situ fenestration requires a high number of specialized devices such as laser equipment, laser fibers, wires, catheters, and balloons.2,12 Additionally, in situ fenestration requires sophisticated tissue manipulation and a long operation time (137 ± 15 min4). Lin et al.13 performed experimental physician-modified laser fenestration and considered that laser fenestration is feasible but that the high risk of tissue damage must be carefully considered. A problem associated with custom-made stent-grafts is the incredibly long waiting time, especially in China. Additionally, deployment of custom-made grafts is associated with some problems.14 The chimney technique is a relatively simple and “on-the-shelf” method for revascularization of aortic arch branches. However, because of the complications of endoleakage and migration, the chimney technique (especially the double chimney technique) should be used judiciously.8 Furthermore, the single-branched stent-graft (Castor; Microport, Shanghai, China) can now be used in China. This stent-graft is convenient and very useful for revascularization of the LSA. The problems associated with this method is the relatively complicated maneuver and the risk of branch twisting.9 Its long-term results need to be established and cautiously evaluated in a larger number of cases.15
In 2015, we began using PMF during aortic arch TEVAR. This is a technically easy, time-saving, and low-cost method to preserve patency of the LSA or LSA and LCCA together. Because of the increased stability and maneuverability of current stent-grafts, it is not difficult to remove some parts of the stent-graft, perform a fenestration, and reload the stent-graft. The mean endovascular procedure time (from first to last DSA) was only 22.8 ± 20.8 minutes in this study. Compared with our previously reported time required for chimney technique fluoroscopy (49 ± 19 min),8 use of the PMF technique shortens the procedure time. The ICU stay and entire hospital stay are also shortened by using PMF. If the distance between the LCCA and LSA is <10 mm, a larger fenestration may be considered to keep both the LCCA and LSA patent. PAU and AD seem to be the most frequent pathologies requiring performance of the PMF technique. The incidence of PAU was relatively high in this cohort; however, it is one acute aortic syndrome that requires treatment by TEVAR when an intervention is indicated.16,17 When performing a larger fenestration of the LCCA and LSA, we usually sterilize the left neck area and prepare a Gore Viabahn stent-graft or Bard Fluency stent-graft for the chimney technique as a bailout procedure if the fenestration fails to keep the branch artery patent.
According to our limited experience, the following technical aspects require particular attention. (1) The radiopaque marker “∞” is very important for locating the fenestration. The marker “∞” between the proximal edge of the fenestration equals the distance between the LCCA and LSA. (2). When we make the fenestration, we keep the metallic strengthening strut in the longitudinal midline of the fenestration. The fenestration is present on both sides of the strengthening strut. When located under fluoroscopy, the strengthening strut is in the larger curvature and at the outermost position of the two-dimensional image (Figure 1(d)). (3) The fenestration should be slightly larger than the branch artery orifice; this can decrease the rate of mis-fitting. (4) When reloading the stent-graft into the outer sheath, twisting of the stent-graft should be avoided. The stent-graft should be placed in its original direction back into the sheath. (5) When delivering the stent-graft along the super-stiff guidewire, rotation of the stent-graft may also occur. The self-axis spinning may result in mis-fitting of the fenestration to the branch orifice. Therefore, LSA/LCCA access needs to be prepared; if fitting of the fenestration to the branch fails, the chimney technique will be performed as a bailout procedure to rescue the patency of the LSA/LCCA.
To our knowledge, the number of PMF procedures in our study is relatively high among previous reports. The merits of this method are as follows. (1) It is technically easy and significantly time-saving. Regardless of whether the chimney technique or in situ fenestration is performed, both of these procedures are more time-consuming than PMF. (2) No sophisticated instruments or devices are needed for PMF. In contrast, extra instruments may be needed to preserve the blood flow during in situ fenestration.18 Furthermore, the higher temperature caused by the laser may increase the risk of stroke up to 7%.19 The only instrument needed for PMF is a sharp knife and a steel rule. The simplicity of this method results in a short learning time and low economic cost. (3) The complication rate of this technique is substantially low because of its simplicity. For example, endoleakage after the chimney technique always raises concerns among endovascular treatment experts.20 Endoleakage,21 the incidence of which may reach 23%,22 has been described in many reports. In the present study, however, the rate of endoleakage was only 3.2% among all PMF procedures. Even in the case of LCCA and LSA double-PMF, no endoleakage was observed in either the perioperative period or mid-term follow-up. In some rare situations, the chimney stent-graft may migrate.7,8,23 However, the PMF technique is not associated with this migration problem. (4) Patients do not require anti-platelet drugs after PMF. Dual antiplatelet drugs (aspirin and/or clopidogrel) are usually administered to patients to increase the patency rate of the branch stent-graft in the chimney technique7,8 or in situ fenestration. Our patients did not require anti-platelet administration. The branch patency rate of PMF was satisfactory.
