Abstract
Loeys-Dietz syndrome (LDS) is a connective tissue disorder with associated systemic vasculopathies including intracranial arterial aneurysm formation and dissections. LDS is a relatively less well-known entity compared with other connective tissue disorders, such as Ehlers-Danlos or Marfan syndrome, and consequently experience in the management of the associated intracranial aneurysms is suboptimal. We present a case of surgical clipping of a middle cerebral artery aneurysm in a patient with LDS. A 46-year-old female with LDS (type III) was found to have a right middle cerebral artery (MCA) bifurcation aneurysm following vascular screening. The decision was made to surgically clip the aneurysm after consultation in our neurovascular multidisciplinary team meeting. A standard right pterional craniotomy was performed and the aneurysm was secured with 2 straight Sugita clips. The temporal M2 branch was noted to be thin walled and this prompted application of the second tandem clip, rather than risk re-positioning the initial clip. In our case, the MCA aneurysm neck was robust enough to take a clip without any complications, and therefore we suggest that the presence of LDS is not an absolute contra-indication to perform open craniotomy and clipping.
Keywords: Loeys-Dietz syndrome, Cerebral aneurysm, Endovascular management, Surgical clipping, Connective tissue disorder
Background
Loeys-Dietz syndrome (LDS) is an autosomal dominant connective tissue disorder resulting in craniofacial malformations and vascular pathologies. The diagnosis is confirmed with genetic molecular testing [1,3,4] and there are 4 main subtypes. Transforming growth factor β receptor I and II (TGFBR1 and TGFBR2) are the chromosomal mutations found in Type I and II LDS. Type III LDS patients have mutations in the mothers against decapentaplegic homolog 3 (SMAD3) gene and mutations in transforming growth factor β 2 ligand gene are present in Type IV LDS [1]. LDS patients’ exhibit defects such as cleft palate, uvula abnormalities, hypertelorism, cardiac valve problems and septal defects, cardiac arrhythmias, pathologic fractures, reduced bone density, osteoarthritis, cartilage degeneration, and type I Chiari malformations [1,2]. The literature describes many other clinical presentations including easy bruising, retinal detachment, cataracts, restrictive lung disease, obstructive sleep apnea and allergic complications such as asthma, food allergy and eczema [1]. The reduced life expectancy of LDS patients is primarily due to cardiovascular manifestations [8], eg, aortic dissection.
Patients with LDS are also known to have extensive intracranial arterial tortuosity and a high risk of developing aneurysms and dissections. Once an intracranial aneurysm is diagnosed and definitive treatment is deemed appropriate it may be successfully treated either by endovascular coiling and/or stenting or clipping via craniotomy [5], [6], [7], [8] to prevent the high case fatality and significant morbidity associated with rupture.
Patient
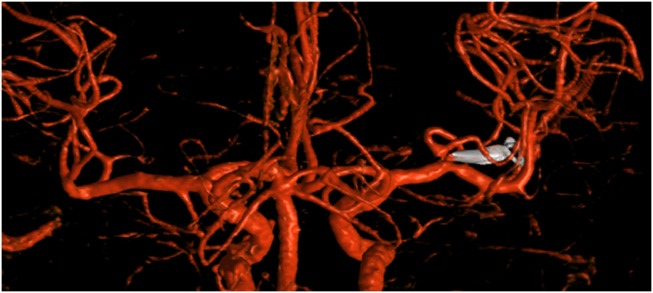
A 46-year-old female was confirmed to have type III LDS following genetic testing. Subsequent vascular screening identified multiple aneurysms: aortic root, splenic, right middle cerebral (MCA) and left posterior cerebral artery. She was subsequently referred to our neurovascular multidisciplinary team meeting for further management. The risk of intra-arterial dissection was deemed significant and because of this an intravenous DSA (as opposed to the usual intra-arterial angiogram) was performed in the first instance. The vascular imaging defined the morphology of the broad-necked 5 mm MCA and the relationship to the M1, the MCA bifurcation and the M2’s. The iv DSA also confirmed abnormal aneurysmal morphology and in the setting of LDS it was deemed appropriate to offer treatment. The management options of an endovascular procedure (Pipeline stent with dual antiplatelet therapy) or open surgical clip ligation were discussed. In our practice, the usual treatment option for a broad-necked MCA aneurysm would be surgery but in the setting of a connective tissue disorder there was consideration of whether the neck of the aneurysm would be sufficiently robust to take the clip. Despite our reservations, open surgery was considered the safest approach.
