Abstract
Objective
To investigate the immunomodulatory effects of the tyrosine kinase inhibitor (TKI) dasatinib on T-cell subtypes in patients with chronic myeloid leukemia (CML).
Methods
T helper (Th) 1, Th2, regulatory T (Treg), and CD8+T cell levels were detected in patients with CML (n = 9) before and after dasatinib treatment. The corresponding response level at the time of a blood test was evaluated.
Results
After dasatinib treatment, six patients achieved a better response level, while three did not show improved response levels. Among the total nine patients, there were no significant differences in Th1, Th2, and Treg cell levels, whereas CD8+T cell levels were significantly increased after dasatinib treatment compared with before treatment. When we analyzed the six patients who obtained a better response level, Th1 and CD8+T cell levels were significantly increased after dasatinib treatment, but Th2 and Treg cell levels did not change. The other three patients who did not have improved response levels showed decreased Th1 cell levels and increased Treg cell levels after treatment.
Conclusions
Dasatinib may increase Th1 and CD8+T cell levels, and decrease Treg cell levels in patients with CML. This finding might be associated with a good therapeutic response to this drug.
Keywords: Chronic myeloid leukemia, tyrosine kinase inhibitor, dasatinib, Th1, regulatory T (Treg) cells, CD8+T
Introduction
Chronic myeloid leukemia (CML) is a malignant disease caused by clonal proliferation of hematopoietic stem cells. The pathogenesis of this disease is related to the formation of the BCR-ABL fusion gene in the Philadelphia chromosome (Ph).1 Proteins encoded by this fusion gene can abnormally increase the activity of tyrosine kinase, resulting in uncontrolled proliferation, blocked differentiation, and inhibited apoptosis of hematopoietic stem cells. This then leads to abnormal hematopoiesis.2
The emergence of tyrosine kinase inhibitors (TKIs) has dramatically improved the efficacy of treating patients with CML, including a prolonged survival period and improved quality of life.3,4 As the first-generation TKI, imatinib has made a great breakthrough in therapeutic efficacy.5 However, because of drug resistance or intolerance, imatinib shows poor therapeutic efficacy in some patients with CML.6,7 Therefore, a second generation of TKIs, such as dasatinib and nilotinib, has gradually been developed and applied in the clinic. These second-generation drugs show encouraging efficacy compared with imatinib, especially in patients who have no improvement with treatment of imatinib.8,9 Studies have shown that second-generation TKIs, such as dasatinib, induce rapid rates of cytogenetic and molecular responses compared with imatinib.10,11 In clinical application and research, researchers have gradually found that dasatinib shows direct anti-tumor effects on CML by inhibiting the activity of tyrosine kinase. Furthermore, dasatinib shows significant immunomodulatory activity, such as stimulating proliferation of natural killer cells and enhancing the cytotoxicity of natural killer cells in vivo, which results in significantly enhanced anti-tumor immune responses.12–15
Currently, immunotherapy strategies that aim at improving the anti-tumor immune responses of the body have achieved remarkable efficacy in treating malignant disease. The immune status of patients with CML is important for choosing an effective drug regimen, evaluating the therapeutic response, and predicting prognosis in patients with CML. There have been many studies on the therapeutic efficacy and prognosis therapy of patients with CML after dasatinib treatment.16 However, the immunomodulatory efficacy and the involved mechanism of dasatinib are not fully understood. Therefore, this study aimed to investigate the immunomodulatory effect of dasatinib and its association with clinical efficacy. We compared changes in levels of T helper (Th) 1, Th2, regulatory T (Treg), and CD8+T cells in patients with CML before and after dasatinib treatment.
