Abstract
Introduction
Accelerated bone loss and osteoporosis are multifactorial comorbidities related to HIV and its treatments; however, their mechanisms remain elusive. Identifying HIV treatments that are differentially linked to osteoporosis risk, and clinical factors associated with HIV-related osteoporosis may enable optimizing anti-retroviral treatment (ART) and anti-osteoporosis therapy in preventing or treating this debilitating complication. This study aims to evaluate the dynamics of bone turnover markers after initiation of two commonly used antiretroviral regimens.
Methods
A prospective matched cohort study. Thirty treatment-naïve male patients (mean age 40 ± 10y) who initiated treatment with truvada (tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC)) + raltegravir or TDF/FTC + efavirenz were included in the study. Control group included 15 treatment-naive HIV patients. Serum morning fasting level of P1NP and CTX were measured 0, 1, 6, and 12 months after treatment initiation in the two study groups, and at 0, 6 and 12 months in the control group.
Results
In both treatment groups, but not in the control group, both markers increased significantly over time with no difference in BTM between patients treated with raltegravir or efavirenz. Levels of P1NP were statistically higher at 6 and 12 months after treatment initiation in both treatment groups compared to the controls, while CTX during treatment increased in both treatment groups but was significantly higher only in the raltegravir treatment group after 12 months. The ratio of area under the curve of P1NP/CTX correlated with CD4 increment.
Conclusions
Treatment initiation with raltegravir or efavirenz combined with TDF/FTC is associated with increased bone turnover. Thus, therapy that optimize bone turnover is needed to reduce bone loss at this vulnerable period and improve long-term bone health.
Keywords: HIV, P1NP, CTX, Raltegravir, Efavirenz, Tenofovir/emtricitabine
1. Introduction
Osteoporosis is an age-related morbidity which is 3.7 times more prevalent in people living with HIV in comparison to the general population, resulting in a 60% higher rate of osteoporotic fractures among HIV patients compared to age and gender matched HIV-negative controls (Triant et al., 2008; Grijsen et al., 2010; Cotter et al., 2014). There are multiple factors responsible for the enhanced osteoporosis risk among this population, such as high prevalence of low body weight, smoking, co-infection with HCV and HBV, chronic inflammation related to HIV infection and antiretroviral therapy (Premaor and Compston, 2018; Hileman et al., 2014; Gohda et al., 2015).
Previous studies have demonstrated that antiretroviral medications (ART) impact bone mass, but many of these studies included multiple regimens and investigated the effect of older medications that are no longer in use (Brown and Qaqish, 2006; Bedimo et al., 2012). Moreover, some of these studies resulted in conflicting data when comparing the effect of different medication classes (McComsey et al., 2011; Duvivier et al., 2009). Most of the studies have used dual-energy X-ray absorptiometry (DXA) to evaluate Bone Mineral Density (BMD). Although this technique is considered the gold standard for diagnosing osteopenia and osteoporosis in clinical use, it does not provide a mechanistic explanation of bone turnover status (enhanced bone resorption or decreased bone formation), and by nature of the imaging technique, can indicate bone abnormalities only after significant damage has occurred. Understanding the mechanistic effect of specific antiretroviral medication on bone turnover is of major importance in light of new availability of both anti-retroviral and anti-osteoporosis treatments.
The aims of this study were to evaluate the impact of two commonly used antiretroviral regimens on bone turnover markers, elucidate the differences between two important classes of antiretroviral medications: non-nucleoside analog reverse transcriptase inhibitors (NNRTIs) vs. integrase inhibitors (INSTI) and to uncover the mechanism of enhanced bone loss after treatment initiation. The two regimens that were studied were based on a nucleotide analog reverse transcriptase inhibitors (NRTIs) backbone combination -tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC)- combined with either raltegravir (INSTI) or efavirenz (NNRTI). Evaluated bone turnover markers (BTM) were procollagen type 1 N-terminal propeptide (P1NP) reflecting bone formation, and C-terminal telopeptide of type 1 collagen (CTX) indicating bone resorption.