The PMF technique had some limitations in the present study. First, there existed a certain rate of whole stent-graft spinning around the longitudinal axis of the stent graft itself. This phenomenon was shown by the strengthening strut but not in the great curvature of the arch. Therefore, we prepared left brachial access for a bailout stent to the LSA in all patients. Fusion imaging is usually used in aortic aneurysm fenestration EVAR.24 When the DSA and CTA images are fused on the screen using this technique, the aorta can be seen in three dimensions and the image of the aorta and its branches can be rotated to view them from different angles. This can help to more accurately fit the position of the fenestration and artery orifice. The initial use of fusion imaging takes more time by the technician. Further, if the magnetic image-guided technique can be used in TEVAR, as in cerebral or coronary interventions,25,26 more precise fenestration fitting is certain to be accomplished. Second, one patient died of stent-graft infection, which is a catastrophic event after TEVAR. We tried to determine whether the infection was related to our PMF maneuver. However, no infection was found during the perioperative period. Additionally, the patient developed no fever during the 1-year follow-up after TEVAR. Thus, he died of a very late stent-graft infection. The incidence of graft-related infection at 1 year was 3.6% (95% CI, 1.7%–5.5%), and that at 2 years was 4.5% (95% CI, 2.4%–6.6%).27 We found no evidence of a relationship between PMF and infection. Finally, one patient developed an LSA thrombosis at 1 month after PMF. The retrograde filling of the distal LSA by blood was perfect, and the patient showed no symptoms. This thrombosis event was discovered at the 1-month CTA examination; however, DSA after TEVAR showed no occlusion of the LSA. The reason for the thrombosis remains unclear. We prescribe antiplatelet drugs for LCCA and LSA fenestration and for patients who undergo bailout chimney stent-graft procedures. Ischemia or stroke after LSA occlusion is also very rare.28
Conclusion
PMF is a simple, “on-the-shelf,” time-saving alternative method to treat pathologies involving the aortic arch. The perioperative complication rate is low, and the short- to mid-term survival rate and fenestrated branch patency rate are satisfactory. When performing PMF-TEVAR, access of the target branch artery (LSA or LCCA) needs to be prepared for bailout stent-graft delivery after fenestration failure.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Natural Science Foundation of China (81500378).
References
- 1.Yuan X, Mitsis A, Tang Y, et al. The IRAD and beyond: what have we unravelled so far? Gen Thorac Cardiovasc Surg 2019; 67: 146–153. [DOI] [PubMed] [Google Scholar]
- 2.Redlinger RE, Jr, Ahanchi SS, Panneton JM. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J Vasc Surg 2013; 58: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 3.Murphy EH, Dimaio JM, Dean W, et al. Endovascular repair of acute traumatic thoracic aortic transection with laser-assisted in-situ fenestration of a stent-graft covering the left subclavian artery. J Endovasc Ther 2009; 16: 457–463. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Zhao Z, Wang R, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. J Am Heart Assoc 2017; 6: pii: e004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahanchi SS, Almaroof B, Stout CL, et al. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J Endovasc Ther 2012; 19: 226–230. [DOI] [PubMed] [Google Scholar]
- 6.Kurimoto Y, Maruyama R, Ujihira K, et al. Thoracic endovascular aortic repair for challenging aortic arch diseases using fenestrated stent grafts from zone 0. Ann Thorac Surg 2015; 100: 24–32. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Shu C, Li QM, et al. First experience with the double chimney technique in the treatment of aortic arch diseases. J Vasc Surg 2017; 5: 30910–30912. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Shu C, Li M, et al. Thoracic Endovascular Aortic Repair with single/double chimney technique for aortic arch pathologies. J Endovasc Ther 2017; 24: 383–393. [DOI] [PubMed] [Google Scholar]