Patients and methods
Baseline characteristics of patients
Nine patients with CML who were treated with dasatinib from January 2018 to December 2018 at Sichuan Provincial People’s Hospital were included in this study. All patients were treated with dasatinib at a dose of 100 mg/day. The levels of Th1, Th2, Treg, and CD8+T cells in each patient with CML were detected before and after dasatinib treatment. A blood test for T-cell subtypes before dasatinib treatment was mostly conducted 1 month before the starting date of dasatinib administration (times of administration for each patient are shown in Table 1), while a blood test for T-cell subtypes after dasatinib treatment was conducted after continued dasatinib treatment of 3 to 5 months (Table 1). During the study, the drug dose was adjusted according to the conditions of blood cells and drug tolerance of patients. Bone marrow morphology, cytogenetics, and molecular biology tests (BCR-ABL transcripts were detected by fluorescence quantitative polymerase chain reaction as previously described17) were performed before and after dasatinib treatment according to the time points of the T-cell subtype test in patients with CML. The Medical Ethics Committee of Sichuan Provincial People’s Hospital approved the study. Informed consent forms were signed and obtained from all of the study’s participants.
Table 1.
Cytogenetic and molecular responses in patients with CML before and after dasatinib treatment.
| No. | Sex | Age (years) | Pre-das treatment |
Time interval |
Cytogenetic response |
Molecular response(BCR-ABL/ABL IS) |
Time of Continued Das treatment |
|---|---|---|---|---|---|---|---|
| 1, 2 | Pre-das, after-das | Pre-das, after-das | |||||
| 1 | Male | 17 | Imatinib for 6 months | 62 days, 12 hours | MCR, CCyR(Ph+ = 40%), (Ph+ = 0%) | 14.097%, 0.087% (MMR) | 5 months |
| 2 | Male | 44 | Imatinib for 43 monthsand then nilotinib for 6 months | 77days, 8 hours | CCyR, CCyR(Ph+ = 0%), (Ph+ = 0%) | 0.000%, 0.000%(MR5), (MR5) | 5 months |
| 3 | Female | 44 | Imatinib for 6 months | 22 days, 12 hours | MCR, CCyR(Ph+ = 50%), (Ph+ = 0%) | 6.657%, 0.010% (MR4) | 4 months |
| 4 | Male | 61 | Imatinib for 53 monthsand then nilotinib for 8 months | 15 days, 6 hours | CCyR, CCyR(Ph+ = 0%), (Ph+ = 0%) | 4.166%, 0.030% (MMR) | 4 months |
| 5 | Male | 32 | Imatinib for 10 months | 3 days, 8 hours | CCyR, CCyR(Ph+ = 0%), (Ph+ = 0%) | 0.246%, 0.000% (MR5) | 3 months |
| 6 | Male | 52 | Imatinib for 2 monthsand then no treatment for 22 months (private economics reason) | 33 days, 5 hours | MCR, CCyR(Ph+ = 100%), (Ph+ = 0%) | 32.000%, 0.000% (MR5) | 3 months |
| 7 | Female | 56 | Imatinib for 12 months | 20 days, 8 hours | MCR, MCR(Ph+ = 52.780%), (Ph+ = 85%) | 16.633%, 3.380% | 4 months |
| 8 | Male | 45 | Imatinib for 3 months | 60 days, 3 hours | MCR, CCyR(Ph+ = 100%), (Ph+ = 0%) | 43.800%, 15.576% | 3 months |
| 9 | Male | 47 | Imatinib for 8 months | 31 days, 8 hours | MCR, MCR(Ph+ = 100%), (Ph+ = 90%) | 52.085%, 41.788% | 3 months |
Time interval 1: time interval between the time point of the first blood test for T-cell subtypes and the start date of dasatinib treatment.
Time interval 2: time interval between the time point of dasatinib intake and the time point of a blood test on the day of the blood test for T-cell subtypes.
Abbreviations: das, dasatinib; pre-das, before treatment with dasatinib; after-das, after treatment with dasatinib; CCyR, complete cytogenetic response; MCR, minor cytogenetic response; MMR, major molecular response; MR4, molecular response 4; MR5, molecular response 5; Ph+, Ph-positive metaphases.
Note: Because of the absence of CD8+T data from the ninth patient, we only analyzed CD8+T data in the first eight patients, including six who achieved better response levels and the other two patients who did not show improved response levels after dasatinib treatment.