2. Methods
All treatment-naïve male patients who were followed at the Hadassah AIDS center and initiated antiretroviral treatment with TDF/FTC combined with raltegravir between 2006 and 2016 were identified using the local AIDS center database. Demographic and clinical data were retrieved from medical records, including age at diagnosis, ethnicity, tobacco and alcohol consumption, CD4 T cell count and viral load (VL) at diagnosis and treatment initiation, nadir CD4 T cell, body mass index (BMI) at treatment initiation and after 12 months, calcium, phosphorus and 25 hydroxyvitamin D (25OHD) levels at treatment initiation. Treatment naïve male patients who initiated antiretroviral treatment with TDF/FTC combined with efavirenz were matched, based on age and CD4 T cell count, to the patients initiating TDF/FTC and raltegravir. In addition, an age-matched control group consisted of untreated HIV positive patients who were followed for at least a year. Frozen serum samples (morning fasting, stored at −80 °C) of all groups were tested for P1NP and CTX levels using electrochemiluminescence immunoassay “ECLIA” COBAS kits (Roche diagnostics, Mannheim Germany, CTX normal range: male 30–50y 16–584 pg/ml 50–70y: 0–704 pg/ml CV (coefficient of variation) 9%, P1NP male 15–59 ng/ml, CV 8%). Tested samples in the two treatment groups included four consecutive time points: 0, 1, 6, 12 months after treatment initiation. The control group included samples collected at 0, 6, 12 months during the follow-up year.
3. Statistics
The primary comparisons in this study were of P1NP and CTX values between treatment groups. In order to detect a difference of 20 ng/ml in P1NP values in a paired comparison, with a 25 ng/ml standard deviation of the differences, assuming a two-sided alpha of 0.05 and a power of 80%, 15 pairs would be required. Similarly, for CTX, a difference of 150 pg/ml, with a standard deviation of 170 pg/ml, alpha 0.05 and 80% power, would require 15 pairs.
These power calculations were performed post-hoc and the estimated difference and variance estimates were derived from the data generated in this study.
Incremental area under the curve (AUC) for CTX and P1NP was computed for each individual using Prizm 8 (GraphPad software), and ratio of AUC P1NP/CTX was calculated. Continuous variables are presented with mean and standard deviation and categorical variables with percentages. Paired comparisons were performed with the Wilcoxon signed-rank test for continuous parameters in each group. CD4 T cell count, VL, P1NP, CTX values and AUC for CTX and P1NP and their ratio were compared between the study groups. Correlation between continuous variables was assessed with the Pearson correlation coefficient. Significance was considered with a p-value equal or below 0.05.
