Abstract
A 49-year-old man was admitted to his local hospital with left leg pain and breathing difficulties. He had negative nasopharyngeal polymerase chain reaction tests for severe acute respiratory syndrome coronavirus 2. Chest X-ray and Computed tomography pulmonary angiogram displayed typical coronavirus disease 2019 (COVID-19) radiological features as ground-glass opacities and bronchovascular thickening. His respiratory symptoms resolved after four days of supportive treatment, whereas his left leg became more painful and discolored. He was referred to our center with acute left leg ischemia. computed tomography angiogram revealed eccentric mural thrombus at the aortic bifurcation, extending into left common iliac and an abrupt occlusion of left popliteal, tibioperoneal, and posterior tibial arteries. He was treated with catheter-directed thrombolysis for 48-hours that achieved successful revascularization of the ischemic limb with no intervention-related complications. At six-week follow-up, he showed full recovery. Our case demonstrates that catheter-directed thrombolysis is a successful and safe treatment option in a COVID-19 patient with acute arterial occlusion.
Background
Since the outbreak of coronavirus disease in 2019 (COVID-19), many reports have described a hypercoagulable state in COVID-19 positive patients. Investigations have shown that the coronavirus infection initiates severe inflammatory responses that lead to blood stasis, endothelial injury, and increased risk of thrombosis and embolism.1, 2, 3 Herein, we report a successful dissolution of COVID-19–induced thrombosis, using standard tissue plasminogen activator catheter-directed thrombolysis (tPA-CDT) without the development of complications in a patient presenting with acute lower limb ischemia.
Case Presentation
A 49-year-old man, previously healthy, who had no prior comorbid diagnosis, contracted the common symptoms of coronavirus infection in early April this year. He described a two-week history of irritating cough, fever, headache, malaise and myalgia. He was self-isolating in his house with his family, as per the government guidance. Toward the end of his period of self-isolation, he experienced worsening shortness of breath and left leg pain that required hospital admission under the medical team at his local district general hospital. He had chest X-ray and computed tomography pulmonary angiogram and was tested twice for COVID-19 by nasal antigen swabs. His lungs showed radiological features of ground-glass opacities and bronchovascular thickening that were highly suggestive of COVID-19 infection; however, both swab tests were negative. The patient stayed in the hospital for four days, during which he required nasal oxygen. His general condition and most of his respiratory COVID-19 symptoms improved. However, his leg pain worsened, and he had trouble walking. At this point, the left leg was found to be cold with no palpable pulses below the groins, impaired sensation but normal power. An urgent CT peripheral angiogram was performed. He was anticoagulated using low-molecular-weight heparin (tinzaparin 15,000 IU) and referred urgently to our regional vascular center.
On arrival to the vascular hub unit, the patient was normotensive with a blood pressure of 125/75 mm Hg, had a sinus-rhythm tachycardia with a heart rate of 125 bpm, oxygen saturation of 95%, tachypnoea of 24 breaths/minute, and a temperature of 38.1°C, scoring (4) on the National Early Warning Score chart.4 The vascular assessment of the lower limbs showed a pale looking left foot, colder than the right side with prolonged capillary refill time (>4 seconds) and minimal calf tenderness. Neurologically, there was sensory impairment affecting the toes with no associated limb weakness. There were palpable bilateral femoral pulses with absent pulses on the left popliteal and pedal arteries. There was a full complement of lower limb pulses on the right leg. The hand-held Doppler examination revealed monophasic signals on the anterior tibial artery. Ankle-brachial pressure index (ABPI) was not measured preoperatively or postoperatively.
