Abstract
Objective
To assess the frequency of coronavirus disease-2019 (COVID-19) and the effect of obstructive sleep apnea (OSA) management on COVID-19 among patients with confirmed OSA.
Design
Cross-sectional telephone interview survey.
Setting
Academic sleep labs.
Participants
Iranian adults ≥ 18 years old with confirmed OSA.
Results
Among 275 participants with OSA, 20% (n = 55) were suspected to have history of COVID-19 but had no positive test, and 18% (n = 51) were in the definite COVID-19 group according to their reported symptoms or confirmed positive test. Having severe OSA (apnea hypopnea index ≥ 30) was associated with an increased risk of definite COVID-19, with an odds ratio (OR) with 95% confidence interval (95% CI) of 2.31 (0.87-5.55) compared to having mild OSA in definite COVID-19 group. Those not undergoing treatment for OSA had an OR (95% CI) of 2.43 (1.26-4.67) for definite COVID-19 compared to those accepting treatment in definite COVID-19 group. Total sleep times (TSTs) were 354, 340, and 320 minutes in healthy, suspected, and COVID-19 groups, respectively; TST was associated with COVID-19 (P-value = .04). Similarly, sleep efficiency (SE) scores were 75.7, 74.2, and 67.9% for the healthy, suspected, and COVID-19 groups, respectively (P-value = .005); Beck Depression scores were 13.8, 13.0, and 17.7, respectively (P-value = .056).
Conclusions
OSA as a proinflammatory condition with multiple comorbidities may be a contributing factor to developing COVID-19. Greater OSA severity, no treatment for OSA, and lower TST and SE were associated with increased COVID-19 prevalence among patients with OSA.
Key Words: COVID-19, Management, Sleep apnea
Introduction
Since December 2019, the coronavirus disease-2019 (COVID-19) outbreak has been a global pandemic affecting more than 180 countries. Due to the increased prevalence of the disease in those with attenuated immune responses,1., 2., 3. immune system function is the most important reported factor in COVID-19 prognosis.
Obstructive sleep apnea (OSA) could reduce sleep quality with intermittent hypoxemia and sleep fragmentation through the night, which is an important factor influencing the immune system.4., 5., 6., 7. Obesity and male sex are risk factors for developing both COVID-198 , 9 and OSA.10 Comorbidities like cardiovascular disease, heart failure, hypertension, cerebrovascular diseases, and diabetes mellitus, which are common in individuals with OSA, are also important risk factors for severe COVID-19.11 , 12
Therefore, we hypothesize that the prevalence of COVID-19 may be different in patients suffering from OSA than in the general population. Furthermore, patients with more severe OSA may be at even greater risk for infection with COVID-19; when the right treatment for OSA is prescribed and followed, the prevalence of COVID-19 could be reduced in this population.
To the best of our knowledge, this is one of the first articles evaluating the importance of OSA management in the coronavirus infection. We designed this study to investigate the association between OSA severity and the development of COVID-19 and to explore whether the prevalence of COVID-19 differs among OSA patients.
Participants and methods
This was a cross-sectional study conducted at Baharloo and Imam Hospital sleep labs. The study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1399.225). Verbal consent was obtained from all study participants. Participants were patients at one of two clinics, diagnosed with OSA according to the International Classification of Sleep Disorders Third Edition (ICSD3) criteria after undergoing overnight polysomnography (PSG). Analysis and interpretation of PSGs have been done by sleep medicine specialists. The scoring criteria to define hypopneas were a minimum of 3% O2 desaturation from pre-event baseline or arousal after a drop of at least 30% in flow sensor for 10 seconds or more.
Approximately 400 patients over age 18 underwent diagnostic in-laboratory PSG at Baharloo sleep clinic and Imam sleep clinic between September 2019 and January 2020 and were found to have OSA as defined by an apnea hypopnea index (AHI) > 5 events/h and clinical symptoms. Telephone interviews were attempted by sleep technicians for 396 patients. Of this group, 98 patients could not be contacted and 23 refused to participate in the study. As a result, 275 participants (approximately 70% of eligible patients) were successfully contacted to obtain COVID-19 history from April 21, 2020 to May 5, 2020. No deaths were reported by the contacted patients or their family members; however, we are not certain that the nonrespondents were alive.