Treatment
A standard right pterional craniotomy was performed following informed consent. The M2’s, M1, MCA bifurcation, and broad-based aneurysm were identified. Dissection of the aneurysm was routine. An approximate 8-mm section of the temporal M2 branch was noted to be extremely thin-walled, extending to the MCA bifurcation and neck of the aneurysm. The neck was secured with a straight Sugita clip; however, on inspection, we could not be sure that the clip had been adequately placed across the entire aneurysm neck. The decision was made to place a second clip across the neck, just distal to the first clip, to avoid repositioning the first clip. There were no operative complications and the patient recovered very well from surgery with no neurologic deficits. The postoperative angiogram confirmed complete obliteration of the aneurysm and we will continue interval surveillance imaging of her remaining cerebral vasculature, including the small left P2 aneurysm.
Discussion
The difficulties of managing intracranial aneurysms in patients with underlying connective tissue disorders mandate a multidisciplinary approach with involvement of both interventional neuroradiology and neurosurgical teams [5], [6], [7], [8]. In LDS patients, endovascular approaches have previously been used for treatment of ICA, basilar, ophthalmic, and anterior communicating artery aneurysms [7,8]. There are 3 papers in the literature that refer to craniotomy and clipping. The surgical approach was chosen in each case given concerns regarding potential endovascular complications, such as arterial dissection and thrombo-embolic sequelae. Hughes et al. reported severe vascular reactivity of the parent vessel during intraoperative dissection and papaverine was used to treat the vasospasm [6]. Such was the reluctance to use temporary clipping with the inherent risks to the ICA vessel wall integrity, temporary chemical circulatory arrest was instigated prior to clipping. Rahme et al. also utilized temporary flow arrest for a similar ophthalmic aneurysm, though in contradistinction, Carr et al. used brief temporary clipping of a PICA without causing significant vessel wall injury in an LDS patient [5,9].
The dangers of operating on aneurysms in patients harboring the more common connective tissue disorders may also influence decisions to treat, operative risks, and technical nuances. A review of 4 Ehlers Danlos syndrome cases that were surgically treated highlighted a patient who died as a result of surgery and this was directly attributable to blood vessel fragility [10]. Additionally, histologic confirmation of focal damage and weakening of the internal elastic lamina of the aneurysm wall has been reported in Marfan syndrome, suggesting that clipping an aneurysm in this setting may also be associated with elevated risks [11].
During our case, we also had the mindset to avoid temporary clipping and fortunately this was not required. Furthermore, the standard dissection of the aneurysm and parent vessels did not result in vasospasm. The aneurysm itself outwardly appeared similar to a standard broad-necked saccular aneurysm but the temporal M2 vessel was notably different, appearing extremely thin-walled, and this abnormality extended to the bifurcation. The first clip was placed across the neck but the reluctance to gently mobilize the aneurysm resulted in an inability to define whether the clip had obliterated the entire aneurysm. Normally, this situation would have prompted repositioning of the clip, but the underlying vascular fragility led to concerns that the junction of neck and parent vessel was less robust and therefore prone to damage. The prospect of a vessel tear at the base of the unruptured MCA aneurysm was such that we favored placement of a second clip just distal to the first. The postoperative iv DSA confirmed the aneurysm was secured (Figs. 1 and 2).
Fig. 1.
Digital subtraction angiography: posterior view of 5 mm right MCA bifurcation aneurysm.
Fig. 2.
Digital subtraction angiography: post clipping.