A clinical diagnosis of Rutherford class IIa marginally threatened acute limb ischemia was established. An urgent multidisciplinary team discussion ensued, between on-call vascular surgeons, interventional radiologists, anesthetists, and intensive care consultants. They concluded that given the unique circumstances due to COVID-19, urgent CDT would be the treatment of choice for his infrainguinal lesion and to avoid dealing with the common iliac lesion. The rationale behind the decision considered the clinical staging of the ischemic limb (IIa Rutherford), the acute duration of limb symptoms (<two weeks), the absence of any known absolute or relative contraindications to thrombolysis, the acute occlusion of the runoff vessels, and the new guidance from the vascular society that recommends endovascular intervention during the pandemic. The patient agreed and consented for thrombolysis after he was informed of the associated benefits and risks.
Investigations
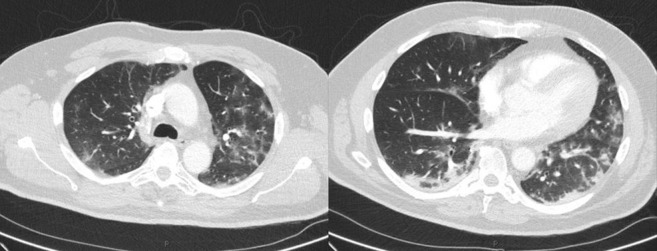
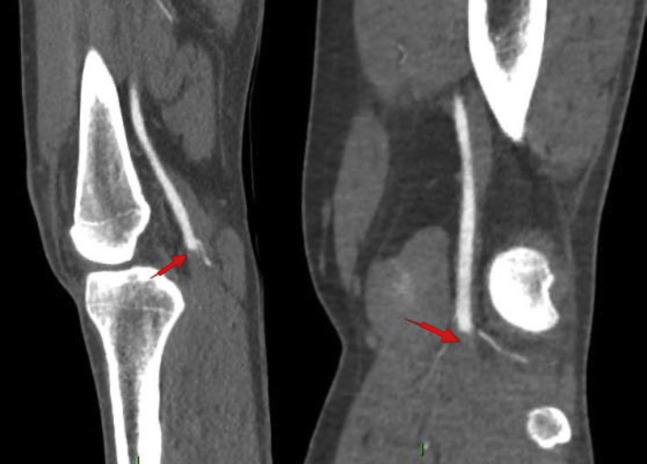
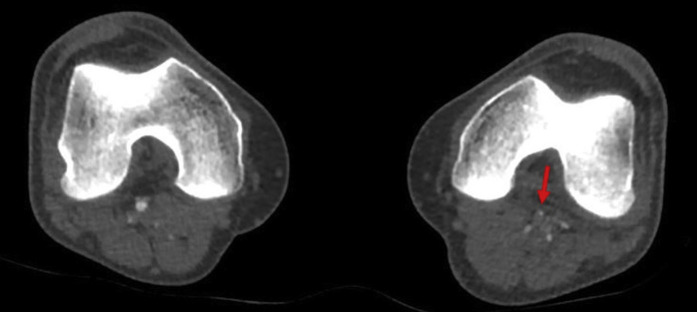
Chest X-ray (Fig. 1 ) and CT pulmonary angiogram (Fig. 2 ) displayed typical radiological features of COVID-19 pneumonia.5 CT angiogram showed eccentric mural thrombus at the aortic bifurcation, extending into the left common iliac artery with luminal narrowing at the origin (Fig. 3 ), abrupt popliteal occlusion extending from the knee joint down to trifurcation, with an absent left posterior tibial artery, and poor run off in the distal anterior tibial and peroneal arteries (Fig. 4, Fig. 5 ). There was no underlying arterial calcification noted.
Fig. 1.