Participants completed a brief questionnaire regarding symptoms of COVID-19 and described their history of infection confirmed by polimerase chain reaction (PCR) and/or computerized tomography (CT) scan, leading to at-home quarantine or hospital admission. The questionnaire asked about sudden onset of cough, shortness of breath, fever, chills, sweating, headache, fatigue, myalgia, conjunctivitis, sore throat, rhinitis, anorexia, nausea, diarrhea, sleepiness, and altered taste and smell in the timeframe from January 2020 to the time of telephone interview. Our sleep technicians described the PCR and CT tests so that participants could report for which test they received a positive result. Participants with dyspnea and/or self-reported COVID-19-positive PCR or CT tests were defined as COVID-19-positive patients.
Participants with only fever, cough, myalgia, and sweating were classified as suspected of COVID-19. Participants who did not report any of the aforementioned symptoms were classified as healthy. Then, the three groups—COVID-19-positive, suspected COVID-19, and healthy—were compared in further data analysis.
OSA treatment was assessed as part of the COVID-19 telephone interviews. OSA treatment was defined as adherence to recommended therapy, including positive airway therapy, upper airway surgery, and oral appliance, with or without alternative therapies (weight loss or position therapy). Self-reported adherence to positive airway pressure (PAP) was defined as a minimum use of 4 hours per night for at least 5 nights per week in the previous month.
Other patient characteristics, including baseline Insomnia Severity Index (ISI) score, Epworth Sleepiness Scale (ESS) score, Beck Depression Inventory II (BDI-II), and self-reported underlying diseases (hypertension, stroke, diabetes, thyroid disease, lung disease, depression, heart disease), were recorded from available medical records of patients at sleep labs. Validated questionnaires in the Persian language, including the ESS,13 ISI,14 and BDI-II15 were administered. The total scores of these questionnaires were used for further data analysis.
Statistical analysis
In the present study, the mean ± standard deviation or frequency and percent were used for continuous and categorical variables, respectively. Student's independent t test and one-way ANOVA analyses were used to compare two and more-than-two continuous variables, respectively. The differences in categorical variables were examined using a chi-square test. We assessed differences in COVID-19 prevalence in terms of apnea severity category and treatment using a multinomial logistic regression model adjusted for age, sex, body mass index (BMI), and pulmonary diseases; the healthy group was used as the reference group. We compared patients with and without treatment for OSA using a trend graph.
Results
The mean age of the 275 patients who completed the study was 49.0 ± 12.7 years, and the mean BMI was 30.4 ± 5.6 kg/m2. A total of 178 (65%) patients were male. Other patient characteristics including comorbidities are also summarized in Table 1 . Fifty-five participants (20%) were categorized in the suspected group, and 51 (18%) were in the COVID-19-positive group. Of those in the COVID-19-positive group, 4 (7%) participants reported admission to the hospital but not the intensive care unit, and the others reported outpatient treatment. In total sleep times (TST), SE, and pulmonary disease, P-values were less than 0.05. TSTs in healthy, suspected, and COVID-19 positive groups were about 354, 340, and 320 minutes, respectively. The trend was similar in the SEs. The percentages of those with pulmonary diseases in healthy, suspected, and COVID-19 positive groups were 8.9%, 9.1%, and 21.6%, respectively. There was also an increasing pattern in ISI score in these three groups.
Table 1.