4. Results
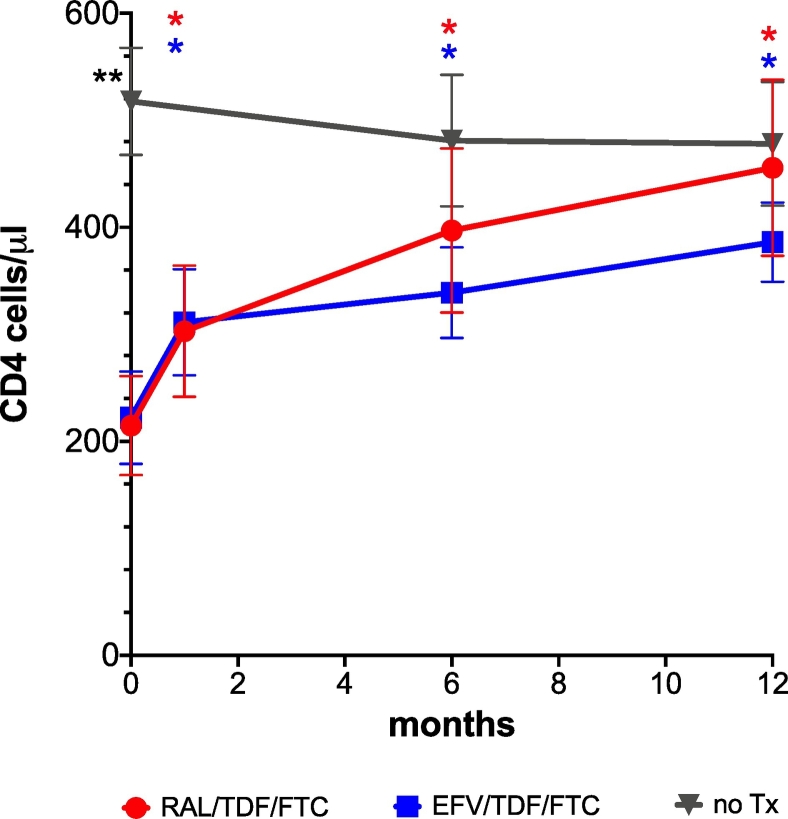
15 patients were included in each study group. Basic characteristics of each group are presented in Table 1. The two treatment groups were similar in nadir CD4, age and CD4 count, 25OHD, calcium phosphorus, BMI at treatment initiation and BMI increment after treatment initiation. VL were higher in the raltegravir group at treatment initiation, and time from diagnosis to treatment initiation was longer in the efavirenz group. Following treatment initiation, CD4 T cell counts increased in both treatment arms and became indistinguishable to the control group level after 12 months of treatment (RAL 456 ± 308 cell/μl, EFV 368 ± 129 cell/μ, Control 478 ± 224 cell/μ. control vs RAL p = 0.85, control vs EFV p = 0.13) (Fig. 1, Supplemental Table 2). As expected, VL decreased in both treatment arms following treatment initiation. 10/14 of patients in the raltegravir group and 13/15 in the efavirenz arm had undetectable viral loads after 12 months of treatment (Fig. 2).
Table 1.
Patient characteristics at treatment initiation. Results are Mean ± SD. Control groups are patients that were not treated during the study duration.
| Control | RAL | EFV | |
|---|---|---|---|
| Age at treatment initiation (y) | 42.4 (14) | 42.5 (10) | 40.7 (8) |
| Ethnicity % (n) | |||
| Israeli Jew | 33.3 (5) | 26.6 (4) | 26.6 (4) |
| Israeli Arab | 0 (0) | 13.3 (2) | 40 (6) |
| Ethiopian Jew | 46.6 (7) | 26.6 (4) | 6.6 (1) |
| Former USSR | 13.3 (2) | 13.3 (2) | 6.6 (1) |
| Other | 6.6 (1) | 20 (3) | 20 (3) |
| Smoking (Y/N) | 4/8 | 5/9 | 5/8 |
| Alcohol (Y/N) | 1/10 | 3/11 | 3/10 |
| Time from diagnosis to treatment initiation (days) | 428 (937) | 858 (1331) | 1301 (1269) |
| CD4-treatment initiation (cells/μl) | 516 (192) | 195 (178) | 220 (173) |
| VL at treatment imitation (copies/ml) | 28,000 (32000) | 204,000 (333,000) | 77,000 (111,000) |
| BMI at treatment initiation kg/M2 | 25.3 (4.2) | 26 (3.6) | 23 (3.2) |
| Nadir CD4 (cells/μl) | 317.3 (156) | 194.8 (157) | 210.6 (142) |
| OH-25 vitamin D (ng/dl) | 27 (18) | 23.2 (14) | 22.7 (13) |
| Albumin-corrected calcium (mmol/L) | 2.0 (0.3) | 2.2 (0.2) | 2.1 (0.3) |
| Phosphorus (mmol/L) | 1 (0.2) | 1 (0.3) | 1 (0.2) |
| P1NP (ng/ml) | 47 (20) | 46 (17) | 55 (32) |
| CTX (pg/ml) | 288 (264) | 341 (296) | 243 (161) |
Fig. 1.
CD4 T cell dynamics during the study period. **CD4 levels were significantly higher before treatment initiation in the control group (control vs RAL p < 0.001, control vs EFV p = 0.002).  CD4 levels increased significantly in the raltegravir/TDF + FTC [RAL] C in each time point-1, 6, 12 in comparison to treatment initiation (p = 0.009, p = 0.004, p < 0.001 respectively).