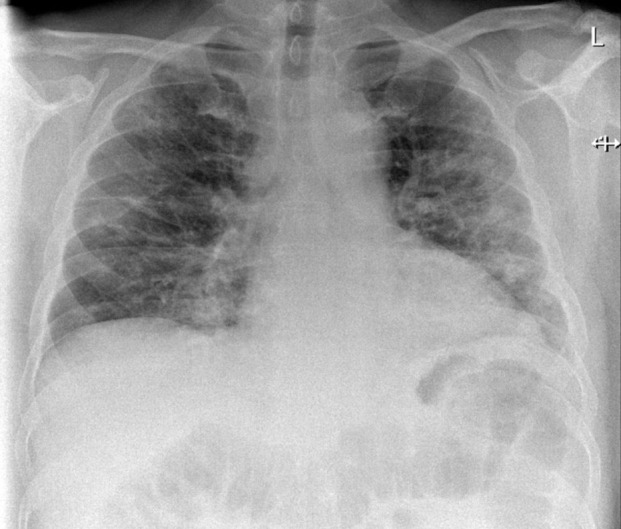
Chest X-ray showing patchy air space shadowing throughout both lung fields with a predominance of peripheral ground-glass changes.
Fig. 2.
CT pulmonary angiogram demonstrated no evidence of acute pulmonary embolus but numerous foci of ground-glass changes affecting all lung lobes, predominantly in a peripheral distribution with patchy consolidation and prominent mediastinal and hilar lymph nodes.
Fig. 3.
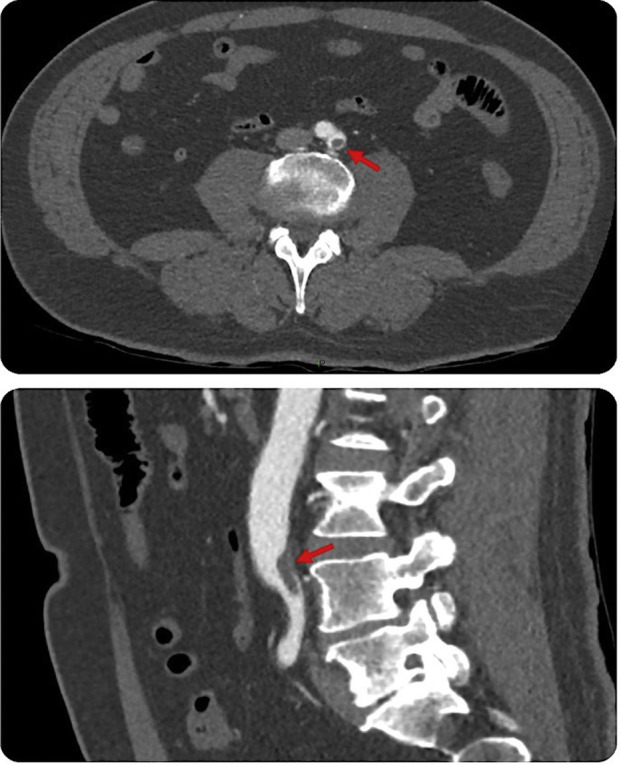
CT peripheral angiogram (axial and sagittal views) shows eccentric mural thrombus at the aortic bifurcation, extending into the left CIA with luminal narrowing at the origin. (Arrows).
Fig. 4.
CT peripheral angiogram (sagittal and coronal views) shows an abrupt occlusion of the left popliteal artery. (Arrows).
Fig. 5.
CT peripheral angiogram (axial view) shows an abrupt occlusion of the left popliteal artery. (Arrow).
Full blood tests were unremarkable except the following abnormalities: raised white blood cells count (11.8 per μL; reference range 4–11 per μL), raised platelet count (520 per μL; reference range 120–400 per μL), mildly raised C-reactive protein (12 mg/L; reference range 0–10 mg/L), and deficient levels of activated partial thromboplastin time ratio (0.9; reference range 2–3).
On admission to the vascular hub unit, a third nasopharyngeal sample was sent and came back negative for SARS-CoV-2. An electrocardiogram confirmed sinus tachycardia and urgent echocardiography was normal, with no cardiac source of embolization detected.