Descriptive characteristics and risk factors for COVID-19 among participants
| Total (n = 275) | Healthy (n = 169) | Suspected COVID-19(n = 55) | COVID-19 + (n = 51) | P-value | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (year) | 49.0 (12.7) | 49.4 (12.6) | 48.6 (12.4) | 48.2 (13.7) | .796 |
| BMI (kg/m2) | 30.4 (5.6) | 30.0 (5.3) | 31.2 (6.1) | 31.0 (5.9) | .265 |
| AHI (per hour) | 48.7 (35.0) | 47.8 (34.9) | 46.9 (35.5) | 53.4 (25.2) | .561 |
| MinSpO2 (%) | 76.7 (10.8) | 77.5(9.9) | 76.4 (11.0) | 74.3 (12.8) | .187 |
| Mean SpO2 (%) | 90.3 (5.1) | 90.7 (4.0) | 88.9 (8.4) | 90.8 (3.4) | .061 |
| SpO2 < 90 (Time%) | 27.4 (30.4) | 26.5 (30.0) | 29.9 (31.8) | 29.0 (30.5) | .817 |
| TST (min) | 345.4 (82.2) | 354.1 (76.9) | 340.2 (91.1) | 320.4 (85.8) | .042 |
| SE | 74.0 (15.1) | 75.7 (13.9) | 74.2 (13.8) | 67.9 (18.5) | .005 |
| BDI-II | 14.3 (11.2) | 13.8 (10.8) | 13.0 (10.3) | 17.7 (13.1) | .056 |
| ISI | 13.0 (5.8) | 12.5 (5.9) | 13.1 (5.2) | 14.6 (5.9) | .085 |
| ESS | 9.8 (5.7) | 9.8 (5.8) | 10.5 (5.5) | 9.0 (5.3) | .409 |
| Frequency (percent) | Frequency (percent) | Frequency (percent) | Frequency (percent) | P-value | |
|---|---|---|---|---|---|
| Sex (men) | 178 (65.0) | 115 (68.5) | 35 (63.6) | 28 (54.9) | .201 |
| Age > 50 | 127 (46.2) | 77(45.6) | 25(45.5) | 25(49.0) | .910 |
| BMI > 35 | 46 (16.7) | 27(16.0) | 9(16.4) | 10(19.6) | .828 |
| Smoking (yes) | 74 (27.2) | 47 (28.0) | 10 (18.5) | 17 (34.0) | .195 |
| Hypertension (yes) | 79 (28.7) | 47 (27.8) | 19 (34.5) | 13 (25.5) | .538 |
| Cardiovascular diseases (yes) | 49 (17.9) | 28 (16.8) | 7(12.7) | 14 (27.5) | .116 |
| Stroke (yes) | 16 (5.8) | 12 (7.1) | 1 (1.8) | 3 (5.9) | .348 |
| Pulmonary diseases (yes) | 31 (11.3) | 15 (8.9) | 5 (9.1) | 11 (21.6) | .036 |
| Diabetes (yes) | 48 (17.5) | 31 (18.3) | 7 (12.7) | 10 (19.6) | .574 |
| Thyroid diseases | 34 (12.5) | 22 (13.2) | 8 (14.5) | 4 (8.0) | .547 |
| Depression | 72 (26.2) | 40 (23.7) | 13 (23.6) | 19 (37.3) | .137 |
AHI, apnea hypopnea index; BDI, Beck Depression Inventory; BMI, body mass index; ESS, Epworth Sleepiness Scale; ISI, Insomnia Severity Index; SD, standard deviation; SE, sleep efficiency; TST, total sleep times.
Frequencies of fever, chills, and sweating reported in the study population were 47 (17.1%), 42 (15.3%), and 27 (9.8%), respectively. Fifty-one patients (18.5%) had a cough, and 27 patients (9.8%) had shortness of breath. Other symptoms included headache (10.2%), fatigue (13.8%), myalgia (15.3%), conjunctivitis (34.9%), sore throat (12.4%), rhinitis (13.8%), anorexia (5.8%), nausea (4%), diarrhea (3.3%), sleepiness (6.9%), and altered taste and smell (6.5%).
As shown in Table 2 , the percentages of COVID-19-positive patients in mild (AHI 5-15 events/h), moderate (AHI 15-30 events/h), and severe OSA (AHI ≥ 30 events/h) groups were 14%, 16.7%, and 20.3%, respectively. In patients who did not receive any treatment, 20.2% were COVID-19 positive vs. 14.8% in those with treatment. The odds of definite COVID-19 were higher greater in participants with severe OSA after adjustment for age, sex, BMI, and pulmonary diseases compared to those with mild OSA, although the association was not statistically significant. Patients who had no treatment for OSA had higher odds (2.43) of being in the definite COVID-19 group than those receiving treatment. Most patients on treatment were using PAP therapy (80%).
Table 2.
Associations of COVID-19 with OSA severity and treatment
| Suspected COVID-19 | COVID-19+ | Suspected COVID-19 | COVID-19+ | ||
|---|---|---|---|---|---|
| Frequency (percent) | Frequency (percent) | Adjusted OR (95% CI)* | Adjusted OR (95% CI)* | ||
| AHI (5-15) | 43 | 7 (16.3) | 6 (14) | 1⁎⁎ | 1 ⁎⁎ |
| AHI (15-30) | 60 | 18 (30) | 10 (16.7) | 1.81 (0.67-4.93) | 1.04 (0.40-2.69) |
| AHI ≥30 | 172 | 30 (17.4) | 35 (20.3) | 1.57 (0.61-4.95) | 2.31 (0.87-5.55) |
| Treatment for apnea | |||||
| Yes | 81 | 26 (32.1) | 12 (14.8) | 1⁎⁎⁎ | 1 ⁎⁎⁎ |
| No | 188 | 28 (14.9) | 38 (20.2) | 0.83 (0.39-1.75) | 2.43 (1.26-4.67) |
OR, odds ratio; AHI, apnea hypopnea index.