Evaluation of clinical efficacy
The definition of a molecular response in patients with CML was based on “The guidelines for diagnosis and treatment of chronic myeloid leukemia in China” (2016 edition).18 A major molecular response was defined as BCR-ABL1IS 0.1%. Molecular response4 was defined as BCR-ABL1IS 0.01%, molecular response 4.5 was defined as BCR-ABL1IS 0.0032%, and molecular response 5 was defined as BCR-ABL1IS 0.001%. The definition of a cytogenetic response was based on “Chronic Myeloid Leukemia, Version 1.2019 Clinical Practice Guidelines in Oncology”.19 A complete cytogenetic response was defined as no Ph-positive metaphases.4 A partial cytogenetic response was defined as 1% to 35% Ph-positive metaphases. A minor cytogenetic response was defined as Ph-positive metaphases >35% to 65%.
Detection of T-cell subtypes
For each patient with CML, the levels of T-cell subtypes, including CD8+T (CD8+T/lymphocyte), Th1 (Th1/CD4+T), Th2 (Th2/CD4+T), and Treg (Treg/CD4+T) cells, were detected before and after dasatinib treatment. Detection was performed using a FACSCanto II Flow Cytometer (BD Corporation, Franklin Lakes, NJ, USA). Immunofluorescent antibody–fluorescein-labeled anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-interferon (IFN)-γ, and anti-interleukin (IL-4) were purchased from BD Corporation. Fluorescein-labeled anti-Foxp3 antibody was purchased from Thermo Fisher Scientific (Waltham, CA, USA). Stimulants, including brefeldin A, ionomycin, and phorbol myristate, were purchased from Sigma-Aldrich (St Louis, MO, USA). Permeabilization medium and fixation medium were purchased from Thermo Fisher Scientific.
The process of flow cytometry staining was as follows. Each patient with CML had 2 to 4 mL of peripheral blood collected for flow cytometry analysis. The Th1 and Th2 ratios (Th1/CD4+T and Th2/CD4+T) were measured as follows. Peripheral blood of patients with CML was mixed with RPMI1640 at an equal volume, and subsequently mixed with brefeldin A, ionomycin, and phorbol myristate acetate for 4 hours at 37°C, 5% CO2. Antibodies for cell surface markers, including fluorescein-labeled anti-CD3 (20 µL) and anti-CD8 (5 µL) antibodies, were then added. The mixture was incubated for 15 minutes at room temperature in the darkness. Erythrocyte lysate solution (BD) was added to this mixture and mixed, followed by a 10-minute incubation to ensure complete lysis of erythrocytes. The mixture was then centrifuged at 395 × g and the supernatant was removed. Subsequently, cells at the bottom of the centrifuge tube were washed with 1×phosphate-buffered saline (PBS) and then fixed by fixation medium for 15 minutes at room temperature in darkness. The cells were then washed once in 3 mL PBS + 0.1% NaN3 + 5% fetal bovine serum (FBS). Permeabilization medium and intracellular antibodies, including anti-IFN-γ antibody (20 µL) and anti-IL-4 antibody (20 µL), were mixed with these cells for incubation of 20 minutes. After washing once in 3 mL PBS + 0.1% NaN3 + 5%FBS, these cells were resuspended in an appropriate volume of 1×PBS solution, and then analyzed by flow cytometry. The surface markers of Th cells are CD3+ CD4+, whereas CD4+ cells significantly decrease or even disappear when stimulated by phorbol myristate acetate for 4 hours. This is because CD4 molecules on the cell surface are endocytosed by the cell, which is induced by phorbol myristate acetate. Therefore, in this study, CD3+ CD8– cells were considered as CD3+ CD4+ cells. Additionally, CD3+ CD8– IFN-γ+ cells were considered as Th1 cells, CD3+ CD8– IL-4+ cells were considered as Th2 cells, and CD3+ CD8– cells were considered as CD4+T cells.