Treatment
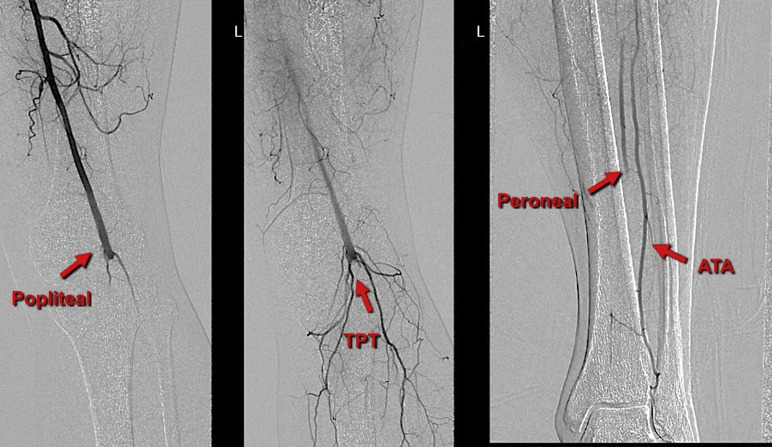
On admission, the patient was started on systemic anticoagulation using heparin infusion, 75 mg aspirin, 80 mg atorvastatin, and analgesia. In the interventional radiology suite, a World Health Organization surgical safety checklist was completed. Under local anesthesia and complete sterile conditions, antegrade access was achieved using an ultrasound-guided puncture to the left common femoral artery. A 5-Fr sheath was inserted, intravenous 5,000 units of unfractionated heparin were given, and an initial digital subtraction angiography was performed (Fig. 6 ). A Fountain-infusion catheter (5-Fr, 90 cm × 20 cm) was then placed down into the tibioperoneal trunk. A 10 mg bolus of tPA was infused followed by continuous tPA infusion at a rate of 1 mg/hr through the Fountain catheter and 0.5 mg/hr through the sheath in addition to continuous heparin infusion at a rate of 1000 U/hr. The postprocedure assessment was uneventful, and the patient moved to the high dependency unit for close observation.
Fig. 6.
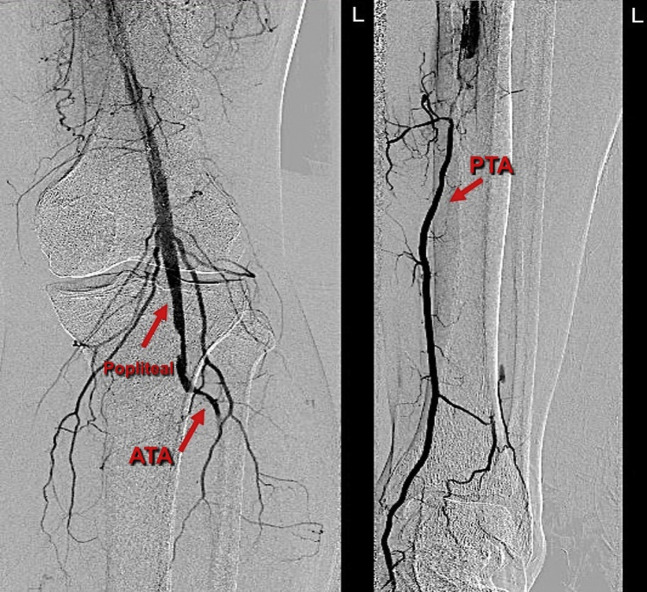
Left lower limb angiogram shows an acute occlusion of the popliteal, tibioperoneal trunk, and poor run off, with segments of reconstitution of the anterior tibial artery (ATA) and peroneal at the mid-distal calf level. (Arrows).