Adjusted odds ratio for age, sex, BMI, and pulmonary diseases.
In the multinominal logistic regression, healthy group (without COVID-19) and patients with AHI 5-15 were considered as reference groups.
In the multinominal logistic regression healthy group (without COVID-19) and patients with treatment are considered as reference groups.
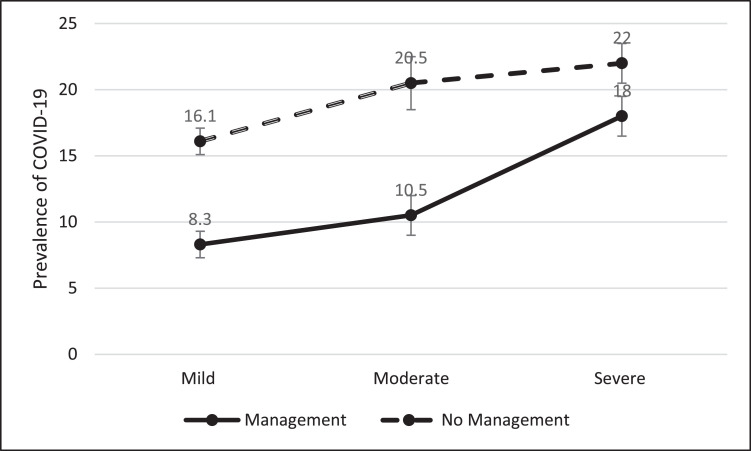
Fig. 1 presents the prevalence of COVID-19 in patients with mild, moderate, and severe OSA in terms of apnea management. The prevalence of COVID-19 was lower in patients in the management group than in patients in the no management group for all categories of OSA severity.
Fig. 1.
Comparison of COVID-19 prevalence among OSA patients according to apnea management.
Discussion
Most of the patients in our study were middle-aged and had high BMIs. The prevalence of COVID-19 was about 18%. We observed that in patients with OSA, COVID-19 was associated with lower TST and SE values. Furthermore, the group reporting the highest BDI-II scores was the COVID-19-positive group. Patients with histories of pulmonary disease were more prone to COVID-19; however, the other underlying diseases did not have statistical associations with the prevalence of COVID-19.
In the present study, men with OSA had a lower chance of reporting COVID-19. The prevalence of COVID-19 was higher in the group with severe OSA at baseline, and the patients who reported treatment for OSA had lower COVID-19 than those not receiving treatment. Although OSA severity was associated with higher prevalence of COVID-19, participants using OSA treatment had a lower prevalence of COVID-19 than those in the no-treatment group.
Because being obese or overweight with underlying diseases is common among our OSA patients, we expected high prevalence of COVID-19 in this population. Based on a meta-analysis, hypertension (21.1%), followed by diabetes (9.7%), cardiovascular disease (8.4%), and respiratory disease (1.5%), were comorbid conditions of COVID-19.16 In the present study, the prevalence of chronic comorbidities with OSA was high; however, perhaps because they were undergoing treatment, the reported prevalence was not as high as we expected. The comorbidities we studied are considered proinflammatory conditions that attenuate patients’ immunity (e.g., against COVID-19).17 However, one of the most important diseases involving multiple organs in this meta-analysis and other investigations is OSA as a separate risk factor for COVID-19 susceptibility. Risk factors for OSA are approximately shared with COVID-19, providing a vulnerable inflammatory state in patients for developing COVID-19. In the sleep heart health cohort, hypertension was the most frequent comorbidity of COVID-19, at approximately 59%, 62%, and 67% in mild, moderate, and severe OSA, respectively. Patients with OSA also are at increased risk of other comorbidities such as stroke,18 daytime hypertension, obesity, impaired glucose metabolism, and diabetes.19 , 20
Furthermore, current research showed that obesity and female sex put people with OSA at higher risk of COVID-19, although the association was not statistically significant. Almost all of our participants were obese or overweight. Consistent with other studies, we observed that obese patients have a higher risk of developing COVID-19. On the other hand, we know that obesity is a known risk factor for OSA.10 Years of research on OSA indicates middle age and obesity as risk factors of OSA. We hypothesized a higher prevalence of COVID-19 in this population because of shared known risk factors. We expected male sex to be a risk factor for COVID-19, but men in our study reported a lower prevalence of COVID-19. This may be due to fewer reported symptoms by men during the interview or the possible influence of OSA on sex susceptibility to COVID-19. These findings warrant the assessment of OSA or consideration of OSA treatment to prevent developing COVID-19 or its mortality. However, more investigation is required among different populations.