To determine the Treg ratio (Treg/CD4+T), after peripheral blood of patients with CML was mixed with RPMI1640 culture medium at an equal volume, antibodies for cell surface markers (fluorescein-labeled anti-CD3 [20 µL], anti-CD4 [20 µL], and anti-CD25 [5 µL] antibodies) were added and incubated for 15 minutes at room temperature in the darkness. Erythrocyte lysate solution was added and mixed, followed by 10 minutes of incubation at room temperature. After washing with 1×PBS, fixation medium was added and the mixture was incubated for 15 minutes at room temperature. The cells were then washed once in 3 mL PBS + 0.1% NaN3 + 5% FBS. The sample was centrifuged for 5 minutes at 300 to 350× g and the supernatant was removed. After the cells were resuspended in 1×PBS solution, permeabilization medium and intracellular anti-Foxp3 antibody [5 µL] were added for incubation of 20 minutes. The cells were then washed once in 3 mL PBS + 0.1% NaN3 + 5% FBS. These cells were resuspended in an appropriate volume of 1×PBS solution and then analyzed by flow cytometry. CD3+ CD4+ CD25+ foxp3+ cells were considered as Treg cells and CD3+ CD4+cells were considered as CD4+T cells.
For the CD8+T ratio (CD8+T/lymphocyte), peripheral blood of patients with CML was incubated with erythrocyte lysate solution for 15–20 minutes. This mixture was centrifuged at 395 × g and the supernatant was removed. Fluorescein-labeled anti-CD3 (20 µL) and anti-CD8 (5 µL) antibodies were then added to the mixture for incubation of 20 minutes at 4°C in darkness. After washing with 1×PBS, these cells were resuspended in an appropriate volume of 1×PBS solution, and then analyzed by flow cytometry. Data analysis was performed using BD Diva analysis software (San Jose, CA, USA).
Statistical analysis
The paired t-test was used to analyze changes in T-cell subsets in patients with CML before and after dasatinib treatment with SPSS 14.0 software (Chicago, IL, USA). All results are shown as mean ± standard deviation (M ± SD). P0.05 was considered to indicate a statistically significant difference.
Results
Patients’ characteristics and treatment responses
In this study, levels of T-cell subsets of nine patients with CML before and after dasatinib treatment were detected. Seven men and two women were included, with a range in age of 17 to 61 years. The median time of treatment with dasatinib was 4 months (3–5 months). By the time of reexamination, six of these nine patients achieved a deeper response level after treatment, while the other three did not show improved response levels after treatment compared with the remission situation before dasatinib treatment (Table 1).
Th1 ratio (Th1/CD4+T)
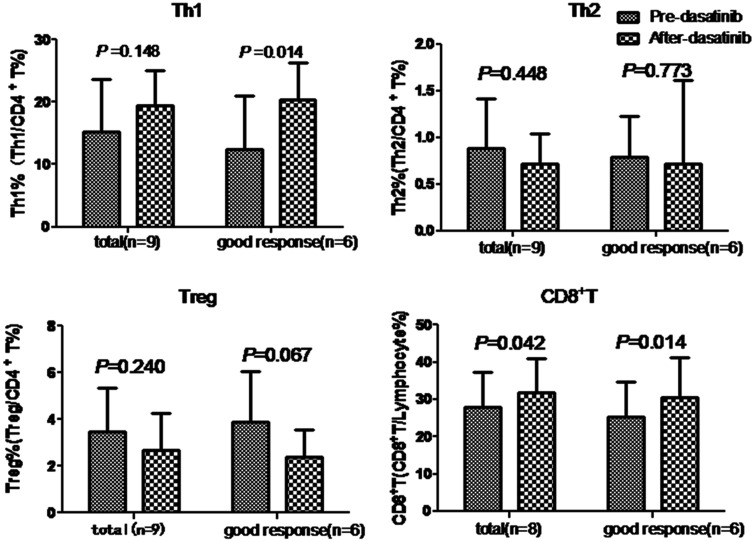
In the nine patients, the mean ( ± standard deviation) Th1 proportion (Th1/CD4+T) was 15.31% ± 8.29% before dasatinib treatment and 19.52% ± 5.61% after treatment, with no significant difference before and after treatment. Among the six patients who obtained a deeper response level after treatment, the proportion of Th1 cells was significantly higher after treatment than before treatment (P = 0.014) (Figure 1). However, the other three patients who did not have improved response levels after treatment showed lower Th1 levels compared with before treatment.