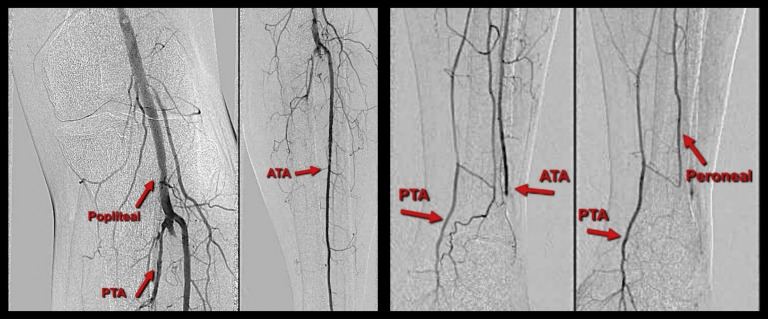
Twenty-four-hour-checkangiography showed resolution of most of the popliteal thrombosis and recanalization of the distal two-thirds of his posterior tibial artery (Fig. 7 ). The catheter was advanced into the proximal posterior tibial artery, and a further 24 hours of tPA-CDT was continued using the same protocol of infusion that was listed previously. A second 24-hour-checkangiography showed complete resolution of the popliteal thrombus and inline flow in the proximal-mid anterior tibial artery. While the proximal peroneal and posterior tibial artery remained occluded, these vessels reformed in the mid-distal calf and filed plantar arch (Fig. 8 ). Despite these suboptimal radiological features (occluded distal anterior tibial artery and proximal posterior tibial artery), a decision was made to stop the procedure as the foot was warm, well perfused with triphasic Doppler signals, and palpable left posterior tibial artery pulses. The catheter and the sheath were removed, and the common femoral artery access site was closed using a 6 Fr Angio-Seal device (Angio-Seal™ Evolution™ Terumo, Europe).
Fig. 7.
24-hr-first check angiogram shows the dissolution of the popliteal thrombus and reconstitution of the mid-distal posterior tibial artery (PTA) with poor views in the anterior tibial artery (ATA) and peroneal arteries. (Arrows).
Fig. 8.
48-hr second check angiogram shows resolution of the popliteal thrombus, patent proximal-mid anterior tibial artery (ATA) that occlude at the level of the ankle, proximally occluded peroneal, and posterior tibial artery (PTA) arteries which reconstitute mid-distal calf and collateral filling the arch. (Arrows).
Outcome and Follow-Up
The patient was continued on heparin infusion for extra 24 hours after thrombolysis followed by combined oral anticoagulation (dabigatran 150 mg twice daily) and a single antiplatelet (aspirin 75 mg OD) for six weeks with a plan to stop anticoagulation after reviewing in the clinic. Before discharge, the patient reported complete resolution of his symptoms and was able to walk pain-free. On clinical examination, the left lower limb was well perfused (pink and warm), capillary refill < 2 seconds, palpable posterior tibial artery, and dorsalis pedis artery pulses with triphasic Doppler signals on both arteries. An ultrasound scan of the left groin showed no evidence of hematoma or bleeding. The patient was discharged home with no early postprocedure complications.
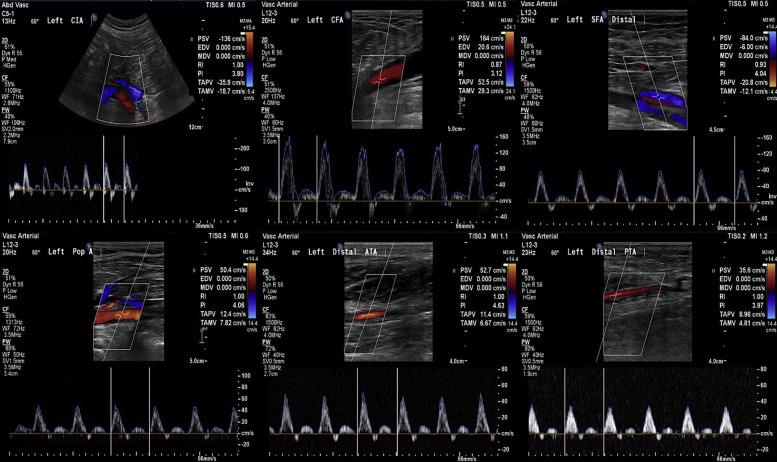
Six weeks later, in the follow-up vascular clinic, the patient showed full recovery of his symptoms with no complication. He had a planned left lower limb arterial duplex scan that showed patent arterial tree; aorta, common and external iliac, common femoral, profunda and superficial femoral, popliteal, tibioperoneal trunk, and the three runoff vessels with triphasic flow signals (Fig. 9 ). The patient has been maintained on dabigatran, aspirin 75 mg, and atorvastatin 80 mg for a further eight weeks.