In the adjusted regression model of risk factors for COVID-19 (age, sex, obesity, pulmonary diseases, OSA severity, and treatment for OSA), severe and moderate OSA were associated with higher clinical odds for developing COVID-19, although the association was not statistically significant, possibly due to low statistical power and small sample size. Intriguingly, the group of OSA patients with no treatment had a significantly higher chance of contracting COVID-19, an important issue that warrants further investigation among the population of OSA patients.
OSA is associated with intermittent nocturnal hypoxemia, sleep fragmentation with possible lower mean oxygen saturation levels at night. OSA is a low-grade chronic inflammatory disease, associated with higher circulating levels of inflammatory markers including such as C-reactive protein, interleukin-6, interleukin-8, tumor necrosis factor alpha, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1, independent of obesity.21., 22., 23. This is a condition that may increase the susceptibility of patients with OSA to COVID-19. Good compliance to CPAP therapy has been associated with the reduction of immune response factors in OSA patients.21
We saw a lower prevalence of COVID-19 in OSA patients who underwent OSA treatment, highlighting the role of OSA management in tackling COVID-19 and the need to attend to sleep disorders in management and prevention strategies and investigations of the novel coronavirus. Besides attenuated immune function, higher odds of developing COVID-19 in the no-treatment group may be related to socioeconomic factors and cultural issues. This portion of participants may have experienced more socioeconomic and financial problems in the lockdown period, limiting their access to health care services for the management of OSA or other comorbid conditions (e.g., PAP device or oral appliance). Thus, lower socioeconomic status can lead to poorer health outcomes in the no-treatment group and conflate the treatment for OSA and risk of COVID-19. Thus, adjustment for the socioeconomic status of OSA patients is recommended in future studies of COVID-19 in this population.
The other factor that may make OSA patients more susceptible to COVID-19 is sleep fragmentation due to repeated respiratory pauses or cortical arousals. We observed that among PSG-recorded parameters, lower baseline TST and SE values have significant associations with COVID-19. Furthermore, those with higher insomnia scores on the ISI scale were more likely to report COVID-19. This issue can be discussed regarding two points of view. First, we know that disrupted sleep may be associated with disrupted immune response.4 Therefore, reported insomnia and sleep fragmentation in OSA, especially in the case of no treatment, can pose more risks to patients with COVID-19, as ample evidence supports the role of healthy sleep in boosting the immune system.4 , 24., 25., 26., 27. Second, another hypothesis involves the main pathogenic mechanism of OSA and its subsequent hypoxemia. Patients who had more severe OSA and those who did not report treatment for their apnea had higher prevalences of COVID-19. Furthermore, those with comorbid pulmonary disease also had a greater prevalence of coronavirus infection, supporting a potential role of hypoxia as a pathway contributing to COVID-19 risk. Putting these together and reviewing the published facts about the COVID-19, we think that OSA can increase risk of COVID-19, a finding that needs additional verification and further investigation. Interesting and useful research in this field would explore the role of management of OSA in decreasing COVID-19 or its severity.
Sample size and limited access to more information about COVID-19-positive patients are limitations of the current study. In addition, we may have unintentionally excluded patients who died. More details of residual AHI in the patients’ PAP reports should be investigated in future studies. Although current findings are clinically important, larger sample sizes in different subgroups may lead to statistically significant findings. Therefore, more research on concomitant OSA and COVID-19 or treatment of OSA in COVID-19 patients could be informative and lifesaving.
Conclusions
Proinflammatory characteristics of OSA may increase the risk of COVID-19. Higher BMI, female sex, more severe OSA, insomnia, and depression, lower TST and SE, and lack of OSA management were associated with higher COVID-19 prevalences among patients with OSA. This is important for treating OSA in these patients as a protective measure against COVID-19. Researchers and practitioners should attend to both diagnosed and undiagnosed OSA because it is prevalent in the population and may increase risk of COVID-19. More investigations with larger sample sizes are recommended.