CD4 levels increased significantly in the raltegravir/TDF + FTC [RAL] C in each time point-1, 6, 12 in comparison to treatment initiation (p = 0.009, p = 0.004, p < 0.001 respectively).  CD4 levels increased significantly in the efavirenz/TDF/FTC [EFV] in each time point-1, 6, 12 in comparison to treatment initiation (p < 0.001, p = 0.003, p < 0.001 respectively). Bars indicate SD.
CD4 levels increased significantly in the efavirenz/TDF/FTC [EFV] in each time point-1, 6, 12 in comparison to treatment initiation (p < 0.001, p = 0.003, p < 0.001 respectively). Bars indicate SD.
Fig. 2.
HIV viral load changes during the study period.
Mean baseline levels of P1NP were similar in all three study groups (RAL 46 ± 17 ng/ml, EFV 55 ± 32 ng/ml, control 47 ± 20 ng/ml). At 6 months following treatment initiation, P1NP was significantly elevated in both treatment groups (95 ± 38 ng/ml in the raltegravir group and 79 ± 38 ng/ml in the efavirenz group) in comparison to the control group (45 ± 17 ng/ml), p = 0.002 and p = 0.005 respectively (Fig. 3, Supplemental Tables 1, 2). There was no significant difference in P1NP levels between the raltegravir and the efavirenz groups (p = 0.3 Fig. 3, Supplemental Table 2). Twelve months following treatment initiation, P1NP levels remained constant in the raltegravir group (91 ± 17 ng/ml) and continued to increase gradually in the efavirenz group (89 ± 44 ng/ml) (Fig. 3). P1NP levels in both treatment groups were significantly higher than their respective baseline levels, and from control group levels at the same time point, but did not differ between groups (p = 0.01, p = 0.001 and p = 0.7 respectively) (Fig. 3, Supplemental Tables 1, 2, 3).
Fig. 3.
P1NP levels during the study period.  P1NP level increased significantly in the raltegravir/TDF/FTC [RAL] group after 6 and 12 months of treatment in comparison to treatment initiation (6 months vs treatment initiation p = 0.006, 12 months vs treatment initiation p = 0.006) and to the control group (RAL vs control 6 months p = 0.002, RAL vs control 12 months p = 0.01).
P1NP level increased significantly in the raltegravir/TDF/FTC [RAL] group after 6 and 12 months of treatment in comparison to treatment initiation (6 months vs treatment initiation p = 0.006, 12 months vs treatment initiation p = 0.006) and to the control group (RAL vs control 6 months p = 0.002, RAL vs control 12 months p = 0.01).  P1NP level increased significantly in the efavirenz/TDF/FTC [EFV] after 6 and 12 months of treatment compared to treatment initiation (6 months vs treatment initiation p = 0.001, 12 months vs treatment initiation p = 0.001) and the control group (EFV vs control 6 months p = 0.005, EFV vs control 12 months p = 0.001).
P1NP level increased significantly in the efavirenz/TDF/FTC [EFV] after 6 and 12 months of treatment compared to treatment initiation (6 months vs treatment initiation p = 0.001, 12 months vs treatment initiation p = 0.001) and the control group (EFV vs control 6 months p = 0.005, EFV vs control 12 months p = 0.001).  P1NP levels were significantly higher in the efavirenz/TDF/FTC group in comparison to raltegravir/TDF/FTC [RAL] after 1 months of treatment. p = 0.004, but not at other time points (6 months p = 0.3, 12 months p = 0.7). Bars indicate SD.
P1NP levels were significantly higher in the efavirenz/TDF/FTC group in comparison to raltegravir/TDF/FTC [RAL] after 1 months of treatment. p = 0.004, but not at other time points (6 months p = 0.3, 12 months p = 0.7). Bars indicate SD.