Fig. 9.
Lt lower limb arterial duplex scan: Six weeks postintervention shows patent arterial tree; common iliac and common and superficial femoral, popliteal, tibioperoneal trunk, and the run-off vessels with triphasic flow signals.
Discussion
In this article, we are reporting one of the earliest cases of successful endovascular management of acute lower limb ischemia that developed in a previously healthy young man after suffering from the acute illness and chest changes consistent with COVID-19.
The novel coronavirus (SARS-CoV-2) has been identified as the cause of COVID-19.2 It mainly affects the respiratory system with symptoms ranging from a mild common cold–like illness to severe viral pneumonia in symptomatic patients. In severely affected patients, the disease may progress to acute respiratory distress syndrome, septic shock, metabolic acidosis, coagulopathy, including disseminated intravascular coagulopathy, and death.6
Our patient experienced an initial deterioration of his respiratory symptoms requiring hospital admission and oxygen therapy. His radiological investigations displayed typical features of COVID-19 pneumonia that matched his initial admission symptoms. The hallmarks of COVID-19 on CT imaging are bilateral and peripheral ground-glass opacities, consolidative pulmonary opacities, and crazy paving patterns.5 These radiological changes in association with the respiratory symptoms are sufficient for diagnosis of COVID-19 even in the absence of positive nasopharyngeal swab.7 A study in Wuhan, China, of 1,014 patients examined the correlation of chest CT and nasal swab testing for COVID-19.7 It concludes that chest CT had higher sensitivity (97%) for diagnosis of COVID-19 as compared with swab testing sensitivity (59%). It also revealed that nearly 30% of patients diagnosed with COVID-19 positive CT chest findings had a negative nasal swab result. It was suggested that a negative swab test does not rule out the disease. Although no robust peer review literature currently exists to reliably calculate the specificity and sensitivity of the nasal swab testing, it is widely agreed on that the testing specificity is high, approximately 98%, and the sensitivity is contested with reports suggesting that it may be as low as 60%.8 The disease prevalence also factors significantly into the negative predictive value of the test result. Unfortunately, the local reported prevalence of COVID-19 is variable and likely unreliable, given limited access to testing at that time. When the patient presented during the early stages of the pandemic, COVID-19 antibody testing was not available in the United Kingdom. Currently, it is only offered to National Health Service and care staff, but not to outpatients, so we cannot perform it for our patient.
The incidence of thromboembolic complications in patients with COVID-19 is as high as 35–45%.9 A recent study from Lombardy, Italy, showed a notable rise in the incidence of acute threatening limb ischemia due to hypercoagulability linked to COVID-19 infection.10 The pathophysiology of COVID-19–induced thrombosis is recently explained.11 Angiotensin-converting enzyme-2 (ACE2) receptor is highly expressed in vascular endothelium and other cells.12 SARS-CoV-2 infiltrates these cells by binding to (ACE2) receptor and therefore potentiates an intense immune response known as the cytokine storm, which precipitates the onset of systemic inflammatory response syndrome (SIRS). The subsequent SIRS will induce an endotheliopathy and hypercoagulability state, leading to both systemic macrothrombosis and microthrombosis.11 Although our patient had no signs of underlying large vessels disease as confirmed with the absence of arterial calcification or stenotic disease in his CT angiogram, he developed intra-arterial thrombosis. We believe that his arterial thrombosis is a consequence of the SIRS that was triggered by the COVID-19 viral pneumonia.