Declaration of conflict of interest
The authors have no conflict of interest to declare.
Acknowledgment
We acknowledge the research deputy of Tehran University of Medical Sciences for their support and fund. Our sincere thanks go to the staff of our sleep clinics at Baharloo and Imam hospitals (Bagheri F, Fallahi F, Fatehi Peikan F, Ghasemi E, Ghodrati Asgarabadi S, Karimzadeh Aghcheh Kandi N, Mirebrahimi M, Mohammadi S, Mohsenikia M, Naziri Alehashem E, Rashidi M, Sheibani P) for their fine contributions in interviewing patients and data entry during the COVID-19 epidemic lockdown. We also would like to thank all of our hero colleagues who sacrificed their lives in the fight against COVID-19. We express our sincere condolences to their families and close friends.
References
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. 2020. 2020.02.10.20021675.
- 4.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staats R, Rodrigues R, Barros A. Decrease of perforin positive CD3+γδ-T cells in patients with obstructive sleep disordered breathing. Sleep Breath. 2018;22(1):211–221. doi: 10.1007/s11325-017-1602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee EJ, Heo W, Kim JY. Alteration of inflammatory mediators in the upper and lower airways under chronic intermittent hypoxia: preliminary animal study. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/4327237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Said EA, Al-Abri MA, Al-Saidi I. Altered blood cytokines, CD4 T cells, NK and neutrophils in patients with obstructive sleep apnea. Immunol Lett. 2017;190:272–278. doi: 10.1016/j.imlet.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Resp Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qingxian, C and Fengjuan, C and Fang, Let al., Obesity and COVID-19 severity in a designated hospital in Shenzhen, China (3/13/2020). Available at SSRN: https://ssrn.com/abstract=3556658 or 10.2139/ssrn.3556658 [DOI] [PubMed]
- 10.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto JA, Ribeiro DK, Cavallini AF, Duarte C, Freitas G. Comorbidities associated with obstructive sleep apnea: a retrospective study. Int Arch Otorhinolaryngol. 2016;20(2):145–150. doi: 10.1055/s-0036-1579546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haghighi KS, Montazeri A, Mehrizi AK. The Epworth Sleepiness Scale: translation and validation study of the Iranian version. Sleep Breath. 2013;17(1):419–426. doi: 10.1007/s11325-012-0646-x. [DOI] [PubMed] [Google Scholar]
- 14.Yazdi Z, Sadeghniiat-Haghighi K, Zohal MA. Validity and reliability of the Iranian version of the insomnia severity index. Malay J Med Sci. 2012;19(4):31. [PMC free article] [PubMed] [Google Scholar]
- 15.Beck A, Steer R, Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the BDI-II. [Google Scholar]
- 16.Hu Y, Sun J, Dai Z. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med. 2012;2 doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieto FJ, Young TB, Lind BK. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 19.Millman RP, Redline S, Carlisle CC, Assaf AR, Levinson PD. Daytime hypertension in obstructive sleep apnea: prevalence and contributing risk factors. Chest. 1991;99(4):861–866. doi: 10.1378/chest.99.4.861. [DOI] [PubMed] [Google Scholar]
- 20.Seicean S, Kirchner HL, Gottlieb DJ. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31(5):1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 21.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28(2):87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Mills PJ, Dimsdale JE. Sleep apnea: a model for studying cytokines, sleep, and sleep disruption. Brain, Behav Immunity. 2004;18(4):298–303. doi: 10.1016/j.bbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Steiropoulos P, Kotsianidis I, Nena E. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep. 2009;32(4):537–543. doi: 10.1093/sleep/32.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.coronavirus Disease 2019 (COVID-19). Manage Anxiety & Stress. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. [updated 2020 April 24; cited 2020 March 12]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/
- 25.Virginia Commonwealth University. Health expert on limiting coronavirus risk: ‘Get sleep, stay hydrated, wash your hands’. VCU University Relations. [updated 2020 March 6; cited 2020 March 12] Available from: https://news.vcu.edu/
- 26.Coronavirus (COVID-19): what you need to do. COVID-19: stay at home guidance. Open Government Licence v3.0 [updated 2020 April 28; cited 2020 March 12] Available from: https://www.gov.uk/government/publications/
- 27.Adequate sleep can help fight COVID-19′. New Straits Times, New Straits Times Press [updated 2020 Feb. 21; cited 2020 March 12]. Available from: https://www.nst.com.my/news/nation/