Abstract
An accessory spleen refers to single or multiple splenic tissues that appear outside the normal spleen position and have structures and functions similar to those of a normal spleen. We herein present a rare case of a 31-year-old woman who was hospitalized because of a 14-year history of sudden left upper abdominal pain after running. Abdominal computed tomography suggested a large soft tissue mass at the left renal hilum surrounded by several enlarged lymph nodes, which was totally different from computed tomography scanning of normal accessory spleen. The mass was resected by robot-assisted laparoscopic surgery. Histopathological examination confirmed the diagnosis of accessory spleen. The incidence of retroperitoneal accessory spleen is very rare, which should be differentiated with retroperitoneal tumors.
Keywords: Accessory spleen, retroperitoneal tumor, imaging, case report, computed tomography, histopathology
Introduction
The spleen is derived from mesenchymal cells of the dorsal mesentery. At the fifth week of embryonic development, an accessory spleen may form if the embryo spleen bud is not fully fused or its single cell is separated from the body of the spleen.1 Accessory spleens are relatively common and are found in about 16% of patients undergoing contrast-enhanced abdominal computed tomography (CT).2 Accessory spleens generally have no obvious clinical symptoms. Most accessory spleens are diagnosed as an incidental finding during a health examination. Accessory spleens are frequently located in the hilum of the spleen or adjacent to the tail of the pancreas.2,3 When the accessory spleen is large, symptoms of compression, gastrointestinal tract pulling, or pain during exercise may appear. Clinical symptoms may also occur when the accessory spleen develops complications such as torsion, necrosis, infarction, or traumatic bleeding. Accessory spleens may mimic lymphadenopathy as well as neoplasms located in the pancreas,4 left suprarenal gland,5 and retroperitoneum.6 This case report describes a giant retroperitoneal accessory spleen misdiagnosed as a retroperitoneal mass. CT showed that the mass was located at the left renal hilum, close to the left renal artery, and surrounded by tortuous vascular shadows. Considering the difficulty of surgical removal of the mass and the inability to obtain a preoperative pathological diagnosis, we used a robotic surgical system to resect the left retroperitoneal mass. Accessory spleens are not uncommon, and most of the imaging findings are typical. However, the imaging findings of the accessory spleen in the present case were very special and difficult to distinguish from a retroperitoneal tumor. An in-depth study of this case can broaden our understanding of accessory spleens. The findings indicate that when we treat a patient with a retroperitoneal mass, we can consciously distinguish the mass from an accessory spleen and thus avoid unnecessary surgical treatment.
Case report
A 31-year-old woman presented with a 14-year history of pain in the left upper abdomen after running. The pain could be relieved after taking a break from running. She was hospitalized after a left retroperitoneal mass was detected during a health examination. The patient reported no discomfort or other medical history. After hospitalization, physical examination and routine tests showed no obvious abnormalities. Abdominal CT revealed a soft tissue mass in the left renal hilum (Figure 1(a)). Contrast-enhanced CT showed that the mass exhibited significant homogeneous enhancement and was close to the left renal artery (Figure 1(b)–(d)). The maximum diameter of the mass was 5.0 cm. Multiple enlarged lymph nodes and circuitous vascular shadows were observed around the mass. No obvious abnormalities were found in the liver, gallbladder, pancreas, spleen, bilateral kidneys, or other organs. The concentrations of plasma adrenocorticotropic hormone, serum cortisol, 24-hour urine catecholamine, plasma renin angiotensin, and serum aldosterone were normal.
Figure 1.
(a) Abdominal computed tomography showed a soft tissue mass in the left renal hilum. The maximum diameter of the mass was 5.0 cm. (b–d) Contrast-enhanced computed tomography showed that the mass exhibited significant homogeneous enhancement and was located close to the left renal artery. The main supply artery of the mass came from the left common iliac artery.
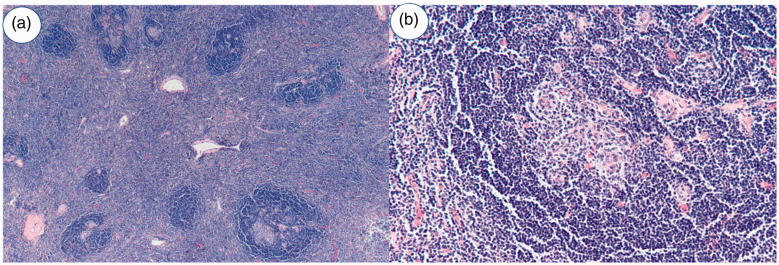
Robot-assisted laparoscopic resection of the left retroperitoneal mass was performed. The resected specimen was examined by histopathology. The mass was a 9- × 5- × 4-cm gray-red nodule with a solid cut plane, tough texture, and clear boundary (Figure 2). Microscopic examination revealed red pulp and white medullary structure in the tissue, which was finally diagnosed as an accessory spleen (Figure 3). The patient was followed up for 6 months postoperatively and developed no further pain or discomfort.
Figure 2.
The specimen was a 9- × 5- × 4-cm gray-red mass.
Figure 3.
Microscopic examination demonstrated cytomorphologic features consistent with conventional splenic tissue. (a) Hematoxylin and eosin, ×40 and (b) Hematoxylin and eosin, ×200.