Figure 1.
Levels of Th1, Th2, Treg, and CD8+T cells before and after dasatinib treatment. Data of the total patients with chronic myeloid leukemia and those of six patients who achieved deeper response levels after dasatinib treatment are shown. Abbreviations: Th, T helper; Treg, T regulatory.
Th2 ratio (Th2/CD4+T)
In the nine patients, the mean Th2 proportion (Th2/CD4+T) was 0.89% ± 0.53% before dasatinib treatment and 0.72% ± 0.32% after treatment, with no significant difference before and after treatment. The Th2 proportion was also not significantly different in the six patients who achieved a deeper response level after dasatinib treatment compared with before treatment (Figure 1
Treg ratio (Treg/CD4+T)
In the nine patients, the mean Treg proportion (Treg/CD4+T) was 3.49% ± 1.87% before dasatinib treatment and 2.69% ± 1.59% after treatment, with no significant difference before and after treatment. In the six patients who achieved a deeper response level after dasatinib treatment, the Treg proportion tended to be lower after dasatinib treatment compared with before treatment (P = 0.067) (Figure 1). However, the other three patients who did not have improved response levels showed increased Treg cell levels after treatment compared with before treatment.
CD8+T ratio (CD8+T/lymphocytes)
Among eight patients, the mean CD8+T proportion (CD8+T/lymphocyte) was significantly lower (28.00% ± 9.38%) before dasatinib treatment compared with after treatment (31.75% ± 9.11%, P = 0.042). Among these eight patients, the CD8+T proportion was significantly increased (P = 0.014) in the six patients who achieved better response levels after dasatinib treatment compared with before treatment, while it was decreased in the other two patients who did not show an improved response level (Figure 1).
Discussion
TKIs, which can directly inhibit the activity of tyrosine kinase, are important for therapeutic efficacy and have become essential drugs for therapy of patients with CML. Dasatinib can enhance the anti-cancer immune responses of patients with CML in vivo because of its immunomodulatory activity. Cancer therapy has entered the new era of immunotherapy. Improving the anti-tumor immunity of patients is important for completely eliminating the last cancer cell. Therefore, dasatinib has good potential in novel immunotherapy strategies.
At present, the body’s anti-tumor immunity is believed to be mainly mediated by cellular immune responses.20 In CD4+T-cell subsets, Th1 cells secrete IFN-γ and mediate activation of the cellular immune responses, Th2 cells mediate activation of the humoral immune responses, and Treg cells mediate immunosuppression. Th1 cells are an important helper T-cell subtype, which can mediate the cellular immune response, and elevated Th1 levels can contribute to promotion of anti-tumor cellular immune responses. Th1 cells promote complete activation of CD8+T cells, which can further directly kill cancer cells. Therefore, the T-cell subsets that play an essential role in the anti-tumor immune responses in vivo are Th1 and CD8+T cells.21 The level of Th2 cells can reflect in part whether the mode of cellular immune responses is dominant. Treg cell levels are important for evaluating the activation level of immune responses. Treg cells play an important role in the negative regulation of immune responses. In normal mice and humans, Treg cells account for 5% to 10% of CD4+T cells in peripheral blood.22 However, in patients with tumors, abnormally elevated Treg cells may inhibit the anti-tumor specific immune responses.23 Therefore, tumor cells are not detected and killed by the immune system, which results in acceleration of tumor progression. In this study, Th1, Th2, Treg, and CD8+T cells were detected before and after treatment with dasatinib to evaluate the anti-tumor immunity of patients with CML and to analyze the association between the above-mentioned immune parameters and the efficacy of dasatinib.
