Abstract
Objective
To study the therapeutic effect and mechanism of action of quercetin in a rat model of osteoarthritis (OA).
Methods
The OA rat model was established by intra-articular injection of papain. Changes in knee diameter, toe volume and histopathology were measured. Levels of interleukin (IL)-β and tumor necrosis factor (TNF)-α were assessed by ELISA. Relative expression of Toll-like receptor (TLR)-4 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) was evaluated by western blotting.
Results
Compared with rats treated with papain alone, changes in knee diameter, toe volume and Makin' s score were less apparent in OA rats treated with quercetin. Levels of serum IL-1β and TNF-α were also reduced in quercetin-treated OA rats. Expression of TLR-4 and NF-κB was significantly suppressed in a dose-dependent manner in quercetin-treated OA rats.
Conclusion
Quercetin exhibited a therapeutic effect in OA rats, which may be related to inhibition of IL-1β and TNF-α production via the TLR-4/NF-κB pathway.
Keywords: Osteoarthritis, quercetin, inflammation, TLR-4/NF-κB, IL-1β, TNF-α
Introduction
Osteoarthritis (OA) is caused by urate crystal deposition in articular capsules, synovial membranes, cartilage, bone and joint tissue. Eventually, lesions and inflammatory reactions develop at the joint surface and in synovial and surrounding tissues.1 Currently available therapies such as non-steroidal anti-inflammatory drugs have serious side effects including gastrointestinal toxicity, nephrotoxicity, and gastrointestinal bleeding.2 Therefore, development of effective and non-toxic OA drugs derived from natural products is an urgent goal. Quercetins, polyphenols present in a variety of fruits and vegetables such as apples, onions and broccoli, have anti-inflammatory, antioxidant and pro-apoptotic activities which can be beneficial for human health.3 Previous studies demonstrated that quercetin had anti-inflammatory effects on chronic adjuvant arthritis in rats, and could significantly reduce the levels of interleukin (IL)-1β and tumor necrosis factor (TNF)-α produced by peritoneal macrophages.4 Toll-like receptor (TLR)-4/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling is a critical pathway in the development of inflammation.5–7
However, the efficacy and mechanism of action of quercetin in OA remain unclear. In the present study, we developed a rat model of OA by intra-articular injection of papain. We evaluated the efficacy of quercetin in OA by assessing knee diameter, toe volume, histopathology of bone joints, and Makin's scores. Last, we measured relative TLR-4 and NF-κB protein expression by western blotting to understand quercetin’s mechanism of action in OA.
Materials and methods
Materials and reagents
Quercetin was purchased from Nanjing Jing Bamboo Biological Science and Technology Co., Ltd. (Nanjing, China). Aspirin was purchased from Hunan Xinhui Pharmaceutical, Ltd. (Changsha, China). A 4% papain solution was purchased from Shanghai Blue Season Biological Co., Ltd. (Shanghai, China). An ELISA kit was purchased from Tianjin Annuo Ruikang Co., Ltd. (Tianjing, China). Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH), anti-TLR-4 and anti-NF-κB antibodies were purchased from Abcam (Cambridge, MA, USA).
Experimental animals
Specific pathogen-free Sprague-Dawley rats with body weights of 200 to 250 g were purchased from Nanjing Medical University. Follow-up experiments were carried out 1 week after adaptive feeding. The study protocol was approved by the animal ethics committee of Kunming Medical University.
OA model and treatment groups
Rats were randomly assigned to one of five groups: (i) the “Normal Control” (NC) group, (ii) the “Model” group injected with saline, (iii) the “Low-Dose” group injected daily with 1 mg/kg quercetin, (iv) the “Middle-Dose” group injected daily with 5 mg/kg quercetin, and (v) the “High-Dose” group injected daily with 10 mg/kg quercetin. Rat in the NC group received no further treatment, while rats in the Model, Low-Dose, Middle-Dose and High-Dose groups were injected with 0.3 mL of 4% papain in the knee joint cavity 1, 4 and 7 days post-quercetin treatment. After 2 weeks, NC and Model group rats were gavaged with normal saline. High-Dose, Middle-Dose and Low-Dose group rats were gavaged with relative quercetin for 14 days.
Joint diameter and toe volume measurements
Prior to and 2 weeks following papain treatment, joint diameters and toe volumes were measured. After gavaging with querceptin, relative data were collected every 4 days.