Bone resorption, as determined by CTX, was similar at baseline in all three arms (RAL 341 ± 296 pg/ml, EFV 243 ± 161 pg/ml, control 288 ± 264 pg/ml). CTX levels of both treatment groups increased during the 12 months of treatment (RAL 449 ± 161 pg/ml, EFV 410 ± 287 pg/ml) while those in the control group remained similar to baseline (258 ± 172 pg/ml). There was no significant difference in the CTX levels in any of the timepoint between the raltegravir and the efavirenz groups (p = 0.26, p = 0.6, p = 0.22, p = 0.53, Supplemental Table 2), but a difference in the kinetics of CTX elevation was noticed between the treatment groups. In the raltegravir group, CTX levels increased after 6 months and only trended to significant elevation (p = 0.09), while it became significantly higher than control levels thereafter at 12 months (p = 0.05). In the efavirenz group, CTX levels increased gradually throughout the 12-month follow-up period, and eventually reached similar levels to those of the raltegravir group but only trended to different from the baseline levels (p = 0.07, 0 months vs 12 months) (Fig. 4, Supplemental Tables 1, 2, 3).
Fig. 4.
CTX levels during the study period.  CTX levels increased significantly in the raltegravir/TDF/FTC [RAL] in 12 months of treatment in comparison to treatment initiation (p = 0.5).
CTX levels increased significantly in the raltegravir/TDF/FTC [RAL] in 12 months of treatment in comparison to treatment initiation (p = 0.5).  CTX levels increased significantly in the raltegravir/TDF/FTC [RAL] at 12 months of treatment in comparison to the control group (untreated patients) (p = 0.02).There was no significant difference in CTX levels between RAL vs EFV in any of time points 0, 1, 6, 12 (p = 0.26, p = 0.6, p = 0.22, p = 0.53 respectively). Bars indicate SD.
CTX levels increased significantly in the raltegravir/TDF/FTC [RAL] at 12 months of treatment in comparison to the control group (untreated patients) (p = 0.02).There was no significant difference in CTX levels between RAL vs EFV in any of time points 0, 1, 6, 12 (p = 0.26, p = 0.6, p = 0.22, p = 0.53 respectively). Bars indicate SD.
P1NP correlated with CTX in both treatment groups but not in the control group. Correlation coefficient was 0.356 (p = 0.009) in the raltegravir group, r = 0.675 (p < 0.0001) in the efavirenz group and r = 0.246 in the control group (p = 0.103, not significantly different from a r = 0 horizontal line).
Incremental AUC of P1NP was significantly higher in both treatment groups in comparison to the control group: RAL 384 ± 222 ng/ml∗months, EFV 259.2 ± 203 ng/ml∗months VS control 16.8 ± 108.7 81 ng/ml∗months (p < 0.001). The incremental AUC of P1NP of patients included in the study, correlated significantly with incremental AUC of CTX (r = 0.501, p < 0.0001), 12 months CD4 increment (r = 0.508, p < 0.0001) and VL decrease in 6 months (r = 0.837, p < 0.0001). In the raltegravir group this parameter correlated with the VL decrease at 1 and 6 months only (r = 0.861 p = 0.001), while in the efavirenz group it correlated with CD4 increment after 12 months of the study (r = 0.543, p = 0.036) but not with viral load reduction. The incremental AUC of CTX was higher in both treatment groups in comparison to the control group: RAL 2033.9 ± 1278.7 pg/ml∗months, EFV 1845 ± 1432 pg/ml∗months, vs 848.58 ± 1076.28 pg/ml∗months in the control group, p = 0.009. Taking together all 45 study participants, the incremental AUC of CTX correlated negatively with the CD4 increment in the first month after treatment initiation (r = −0.421, p = 0.036). Interestingly, when analyzing each group separately, this correlation was significant only in the raltegravir group (r = −0.606, p = 0.037).
The incremental AUC P1NP/CTX ratio in the whole study group correlated with first month CD4 increment (r = 0.556, p = 0.004) and with 12 months CD4 increment (r = 0.362, p = 0.017). In the subgroups analysis the ratio significantly correlated with the 12 months CD4 increment in the raltegravir group (r = 0.559, p = 0.038) and only with the first month CD4 increment in the efavirenz group (r = 0.603, p = 0.029).