Among our nine patients, Th1 levels appeared to be higher after dasatinib treatment compared with those before treatment, but this was not significant. However, among the six patients who achieved a better response level after dasatinib treatment, Th1 levels were significantly higher after treatment compared with before treatment. The other three patients did not show an improved response level after dasatinib treatment. Interestingly, these three patients showed lower Th1 levels after dasatinib treatment compared with before treatment. CD8+T cell levels of patients were significantly higher after dasatinib treatment compared with before treatment. Interestingly, six of these eight patients achieved a deeper response level after dasatinib treatment, and they all showed simultaneously increased CD8+T levels after treatment compared with before treatment. However, the other two patients who did not show an improved response level after dasatinib treatment showed decreased CD8+T levels after treatment. There was no significant difference in Th2 levels between before and after dasatinib treatment This lack of change in Th2 levels could be because Th2 induces humoral immune responses, which are not the dominant type of immune responses in vivo in patients with CML Cellular immune responses in patients are preferred and are more dominant in vivo. Treg cells are a T-cell subtype that plays a role in immunosuppression. After treatment with dasatinib, Treg cell levels of all nine patients were lower compared with Treg cell levels before treatment, but this difference was not significant. When we analyzed Treg cell levels of the six patients who achieved better response levels after treatment, Treg cell levels tended to be lower after dasatinib treatment compared with before treatment. This finding may have been related to the limited number of enrolled patients. In the future, we will continue to follow-up the patients and increase the number of enrolled patients. We will also further investigate and confirm the effect of dasatinib on Treg cells. Our findings suggest that dasatinib reduces Treg cell levels, thereby reducing their inhibitory effect on immune responses, which in turn contributes to stronger activation of the immune response in vivo. Dasatinib has a clinically significant and direct effect on immune effector cells, resulting in rapid lymphocyte mobilization, activation, and transmigration.24,25
In our study, after treatment with dasatinib, patients with elevated levels of Th1 and CD8+T cells and decreased Treg cell levels achieved better, deeper, and more ideal remission in their clinical status. However, the unsatisfactory immune status after dasatinib treatment showed that the clinical status was not alleviated after treatment. Therefore, patients may be insensitive to dasatinib treatment or dasatinib treatment is ineffective. At present, the combination of immunomodulatory drugs with traditional anti-tumor regimens is a promising strategy for treating malignant tumors.26 However, currently, research and application of this strategy are still developing. The current study showed that dasatinib had an immunomodulatory effect on patients with CML. Therefore, dasatinib is a promising immunomodulatory drug for combination with current anti-tumor drugs for enhancement of anti-tumor efficacy.
Conclusion
Our clinical observations indicate that Th1, CD8+T, and Treg cell levels may be potential immune markers for evaluating the therapeutic response to dasatinib and predicting clinical remission. The immune markers Th1, CD8+T, and Treg are easily detected (by flow cytometry) and highly practical in the clinic with a low cost of evaluation, and thus may be potential candidates for clinical monitoring. Changes in Th1, CD8+T, and Treg cell levels not only partly help to evaluate anti-tumor immunity of patients with CML, but also may be useful for predicting these patients’ prognosis and adjusting clinical drug regimens.
Acknowledgements
We thank Beijing Hightrust Diagnostics Co., Ltd. (Beijing) for consultation of the technique of flow cytometry.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the International Cooperation and Exchange Program of Science and Technology Department of Sichuan Province (No. 2018HH0114), Sichuan Academy of Medical Sciences Sichuan Provincial People’s Hospital Youth Talent Fund (No. 2017QN03), and the University of Electronic Science and Technology of China Central University Basic Research Expenses Project (No. 2672018ZYGX2018J101).