ELISA
After administration of querceptin for 14 days, the rats were anesthetized by injection of 10% chloral hydrate (0.33 mL/100 g) then sacrificed by decapitation. Blood samples were collected from the secondary aorta, centrifuged for 30 minutes at 8000 RPM, and the supernatants were stored at −20°C. Levels of IL-1β and TNF-α were measured using ELISA kits.
Histopathology
Rat joints were collected and fixed in 10% formalin solution for 24 hours. The tissues were dehydrated, embedded, sliced, dewaxed and rehydrated. After hematoxylin and eosin (H&E) staining, dehydration and mounting, pathological changes were observed using an optical microscope.
Western blotting
Joint tissues were collected and total protein was extracted using 1% radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein concentrations were measured using a bicinchoninic acid assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Fifty micrograms of each sample were prepared on ice, then separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking with 5% (w/v) skim milk for 2 hours and washing with Tris-buffered saline containing 0.1% Tween-20, anti-GAPDH (diluted 1:1000, ab181602), anti-TLR-4 (diluted 1:500, ab8378) and anti-NF-κB (p65) (diluted 1:500, ab16502) antibodies were added and incubated overnight at 4°C. The membranes were washed again, then incubated with secondary antibody for 1 hour. After a final wash, blots were developed using chemiluminescence in a dark room and imaged using a gel imaging system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Results were presented as means ± standard deviations. Differences between groups were assessed using one-way analysis of variance with Fisher’s least significant difference post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Changes in joint diameter
Rats (n = 45) were randomly assigned to one of five treatment groups. After drug administration for 8 days, rat joint diameters in the Middle- and High-Dose groups were significantly smaller compared with the Model group (P = 0.043 and P < 0.01, respectively). After administering drug for 12 days, rat joint diameters in the Low-, Middle- and High-Dose groups were significantly smaller compared with the Model group (P = 0.023 and P = 0.006, respectively). There were significant differences among the Low-, Middle- and High-Dose groups (P < 0.05). The relative data are shown in Table 1. These results showed that low, middle and high doses of quercetin could improve OA symptoms in a dose-dependent manner.
Table 1.
Changes in knee diameters of osteoarthritis rats (means ± standard deviations, n = 8) expressed in D/mm.
| Group | Dose (mg/kg) | Pre-papain injection | Post-papain injection |
Quercetin administration |
||
|---|---|---|---|---|---|---|
| 4 days | 8 days | 12 days | ||||
| NC | – | 6.59 ± 0.30 | 6.61 ± 0.35 | 6.63 ± 0.08 | 6.65 ± 0.08 | 6.64 ± 0.08 |
| Model | – | 6.33 ± 0.27 | 7.39 ± 0.33** | 7.41 ± 0.09** | 7.41 ± 0.06** | 7.46 ± 0.10** |
| Quercetin | ||||||
| Low-Dose | 10 | 6.41 ± 0.29 | 7.39 ± 0.20** | 7.38 ± 0.08 | 7.27 ± 0.04 | 7.10 ± 0.07# |
| Middle-Dose | 20 | 6.49 ± 0.35 | 7.36 ± 0.28** | 7.31 ± 0.07##,* | 7.21 ± 0.07##,* | 6.89 ± 0.06##,* |
| High-Dose | 50 | 6.67 ± 0.29 | 7.30 ± 0.25** | 7.26 ± 0.08###,**,$ | 7.00 ± 0.058###,**,$ | 6.82 ± 0.078###,**,$ |
**: P < 0.01 compared with NC group.
#: P < 0.05; ##: P < 0.01; ###: P < 0.001 compared with Model group.
*: P < 0.05; **: P < 0.01 compared with Low-Dose group.
$: P < 0.05 compared with Middle-Dose group.
Changes in toe volume
Compared with the Model group, toe volumes in the Middle- and High-Dose groups were significantly lower after 4 days of drug administration (P < 0.05, respectively). However, toe volumes in Low-Dose group rats were not significantly different from those of Model group rats. The toe volumes of the Low-, Middle- and High-Dose groups were significantly lower compared with the Model group after 8 and 12 days of drug administration (P < 0.05). The relative data are shown in Table 2. These results showed that low, middle and high doses of quercetin had anti-inflammatory effects after 8 days of treatment.
Table 2.