- 9.Huang H, Jiao Y, Zhang Y, et al. Implantation of unibody single-branched stent graft for patients with type B aortic dissections involving the left subclavian artery: 1-year follow-up outcomes. Cardiovasc Intervent Radiol 2017; 40: 1678–1686. [DOI] [PubMed] [Google Scholar]
- 10.Lee TC, Andersen ND, Williams JB, et al. Results with a selective revascularization strategy for left subclavian artery coverage during the thoracic endovascular aortic repair. Ann Thorac Surg 2011; 92: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizvi AZ, Murad MH, Fairman RM, et al. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg 2009; 50: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 12.McWilliams RG, Murphy M, Hartley D, et al. Harris In situ stent-graft fenestration to preserve the left subclavian artery. J Endovasc Ther 2004; 11: 170–174. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Udgiri N, Guidoin R, et al. Physician modified laser fenestration of aortic stent-grafts: a qualitative analysis under scanning electron microscope. Artif Organs 2016; 40: E241–E252. [DOI] [PubMed] [Google Scholar]
- 14.Varcoe RL, Lennox AF. Fenestrated aortic arch stent-graft malfunction with endovascular salvage. J Endovasc Ther 2013; 20: 242–248. [DOI] [PubMed] [Google Scholar]
- 15.Lu Q, Feng J, Zhou J, et al. Endovascular repair by customized branched stent-graft: a promising treatment for chronic aortic dissection involving the arch branches. J Thorac Cardiovasc Surg 2015; 150: 1632–1638. [DOI] [PubMed] [Google Scholar]
- 16.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010; 121: e266–e369. [DOI] [PubMed] [Google Scholar]
- 17.D'Annoville T, Ozdemir BA, Alric P, et al. Thoracic endovascular aortic repair for penetrating aortic ulcer: literature review. Ann Thorac Surg 2016; 101: 2272–2278. [DOI] [PubMed] [Google Scholar]
- 18.Xiong J, Guo W, Liu X, et al. Novel temporary endovascular shunt technique to assist in situ fenestration for endovascular reconstruction of the distal aortic arch. J Vasc Surg 2015; 62: 226–228. [DOI] [PubMed] [Google Scholar]
- 19.Crawford SA, Sanford RM, Forbes TL, et al. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J Vasc Surg 2016; 64: 244–250. [DOI] [PubMed] [Google Scholar]
- 20.Lindblad B, Bin Jabr A, Holst J, et al. Chimney grafts in aortic stent grafting: hazardous or useful technique? Systematic review of current data. Eur J Vasc Endovasc Surg 2015; 50: 722–732. [DOI] [PubMed] [Google Scholar]
- 21.Voskresensky I, Scali ST, Feezor RJ, et al. Outcomes of thoracic endovascular aortic repair using aortic arch chimney stents in high-risk patients. J Vasc Surg 2017; 66: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangialardi N, Serrao E, Kasemi H, et al. Chimney technique for aortic arch pathologies: an 11-year single-center experience. J Endovasc Ther 2014; 21: 322–323. [DOI] [PubMed] [Google Scholar]
- 23.Leopardi M, Tshomba Y, Castiglioni A, et al. Late retrograde migration of a left subclavian artery chimney stent-graft into the innominate artery. J Endovasc Ther 2016; 23: 666–669. [DOI] [PubMed] [Google Scholar]
- 24.Tacher V, Lin M, Desgranges P, et al. Image guidance for endovascular repair of complex aortic aneurysms: comparison of two-dimensional and three-dimensional angiography and image fusion. J Vasc Interv Radiol 2013; 24: 1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuribara T, Haraguchi K, Ogane K, et al. 3D-FIESTA magnetic resonance angiography fusion imaging of distal segment of occluded middle cerebral artery. Neurol Med Chir (Tokyo) 2015; 55: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsekos NV, Atalar E, Li D, et al. Magneti resonance imaging-guided coronary interventions. J Magn Reson Imaging 2004; 19: 734–749. [DOI] [PubMed] [Google Scholar]
- 27.Berger P, Vaartjes I, Moll FL, et al. Cumulative incidence of graft infection after primary prosthetic aortic reconstruction in the endovascular era. Eur J Vasc Endovasc Surg 2015; 49: 581–585. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009; 50: 1155–1158. [DOI] [PubMed] [Google Scholar]