Conclusion
To our knowledge, this is the first reported surgical clipping of a middle cerebral artery aneurysm in the setting of LDS. LDS is a less well-known connective tissue disorder but is also associated with intracranial aneurysm formation. The management of the intracranial disease requires an understanding of the published literature and close discussion between the interventional neuroradiologists and the cerebrovascular neurosurgeons. Endovascular management of the intracranial aneurysms in LDS is well reported [7,8] and we add our surgical case to the growing literature of LDS patients treated with open craniotomy. Although clipping is a viable option the increased risks associated with temporary clipping and vessel fragility have to be considered with aneurysms in the clinical setting of a connective tissue disorder.
Patient consent
Patient consent for publication of this case was obtained.
References
- 1.MacCarrick G., Black J.H., Bowdin S., El-Hamamsy I., Frischmeyer-Guerrerio P.A., Guerrerio A.L. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16(8):576–587. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loughborough W.W., Minhas K.S., Rodrigues J.C.L., Lyen S.M., Burt H.E., Manghat N.E. Cardiovascular manifestations and complications of Loeys-Dietz syndrome: CT and MR imaging findings. Radiographics. 2018;38(1):275–286. doi: 10.1148/rg.2018170120. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.T., Brinjikji W., Kallmes D.F. Prevalence of intracranial aneurysms in patients with connective tissue diseases: a retrospective study. AJNR Am J Neuroradiol. 2016;37(8):1422–1426. doi: 10.3174/ajnr.A4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeys B.L., Schwarze U., Holm T., Callewaert B.L., Thomas G.H., Pannu H. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355(8):788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 5.Carr SB, Imbarrato G, Breeze RE, Wilkinson CC. Clip ligation for ruptured intracranial aneurysm in a child with Loeys-Dietz syndrome: case report. Journal of neurosurgery. Pediatrics, 2018;21(4):375. doi: 10.3171/2017.10.peds17193 PMID: 29350591. [DOI] [PubMed]
- 6.Hughes B.D., Powers C.J., Zomorodi A.R. Clipping of a cerebral aneurysm in a patient with Loeys-Dietz syndrome: case report. Neurosurgery. 2011;69(3):E746–E755. doi: 10.1227/NEU.0b013e31821964a3. discussion E55. [DOI] [PubMed] [Google Scholar]
- 7.Colby G.P., Lin L.M., Zeiler S.R., Coon A.L. Curative reconstruction of a cerebral aneurysm by flow diversion with the pipeline embolization device in a patient with Loeys-Dietz syndrome. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204412. [DOI] [PMC free article] [PubMed] [Google Scholar]; Published online: [16 Oct 2014] doi:10.1136/bcr-2014-204412.
- 8.Levitt M.R., Morton R.P., Mai J.C., Ghodke B., Hallam D.K. Endovascular treatment of intracranial aneurysms in Loeys-Dietz syndrome. J Neurointerv Surg. 2012;4(6):e37. doi: 10.1136/neurintsurg-2011-010138. [DOI] [PubMed] [Google Scholar]
- 9.Rahme R.J., Adel J.G., Bendok B.R., Bebawy J.F., Gupta D.K., Batjer H.H. Association of intracranial aneurysm and Loeys-Dietz syndrome: case illustration, management, and literature review. Neurosurgery. 2011;69(2):E488–EE93. doi: 10.1227/NEU.0b013e318218cf55. [DOI] [PubMed] [Google Scholar]
- 10.Schievink W.I., Link M.J., Piepgras D.G., Spetzler R.F. Intracranial aneurysm surgery in Ehlers-Danlos syndrome type IV. Neurosurgery. 2002;51(3):607–611. discussion 11-3. [PubMed] [Google Scholar]
- 11.Kubo Y., Ogasawara K., Kurose A., Kakino S., Tomitsuka N., Ogawa A. Ruptured cerebral fusiform aneurysm with mucopolysaccharide deposits in the tunica media in a patient with Marfan syndrome. J Neurosurg. 2009;110(3):518–520. doi: 10.3171/2007.12.17654. [DOI] [PubMed] [Google Scholar]