5. Discussion
This study is the first to directly compare the dynamics of bone turnover markers in treatment-naïve HIV patients initiating treatment with two commonly used antiretroviral regimens, raltegravir versus efavirenz combined with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), as compared to HIV patients followed without treatment. Our findings indicate that the general effects on BTM between patients treated with raltegravir and those treated with efavirenz are comparable, with both treatment regimens inducing a pronounced increase in BTM even in the early phases of treatment. An important finding stemming from our comparison is the correlation found between the AUC P1NP/CTX ratio and CD4 T cell increment. Beyond the absolute values of P1NP and CTX correlating with bone formation and resorption, respectively, the ratio P1NP/CTX is intuitively predictive of a shift either toward bone formation if elevated, or toward bone resorption if low (Fisher et al., 2018). This finding is of great clinical importance as it suggests that immunological non-responder HIV patients feature an impaired bone formation in comparison to bone resorption and thus are even at higher risk to accelerated osteoporosis than previously contemplated. As this immunological phenomenon is typical to HIV late presenters, we suggest to further investigate this finding by larger prospective studies. If our results will be corroborated by others, we suggest that this subgroup should be closely monitored and supplemented with calcium, vitamin D and if the fracture risk is elevated as evaluated by DXA and/or online Fracture Risk Assessment Tool (FRAX) (Kanis, n.d.), anti-resorptive therapy (bisphosphonates or denosumab) may be indicated.
Previous studies suggested that osteopenia and osteoporosis are more pronounced in HIV patients treated with regimens that contain, as a backbone, TDF/FTC in comparison to abacavir/lamivudine (McComsey et al., 2011; Stellbrink et al., 2010; Haskelberg et al., 2012). Fractures observed in patients treated with these regimens mainly involved the femoral neck, in contrast to those in patients treated with protease inhibitors (PIs) in which fractures were mostly localized to the lumbar spine (Duvivier et al., 2009). Several studies indicated that raltegravir is less detrimental to BMD than other antiretroviral medications as TDF, and darunavir both in comparison studies (Bernardino et al., 2015; Brown et al., 2015) and in switch studies (Bloch et al., 2014; Curran et al., 2012). LaFleur reported that efavirenz in combination with TDF/FTC was associated with lower fracture risk compared to other TDF containing regimens but raltegravir was not investigated (LaFleur et al., 2018). Thus to date there no study directly compared INSTI to NNRTI with the same NRTI back bone as in our study, with respect to their impact on bone density and turn-over.
Evaluation of different BTM comparing HIV treatment-naïve patients to HIV-negative or HIV-treated patients is difficult due to heterogeneity of treatment regimens and time of measurement (Almansouri et al., 2016; Marques de Menezes et al., 2013). A recent study further evaluated the effect of HIV seroconversion and treatment initiation upon BTM including PINP, CTX, sclerostin, osteocalcin and vitamin D, and demonstrated by multivariate analysis that osteocalcin levels decreased after HIV infection, while sclerostin, an inhibitor of bone formation via Wnt pathway decreased after ART initiation. These results suggest that sclerostin may be a key factor involved in bone metabolic disorder associated with HIV infection. In this study, CTX and P1NP levels did not change between the pre-seroconversion, pre-ART and post-ART periods, but the sample size was small, and the time lag between the pre-ART and Post-ART was exceedingly long (5 years). Additionally, only 7.7% of patients were treated with tenofovir, and 65% of the patients started treatment before integrase inhibitors became available (Slama et al., 2017). Moyle et al. showed that treatment initiation induced elevation of both CTX and P1NP in efavirenz-based ART. TDF/FTC induced higher levels of both P1NP and CTX than ABC/3TC in every time point up to 96 weeks, with the levels of both parameters peaking at 24 months and thereafter declining (Moyle et al., 2013). Similar kinetics were demonstrated by the ASSERT (Stellbrink et al., 2010) and by Vlot et al. (2018) and Zhang et al. (2013).