Changes in toe volumes of osteoarthritis rats (mean ± standard deviations, n = 8) expressed in ml.
| Group | Dose(mg/kg) | Pre-papain injection | Post-papain injection |
Quercetin administration |
||
|---|---|---|---|---|---|---|
| 4 days | 8 days | 12 days | ||||
| NC | – | 1.31 ± 0.07 | 1.29 ± 0.05 | 1.33 ± 0.07 | 1.29 ± 0.06 | 1.31 ± 0.06 |
| Model | – | 1.30 ± 0.07 | 1.61 ± 0.06** | 1.65 ± 0.03** | 1.65 ± 0.03** | 1.65 ± 0.04** |
| Quercetin | ||||||
| Low-Dose | 10 | 1.32 ± 0.06 | 1.62 ± 0.05** | 1.62 ± 0.02# | 1.58 ± 0.02# | 1.52 ± 0.02# |
| Middle-Dose | 20 | 1.31 ± 0.07 | 1.64 ± 0.05** | 1.55 ± 0.03##,* | 1.52 ± 0.01##,* | 1.47 ± 0.015##,* |
| High-Dose | 50 | 1.30 ± 0.06 | 1.55 ± 0.04** | 1.50 ± 0.03###,**,$ | 1.47 ± 0.01###,**,$ | 1.43 ± 0.01###,**,$ |
**: P < 0.01 compared with NC group.
#: P < 0.05; ##: P < 0.01; ###: P < 0.001 compared with Model group.
*: P < 0.05; **: P < 0.01 compared with Low-Dose group.
$: P < 0.05 compared with Middle-Dose group.
Quercetin affected serum IL-1β and TNF-α levels
Compared with the NC group, serum IL-1β and TNF-α concentrations in the Model group were significantly elevated (P = 0.032. However, serum IL-1β and TNF-α concentrations in the Low-, Middle- and High-Dose groups were significantly lower compared with those of the Model group (all P < 0.05). The relative data are shown in Figure 1.
Figure 1.
IL-1β and TNF-α concentrations in different treatment groups.
NC: rats were untreated; Model: rats were injected with 0.3 mL of 4% papain into the knee joint cavity on days 1, 4 and 7 to induce OA; Low-Dose: papain-treated rats were injected daily with 1 mg/kg quercetin; Middle-Dose: papain-treated rats were injected daily with 5 mg/kg quercetin; High-Dose: papain-treated rats were injected daily with 10 mg/kg quercetin.
#: P < 0.05 compared with NC group.
*: P < 0.05 compared with Model group.
**: P < 0.05 compared with Low-Dose group.
$: P < 0.05 compared with Middle-Dose group.
Histopathological assessment of quercetin treatment
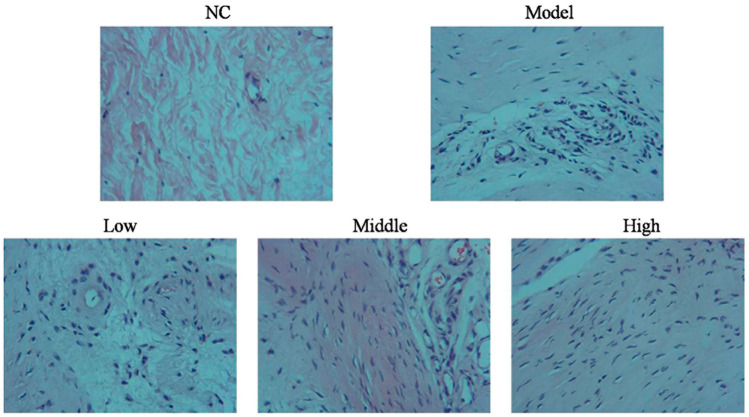
After H&E staining, the cartilage surface in rats of the NC group was smooth, without cracks or defects. The chondrocytes were neatly arranged, and four structural layers were clearly discernible. The tidal line was clear and intact, and the cartilage matrix was evenly stained pink. In rats of the Model group, the cartilage surface was not flat and showed defects. The cartilage cells were disordered and showed a radiation layer and a calcified layer with severe defects. Structures were unclear and showed evidence of tidal line destruction. The number of cartilage cells and deep cells were severely reduced, and a large number of empty cells appeared reflecting severe cartilage lacunae. The matrix was fractured and cells formed clusters, with some sections showing mounded edges and uplifted bone with long, uneven staining. In rats of the Low-Dose group, the cartilage surface was relatively smooth, although the cartilage cells were relatively disordered. Calcified layer defects, tidal line damage, and decreases in cartilage cell numbers were evident. Moreover, a large number of empty matrix cells were observed reflecting cartilage lacunae, and cracks and uneven staining were present. In rats of the Middle-Dose group, the cartilage surface was smooth, with relatively clear structures and a complete tidal line. A small number of empty cartilage lacunae and cell matrix cracks were observed, but staining was uniform. In rats of the High-Dose group, the cartilage surface was smooth and the chondrocytes were arranged relatively neatly. The structures of the four layers were clear, the tidal line was nearly complete and staining was uniform. These data are shown in Figure 2.