Doppler segmental limb pressures are generally of no benefit in acute arterial occlusions. The criteria developed for chronic ischemia do not apply in acute settings. The ABPI measurement has little relationship to that considered for chronic critical limb ischemia, and there is no way to predict the ultimate ABPI in the acute period. If the ABPI can be measured, the limb is a nonthreatened extremity.
Laboratory investigations have confirmed that COVID-19 infection is associated with initial coagulopathy in the form of prominent elevation of D-dimer and fibrin/fibrinogen degradation products. In contrast, abnormalities in prothrombin time, partial thromboplastin time, and platelet counts are relatively uncommon on initial presentations.13, 14, 15 Our patient had abnormal partial thromboplastin time and thrombocytosis on admission, but the D-dimer and fibrinogen tests were not performed. The released proinflammatory cytokines from his lung macrophage system secondary to COVID-19 pneumonia could contribute to his thrombocytosis.16 No thrombophilia tests were performed due to the absence of any patient's history of any previous thromboembolic diseases as well as the negative family history of any related blood disorders.
It is established that all patients with acute limb ischemia should immediately commence on parenteral anticoagulation to prevent extension of the thrombus, to conserve the patency of the collaterals, to reduce the incidence of cardiovascular events, and to improve prognosis by preventing early recurrent thrombosis.17 , 18 In addition, any delays in reinstating blood supply to the ischemic tissues could trigger a significant second hit with the augmented systemic inflammatory response after reperfusion. Our patient probably benefited from the initial heparin treatment because of its anti-inflammatory effect, which is protective to the lung endothelium. The latter is mediated by neutralizing the damage-associated molecular patterns.19
The patient was eligible for catheter-directed thrombolysis, catheter-directed suction thrombectomy, or open embolectomy. However, the decision to select the most appropriate revascularization option was challenging. Open surgery is invasive and associated with higher morbidity and mortality. In the COVID-19 era, open surgery with general anesthesia would be considered as an aerosol-generating procedure that requires full personal protective equipment with increased risks on both patients and the theater team. General anesthesia was reported to increase postoperative mortality in patients COVID-19.20 An endovascular approach, as the first-line option, was recommended by the Vascular Society of Great Britain and Ireland guidelines during the COVID-19 pandemic.21
Currently, the evidence for endovascular treatment for COVID-19–induced arterial thrombosis is lacking. However, it is our opinion that CDT would minimize the intimal trauma and the risk of recurrent thrombosis, which could otherwise occur as a result of mechanical thrombectomy. Although CDT is less invasive, it has the risks of failure, myocardial infarction, cerebrovascular accidents, bleeding, and distal embolization.17 Specifically, CDT could trigger bleeding in COVID-19–induced lung disease. Indeed, hemoptysis has been reported as one of the symptoms of the COVID-19.1
While the general bleeding risks of CDT are known to us, and to reduce risks, our center's protocol is to recover and observe all patients with arterial thrombolysis in the high-dependency unit. It is reasonable to use individualized risk stratification to balance between risks of thrombosis and bleeding.22 , 23 Thus, a multidisciplinary consensus between relevant clinicians and patients is paramount in times of clinical uncertainties and outcomes should be documented in the patients' records.
In this case, we intentionally decided not to directly treat the left common iliac artery thrombus, as it was not flow limiting. Further, we expected that the systemic effect of tPA and the intravenous heparin would stabilize the common iliac thrombus.
In a similar recent case using urokinase, CDT failed to achieve successful revascularization of a patient with tibial emobolization; hence, a retrograde pedal embolectomy was required to reperfuse an ischemic leg.24 The use of different techniques and another thrombolytic agent may explain the difference in the outcome. We placed an antegrade sheath and the fountain catheter into the popliteal thrombus to maximize the thrombolysis effect. Our protocol is to use tPA in both the sheath and catheter. In our case study, 48 hours of tPA-CDT was able to dissolve most of the thrombus with remaining short lesion in the proximal posterior tibial artery. However, with continuous anticoagulation for six weeks, the infrainguinal thrombus resolved ultimately.
We used dabigatran as an oral anticoagulant in addition to aspirin for six weeks. However, dabigatran is not licenced for use in arterial thrombosis. This underpins the fact that there is insufficient data available for pharmacological management of patients with acute limb ischemia secondary to COVID-19 infection. Dabigatran was used for its convenience as the patient did not need monitoring or to attend anticoagulation clinics given the COVID-19 restrictions. In additioin, the use of anticoagulant with single antiplatelet provides better protection from recurrence of arterial thrombosis than a single modality, albeit with a higher risk of bleeding. Therefore, we decided to use a direct oral anticoagulant, that is dabigatran, that has an antidote if needed.
Recently, it was brought to our attention the establishment of an international effort known as the “Vascular Surgery COVID-19 Collaborative” (VASCC).25 Within the VASCC, two distinct projects were created to provide vascular surgeons from around the world with the opportunity to study the major issues facing our specialty. In particular, project 2: “Thrombotic complications of COVID-19” aims to investigate further and clarify the thrombotic manifestations of the disease and to underline management practices under such circumstances. This project will hopefully provide a large case basis to address this critical issue.
Current literature consists only of single centers experience. In our report, we demonstrated that tPA-CDT was successful in dissolving thrombosis caused by COVID-19 infection, without complication and should be considered as a safe treatment option in the presence of pulmonary COVID-19 changes. At a time when the disease process has not been fully understood, this case adds to the body of evidence that supports the Vascular Society guidance of an endovascular approach in the management of COVID-19 vascular events.
Learning Points.
-
•
Coronavirus disease 2019 (COVID-19) is a prothrombotic disease and may predispose patients to arterial thrombosis and acute limb ischemia.
-
•
Tissue plasminogen activator catheter-directed thrombolysis (tPA–CDT) is a successful modality in treating arterial thrombosis secondary to COVID-19 infection.
-
•
Acute limb ischemia could still develop during the recovery phase of a COVID-19 infection.
Footnotes
Declarations of interest: None.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Rio C., Malani P.N. 2019 Novel coronavirus—important information for clinicians. JAMA. 2020;323:1039–1040. doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 4.Royal College of Physicians National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London: RCP. 2017. https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 Available at:
- 5.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26] Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zitek T. The appropriate use of testing for COVID-19. West J Emerg Med. 2020;21:470–472. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Moore M., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han H., Yang L., Liu R., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1118–1119. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 15.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conti P., Ronconi G., Caraffa A., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 17.Berridge D.C., Kessel D.O., Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2013:CD002784. doi: 10.1002/14651858.CD002784.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Björck M., Earnshaw J.J., Acosta S., et al. Editor's choice - European society for vascular surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Mu S., Li X., et al. Unfractionated Heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. J Surg Res. 2019;238:175–185. doi: 10.1016/j.jss.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 20.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VSGBI guidance dated 27th March The management of vascular patients during the current COVID-19 pandemic. 2020. https://fssa.org.uk/_userfiles/pages/files/covid19/prioritisation_master_240720.pdf Available at:
- 22.Cohen A.T., Harrington R.A., Goldhaber S.Z., et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534–544. doi: 10.1056/NEJMoa1601747. [DOI] [PubMed] [Google Scholar]
- 23.Spyropoulos A.C., Ageno W., Albers G.W., et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 24.Andrea V., Gianluca F., Rodolfo P., et al. Unheralded lower limb threatening ischemia in a COVID-19 patient. Int J Infect Dis. 2020;96:590–592. doi: 10.1016/j.ijid.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Oria M., Mills J.L., Cohnert T., et al. On behalf of the vascular surgery COVID-19 collaborative (VASCC), the “vascular surgery COVID-19 collaborative” (VASCC) Eur J Vasc Endovasc Surg. 2020;60:489–490. doi: 10.1016/j.ejvs.2020.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]