Discussion
The density of an accessory spleen is similar to that of the normal spleen, and its structure is consistent with splenic tissue. The blood supply of the accessory spleen is mainly provided by the splenic vessels.2 Compensatory hyperplasia of the accessory spleen after splenectomy has been reported in the literature.7 An accessory spleen is usually detected by CT. Plain CT of the accessory spleen shows round or elliptical soft tissue nodules of varying sizes and with both smooth and sharp edges. The size of the accessory spleen is usually about 1 cm in diameter.2 After enhancement, the change in the CT value of the accessory spleen is similar to that of the main spleen.2 The main spleen and accessory spleen are histologically composed of red pulp and white pulp. Red pulp is composed of several blood sinuses, while white pulp is located between red pulp and consists of reticular endothelial system cells. Therefore, the distribution of red and white pulp leads to flower-spot-like enhancement in the arterial phase of enhanced CT. Part of the accessory spleen may be uniformly enhanced because of its small size. In the present case, two imaging features of the accessory spleen led to the diagnostic difficulty. First, the main supply artery of the accessory spleen came from the left common iliac artery instead of the splenic artery. Second, the size of the accessory spleen in this case was much larger than usual, and the arterial phase of enhanced scanning was significantly homogeneous, which was totally different from the enhancement pattern of the main spleen. Therefore, the accessory spleen in this case was very unique. Technetium-99m heat-damaged red blood cell scintigraphy is considered the gold standard for the differentiation of accessory splenic tissue.8 However, the use of this special auxiliary inspection requires a high index of suspicion.
According to the results of contrast-enhanced CT, we initially believed that this patient might have had an ectopic pheochromocytoma. However, the adrenocortical hormone and 24-hour urine catecholamine concentrations were normal, and the patient had experienced no hypertension, amaurosis, palpitation, dizziness, hidrosis, or other abnormalities since symptom onset. Therefore, the diagnosis of ectopic pheochromocytoma was excluded. The mass in this case was also differentiated from lymphoma, which can occur in any part of the body, including the lymph nodes, tonsils, spleen, and bone marrow. Painless, progressive lymphadenopathy and a local mass, as well as systemic symptoms including fever, weight loss, and night sweats, are the characteristic clinical manifestations of lymphoma. In a study by Hagtvedt et al.,9 contrast-enhanced CT of retroperitoneal lymphoma showed low or moderate enhancement. In the present case, no suspicious lesions were found in other parts of the patient’s body, and there were no systemic symptoms. Contrast-enhanced CT demonstrated significant homogeneous enhancement of the mass. According to the patient’s history and contrast-enhanced CT findings, we considered lymphoma to be unlikely, and further pathological examination was consequently required for the final diagnosis. Laparoscopic or retroperitoneal laparoscopic resection of masses is less invasive and helps to confirm the diagnosis.10 In this case, robot-assisted laparoscopic surgery was chosen to avoid unnecessary injury. No perioperative complications occurred.
Conclusion
This case report describes a rare case of a giant left retroperitoneal mass that was treated by robot-assisted laparoscopic resection. The final pathological diagnosis was an accessory spleen. Although the imaging findings of the accessory spleen in this case differed from those of a classic accessory spleen, an accessory spleen must still be included as a differential diagnosis of retroperitoneal masses in such cases.
Author contributions
Conception and design: Xiaogong Li and Hongqian Guo
Medical record data: Youjian Li and Weijian Li
Image analysis: Xiaogong Li and Hongqian Guo
Manuscript drafting or revising: Youjian Li and Xuefeng Qiu
Ethics
Ethics committee approval was not required because we presented only one case of a rare condition. Written informed consent was obtained from the patient.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Dodds WJ, Taylor AJ, Erickson SJ, et al. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol 1990; 155: 805–810. [DOI] [PubMed] [Google Scholar]
- 2.Mortelé KJ, Bart M, Silverman SG. CT features of the accessory spleen. AJR Am J Roentgenol 2004; 183: 1653–1657. [DOI] [PubMed] [Google Scholar]
- 3.Gayer G, Zissin R, Apter S, et al. CT findings in congenital anomalies of the spleen. Br J Radiol 2001; 74: 767. [DOI] [PubMed] [Google Scholar]
- 4.Lehtinen SJ, Schammel CM, Devane M, et al. Intrapancreatic accessory spleen presenting as a pancreatic mass. J Gastrointest Oncol 2013; 4: E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CH, Wu HC, Chang CH. An accessory spleen mimics a left adrenal carcinoma. MedGenMed 2005; 7: 9. [PMC free article] [PubMed] [Google Scholar]
- 6.Toutziaris C, Kampantais S, Christopoulos P, et al. Compensatory enlargement of an accessory spleen mimicking a retroperitoneal tumor: a case report. Hippokratia 2013; 17: 185–186. [PMC free article] [PubMed] [Google Scholar]
- 7.Tjaden C, Werner J, Buechler MW, et al. Reactive hypertrophy of an accessory spleen mimicking tumour recurrence of metastatic renal cell carcinoma. Asian J Surg 2011; 34: 50–52. [DOI] [PubMed] [Google Scholar]
- 8.Spencer LA, Spizarny DL, Williams TR. Imaging features of intrapancreatic accessory spleen. Br J Radiol 2010; 83: 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagtvedt T, Aalokken TM, Smith HJ, et al. Enhancement characteristics of retroperitoneal lymphomatous lymph nodes. Acta Radiol 2013; 54: 333–339. [DOI] [PubMed] [Google Scholar]
- 10.Hara I, Tanaka K, Yamada Y, et al. Usefulness of laparo- or retroperitoneoscopic biopsy for retroperitoneal lymph node swelling of unknown origin. Int J Urol 2010; 14: 466–469. [DOI] [PubMed] [Google Scholar]