Figure 2.
Histopathology in different treatment groups observed by hematoxylin and eosin staining (200×)
NC: rats were untreated; Model: rats were injected with 0.3 mL of 4% papain into the knee joint cavity on days 1, 4 and 7 to induce osteoarthritis; Low-Dose: papain-treated rats were injected daily with 1 mg/kg quercetin; Middle-Dose: papain-treated rats were injected daily with 5 mg/kg quercetin; High-Dose: papain-treated rats were injected daily with 10 mg/kg quercetin.
Quercetin affected relative TLR-4 and NF-κB protein expression
Compared with the NC group, TLR-4 and NF-κB expression in the Model group was significantly enhanced (P = 0.025. However, TLR-4 and NF-κB expression in the quercetin treatment groups (Low-, Middle- and High-Dose) was significantly downregulated compared with the Model group (all P < 0.05). There were significant differences in expression among the Low-, Middle- and High-Dose groups (P < 0.05). The relative data are shown in Figure 3.
Figure 3.
Relative protein expression in different treatment groups.
NC: rats were untreated; Model: rats were injected with 0.3 mL of 4% papain into the knee joint cavity on days 1, 4 and 7 to induce osteoarthritis; Low-Dose: papain-treated rats were injected daily with 1 mg/kg quercetin; Middle-Dose: papain-treated rats were injected daily with 5 mg/kg quercetin; High-Dose: papain-treated rats were injected daily with 10 mg/kg quercetin.
#: P < 0.05 compared with NC group.
*: P < 0.05 compared with Model group.
**: P < 0.05 compared with Low-Dose group.
$: P < 0.05 compared with Middle-Dose group.
Discussion
Six methods have been used to develop rat models of OA.8 In our study, we used papain injection into the articular cavity to induce OA. This model is simple, convenient, reproducible and suitable for animal experiments. Joint diameters and toe volumes in rats of the Model group were significantly different compared with those of NC group rats. Thus, our model successfully recapitulated many of the features of OA. OA symptoms (joint diameters and toes volume) were improved upon quercetin treatment, while levels of inflammatory mediators (IL-1β, TNF-α) were diminished.
IL-1β contributes to tissue damage and is one of the most important inflammatory factors in the pathology of OA.9–11 IL-1β can inhibit synthesis of extracellular matrix and accelerate its degradation, resulting in chondrocyte apoptosis.12,13 This cytokine also stimulates the expression of matrix metalloproteinase family proteins,14,15 promotes cartilage degeneration, and inhibits its repair, thereby accelerating OA.16,17 TNF-α is mainly derived from macrophages in the peripheral blood and synovium, which are involved in the immune responses that mediate inflammatory responses.18 TNF-α is also an important cytokine in the pathogenesis of OA.19,20 TNF-α can activate many types of cells, stimulate high expression of metalloproteinases, promote production of prostaglandin E2 in synovial cells, and promote the destruction of bone and cartilage. The results of our experiments showed that all doses of quercetin tested could reduce the levels of IL-1β and TNF-α in OA rats to some degree. Thus, the mechanism of action of quercetin in the treatment of OA may involve regulation of IL-1β and TNF-α production.
TLR4 is a PRR and a major transmitter of inflammatory signals. TLR4 activity plays a key role in inflammation initiation and development.21–23 TLR4 activation triggers downstream NF-κB signaling, leading to NF-κB translocation from the cytoplasm to the nucleus, upregulation of IL-1β and TNF-α expression, and OA inflammation.24 Our results showed that the TLR-4/NF-κB pathway was stimulated in rats of the Model group. However, in quercetin-treated rats, TLR-4/NF-κB signaling was suppressed; this could provide one explanation for inhibition of IL-1β and TNF-α secretion in these animals.