The results of the above studies are consistent with the main results of our study, which demonstrates similar impacts of ART on bone resorption and formation processes in two highly popular new regimens. These results call for consideration to use antiresorptive therapies together with ART, which may include bisphosphonates either oral or intravenous yearly zoledronic acid (Hoy et al., 2018; Ofotokun et al., 2016; Ofotokun et al., 2019; Carr et al., 2019), or the humanized anti-RANKL antibody denosumab, in limiting the occurrence of treatment-associated osteopenia. Alternatively, the recently FDA-approved anabolic anti-osteoporosis anti-sclerostin monoclonal antibody romosozumab (Markham, 2019) may also be a conceptually interesting option as co-treatment in HIV patients, as it may boost bone formation synergistically with initiation of ART, thereby overcoming increased bone resorption in HIV patients.
Our study has several limitations, including the observational nature of the study, the small sample size, the lack of DXA in evaluating the change in bone mineral density during therapy and the different ethnicities of patients included in the study (73.3% Caucasians, 26.7% Ethiopian origin Jews), as different baseline profile of BTM between the different ethnic groups were observed in other studies (Leder et al., 2007). However, baselines levels of both CTX and P1NP did not differ between groups. Further studies are needed to elucidate the impact of medications on BTM in larger cohorts of different ethnicities and include DXA data which will be more valuable in larger cohorts. Our study evaluated only CTX and P1NP but did not assess other markers related to bone turnover, as sclerostin, PTH, FGF23, and renal tubular functions. Despite this limitation, 25OHD levels were similar in control and treatment groups and calcium as well as phosphorus levels were within normal limits during the study and did not differ between groups. Additionally, we did not include a DRV/r combined with TDF/FTC group and could not compare raltegravir or efavirenz combined with ABC/3TC, as these regimens are hardly used in our clinic.
With these limitations notwithstanding, our study indicates that raltegravir and efavirenz combined with TDF/FTC feature similar adverse effects on bone metabolism, with both treatments inducing an early increase in BTM and that patients who are immunological non responders might have a higher risk for osteoporosis. Further studies investigating the effect of initiation TAF/FTC (Sax et al., 2015) combined with INSTI, NNRTI or PI are needed to further refine the differentiation between these regimens. In line with these findings we suggest exploring the potential impact of concomitant time-limited anti-resorptive treatment in patients initiated with all antiretroviral treatment, but also to consider the interesting potential of anti-sclerostin anabolic therapy. Such treatment may prevent or ameliorate osteoporosis and its complications in people living with HIV. These different possibilities should be evaluated in clinical trials to distinguish best safe and efficient regimens.
CRediT authorship contribution statement
Yonatan Oster: Investigation, Methodology, Data curation, Writing - review & editing, Visualization. Matan J. Cohen: Formal analysis. Rivka Dresner-Pollak: Conceptualization. Auryan Szalat: Writing - review & editing, Visualization. Hila Elinav: Conceptualization, Resources, Writing - original draft, Writing - review & editing, Funding acquisition, Visualization.
Declaration of competing interest
All authors declare that they do not have any conflict of interest.
Footnotes
The study was not funded by any external source.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2020.100727.
Contributor Information
Yonatan Oster, Email: yonatano@hadassah.org.il.
Hila Elinav, Email: hila.elinav@gmail.com.