References
- 1.Shtivelman E, Lifshitz B, Gale RP, et al. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 1985; 315: 550–554. [DOI] [PubMed] [Google Scholar]
- 2.Melo JV. The molecular biology of chronic myeloid leukaemia. Cancer Sci 1996; 103: 751–756. [PubMed] [Google Scholar]
- 3.Iriyama N, Tokuhira M, Takaku T, et al. Incidences and outcomes of therapy-related chronic myeloid leukemia in the era of tyrosine kinase inhibitors: surveillance of the CML Cooperative study group. Leuk Res 2017; 54: 55–58. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, O'Brien S, Jabbour E, et al. Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. J Clin Oncol 2011; 29: 3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cojbasic I, Macukanovic-Golubovic L. Prognostic factors associated with complete cytogenetic response in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Srp Arh Celok Lek 2010; 138: 305–308. [DOI] [PubMed] [Google Scholar]
- 6.Berman E, Jhanwar S, Hedvat C, et al. Resistance to imatinib in patients with chronic myelogenous leukemia and the splice variant BCR-ABL1 35INS. Leuk Res 2016; 49: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azad NA, Shah ZA, Pandith AA, et al. Analysis of ABL kinase domain mutations as a probable cause of imatinib resistance in chronic myeloid leukemia patients of Kashmir. Meta Gene 2018; 17: 93–98. [Google Scholar]
- 8.Okada M, Imagawa J, Tanaka H, et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk 2018; 18: 353–360. [DOI] [PubMed] [Google Scholar]
- 9.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006; 354: 2531–2541. [DOI] [PubMed] [Google Scholar]
- 10.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of dasision: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol 2016; 34: 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelison AM, Kantarjian H, Cortes J, et al. Outcome of treatment of chronic myeloid leukemia with second-generation tyrosine kinase inhibitors after imatinib failure. Clin Lymphoma Myeloma Leuk 2011; 11: S101–S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchiyama T, Sato N, Narita M, et al. Direct effect of dasatinib on proliferation and cytotoxicity of natural killer cells in in vitro study. Hematol Oncol 2013; 31: 156–163. [DOI] [PubMed] [Google Scholar]
- 13.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009; 23: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 14.Najima Y, Yoshida C, Iriyama N, et al. Regulatory T cell inhibition by dasatinib is associated with natural killer cell differentiation and a favorable molecular response—The final results of the D-first study. Leuk Res 2018; 66: 66–72. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Kamel-Reid S, Chang H, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica 2009; 94: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasic I, Lipton JH. Current approach to the treatment of chronic myeloid leukaemia. Leuk Res 2017; 55: 65–78. [DOI] [PubMed] [Google Scholar]
- 17.Elmaagacli AH, Beelen DW, Opalka B, et al. The amount of BCR-ABL fusion transcripts detected by the real-time quantitative polymerase chain reaction method in patients with Philadelphia chromosome positive chronic myeloid leukemia correlates with the disease stage. Ann Hematol 2000; 79: 424–431. [DOI] [PubMed] [Google Scholar]
- 18.Jianxiang W. The guidelines for diagnosis and treatment of chronic myelogenous leukemia in China (2016 edition) . Chin J Hematol 2016; 37: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radich JP, Deininger M, Abboud CN, et al. Chronic myeloid leukemia, version 1.2019 clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018; 16: 1108–1135. [DOI] [PubMed] [Google Scholar]
- 20.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Canc Immunol Immunother 2005; 54: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 2015; 135: 626–635. [DOI] [PubMed] [Google Scholar]
- 22.Zahran AM, Badrawy H, Ibrahim A. Prognostic value of regulatory T cells in newly diagnosed chronic myeloid leukemia patients. Int J Clin Oncol 2014; 19: 753–760. [DOI] [PubMed] [Google Scholar]
- 23.Ha TY. The role of regulatory T cells in cancer. Immune Network 2009; 9: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustjoki S, Auvinen K, Kreutzman A, et al. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia 2013; 27: 914–924. [DOI] [PubMed] [Google Scholar]
- 25.Kreutzman A, Juvonen V, Kairisto V, et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010; 116: 772–782. [DOI] [PubMed] [Google Scholar]
- 26.Frazier JL, Han JE, Lim M, et al. Immunotherapy combined with chemotherapy in the treatment of tumors. Neurosurg Clin N Am 2010; 21: 187–194. [DOI] [PubMed] [Google Scholar]