In conclusion, quercetin improved OA via dose-dependent effects on the TLR-4/NF-κB pathway in vivo.
Declaration of conflicting interest
The authors have no conflicts of interest to declare.
Ethics and consent
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University.
Funding
This work received no specific funding from any funding agencies in the public, commercial, or not-for-profit sectors.
ORCID iD
Jun Zhang https://orcid.org/0000-0003-0285-6825
References
- 1.Gentle MJ. Sodium urate arthritis: effects on the sensory properties of articular afferents in the chicken. Pain 1997; 70: 245–251. [DOI] [PubMed] [Google Scholar]
- 2.Martin WJ, Herst PM, Chia EW, et al. Sesquiterpene dialdehydes inhibit MSU crystal-induced superoxide production by infiltrating neutrophils in an in vivo model of gouty inflammation. Free Radic Biol Med 2009; 47: 616–621. [DOI] [PubMed] [Google Scholar]
- 3.Kawabata K, Kawai Y, Terao J. Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J Nutr Biochem 2010; 21: 374–380. [DOI] [PubMed] [Google Scholar]
- 4.Mamani-Matsuda M, Kauss T, Al-Kharrat A, et al. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage in amatory mediators. Biochem Pharmacol 2006; 72: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Zhang RQ, Wei XG, et al. Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac J Trop Med 2016; 9: 503–507. [DOI] [PubMed] [Google Scholar]
- 6.Zhou CH, Wang CX, Xie GB, et al. Fisetin alleviates early brain injury following experimental subarachnoid hemorrhage in rats possibly by suppressing TLR 4/NF-κB signaling pathway. Brain Res 2015; 1629: 250–259. [DOI] [PubMed] [Google Scholar]
- 7.Luo Q, Yan X, Bobrovskaya L, et al. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol Cell Biochem 2017; 428: 129–137. [DOI] [PubMed] [Google Scholar]
- 8.Finn A, Angeby Möller K, Gustafsson C, et al. Influence of model and matrix on cytokine profile in rat and human. Rheumatology (Oxford) 2014; 53: 2297–2305. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Qiao J, Zhao X, et al. Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation 2015; 38: 2235–2241. [DOI] [PubMed] [Google Scholar]
- 10.Fan C, Zhao X, Guo X, et al. P2X4 promotes interleukin‑1β production in osteoarthritis via NLRP1. Mol Med Rep 2014; 9: 340–344. [DOI] [PubMed] [Google Scholar]
- 11.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014; 2014: 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 2015; 16: 26035–26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alqahtani MH, Mobasheri A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci 2015; 16: 20560–20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng GQ, Chen AB, Li W, et al. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res 2015; 14: 14811–14822. [DOI] [PubMed] [Google Scholar]
- 15.Chen WX, Shan FJ, Jin HT, et al. Research on application of determination of MMP-13 in osteoarthritis. Zhongguo Gu Shang 2014; 27: 617–620. [PubMed] [Google Scholar]
- 16.Zhang Q, Ji Q, Wang X, et al. SOX9 is a regulator of ADAMTSs-induced cartilage degeneration at the early stage of human osteoarthritis. Osteoarthritis Cartilage 2015; 23: 2259–2268. [DOI] [PubMed] [Google Scholar]
- 17.Guermazi A, Alizai H, Crema MD, et al. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 1639–1653. [DOI] [PubMed] [Google Scholar]
- 18.Laureys G, Gerlo S, Spooren A, et al. β2-adrenergic agonists modulate TNF-α induced astrocytic inflammatory gene expression and brain inflammatory cell populations. J Neuroinflammation 2014; 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li ZC, Han N, Li X, et al. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. Int J Clin Exp Pathol 2015; 8: 2555–2564. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YP, Liu B, Tian QY, et al. Progranulin protects against osteoarthritis through interacting with TNF-α and β-Catenin signalling. Ann Rheum Dis 2015; 74: 2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev 2015; 36: 245–271. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Yan S, Wang H, et al. IL-29 enhances LPS/TLR4-mediated inflammation in rheumatoid arthritis. Cell Physiol Biochem 2015; 37: 27–34. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh M, Subramani J, Rahman MM, et al. CD13 restricts TLR4 endocytic signal transduction in inflammation. J Immunol 2015; 194: 4466–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 2007; 317: 124–127. [DOI] [PubMed] [Google Scholar]