Appendix A. Supplementary data
Supplementary tables
References
- Almansouri A.Y. Serum sclerostin levels in patients with human immunodeficiency virus infection and their association with bone turnover markers and bone mineral densitometry. J Bone Metab. 2016;23:16–22. doi: 10.11005/jbm.2016.23.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedimo R., Maalouf N.M., Zhang S., Drechsler H., Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- Bernardino J.I. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2015;2:e464–e473. doi: 10.1016/S2352-3018(15)00181-2. [DOI] [PubMed] [Google Scholar]
- Bloch M. Switch from tenofovir to raltegravir increases low bone mineral density and decreases markers of bone turnover over 48 weeks. HIV Med. 2014;15:373–380. doi: 10.1111/hiv.12123. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Qaqish R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- Brown T.T. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J. Infect. Dis. 2015;212:1241–1249. doi: 10.1093/infdis/jiv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A. Prolonged effect of zoledronic acid on bone mineral density and turnover in HIV-infected adults on tenofovir: a randomized, open-label study. J. Bone Miner. Res. 2019 doi: 10.1002/jbmr.3834. [DOI] [PubMed] [Google Scholar]
- Cotter A.G. Relative contribution of HIV infection, demographics and body mass index to bone mineral density. AIDS. 2014;28:2051–2060. doi: 10.1097/QAD.0000000000000353. [DOI] [PubMed] [Google Scholar]
- Curran A. Body composition changes after switching from protease inhibitors to raltegravir: SPIRAL-LIP substudy. AIDS. 2012;26:475–481. doi: 10.1097/QAD.0b013e32834f3507. [DOI] [PubMed] [Google Scholar]
- Duvivier C. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23:817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- Fisher A., Fisher L., Srikusalanukul W., Smith P.N. Bone turnover status: classification model and clinical implications. Int. J. Med. Sci. 2018;15:323–338. doi: 10.7150/ijms.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohda J. HIV-1 replicates in human osteoclasts and enhances their differentiation in vitro. Retrovirology. 2015;12:12. doi: 10.1186/s12977-015-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijsen M.L. High prevalence of reduced bone mineral density in primary HIV-1-infected men. AIDS. 2010;24:2233–2238. doi: 10.1097/QAD.0b013e32833c93fe. [DOI] [PubMed] [Google Scholar]
- Haskelberg H. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman C.O., Labbato D.E., Storer N.J., Tangpricha V., McComsey G.A. Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS. 2014;28:1759–1767. doi: 10.1097/QAD.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy J.F. Zoledronic acid is superior to tenofovir disoproxil fumarate-switching for low bone mineral density in adults with HIV. AIDS. 2018;32:1967–1975. doi: 10.1097/QAD.0000000000001911. [DOI] [PubMed] [Google Scholar]
- Kanis D.J.A. https://www.sheffield.ac.uk/FRAX/index.aspx
- LaFleur J. Tenofovir-associated bone adverse outcomes among a US National Historical Cohort of HIV-infected veterans: risk modification by concomitant antiretrovirals. Infect. Dis. Ther. 2018;7:293–308. doi: 10.1007/s40121-018-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder B.Z., Araujo A.B., Travison T.G., McKinlay J.B. Racial and ethnic differences in bone turnover markers in men. J. Clin. Endocrinol. Metab. 2007;92:3453–3457. doi: 10.1210/jc.2006-2695. [DOI] [PubMed] [Google Scholar]
- Markham A. Romosozumab: first global approval. Drugs. 2019;79:471–476. doi: 10.1007/s40265-019-01072-6. [DOI] [PubMed] [Google Scholar]
- Marques de Menezes E.G. Impact of antiretroviral therapy on bone metabolism markers in HIV-seropositive patients. Bone. 2013;57:62–67. doi: 10.1016/j.bone.2013.07.019. [DOI] [PubMed] [Google Scholar]
- McComsey G.A. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J. Infect. Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle G.J. 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir. Ther. 2013;18:905–913. doi: 10.3851/IMP2667. [DOI] [PubMed] [Google Scholar]
- Ofotokun I. A single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin. Infect. Dis. 2016;63:663–671. doi: 10.1093/cid/ciw331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofotokun I. Antiretroviral therapy-induced bone loss is durably suppressed by a single dose of zoledronic acid in treatment-naive persons with HIV infection: a phase IIB trial. Clin. Infect. Dis. 2019 doi: 10.1093/cid/ciz1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaor M.O., Compston J.E. The hidden burden of fractures in people living with HIV. JBMR Plus. 2018;2:247–256. doi: 10.1002/jbm4.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax P.E. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- Slama L. Changes in bone turnover markers with HIV seroconversion and ART initiation. J. Antimicrob. Chemother. 2017;72:1456–1461. doi: 10.1093/jac/dkx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellbrink H.J. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010;51:963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- Triant V.A., Brown T.T., Lee H., Grinspoon S.K. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J. Clin. Endocrinol. Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot M.C. Effect of antiretroviral therapy on bone turnover and bone mineral density in men with primary HIV-1 infection. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Bone turnover and bone mineral density in HIV-1 infected Chinese taking highly active antiretroviral therapy -a prospective observational study. BMC Musculoskelet. Disord. 2013;14:224. doi: 10.1186/1471-2474-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables