Graphical abstract
Keywords: COVID-19, Therapeutic plasma exchange, Convalescent plasma, ARDS, SARS-CoV-2, Critical COVID-19
Abstract
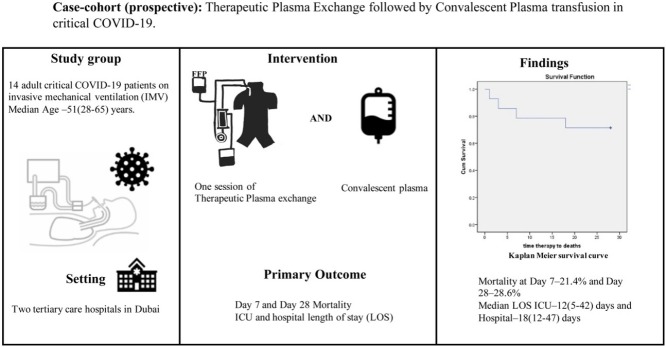
The pathophysiology of severe coronavirus disease 2019 (COVID-19) is primarily a host immune interplay to virus invasion. The therapeutic options have been explored either against hyperinflammation from dysregulated adaptive immunity or direct virus neutralization using antibodies from convalescent plasma (CP) of a recovered patient. The therapeutic plasma exchange (TPE) for removal of excessive inflammatory cytokines has been tried with success in COVID-19. We undertook this exploratory study to evaluate safety and efficacy of TPE followed by CP transfusion in 14 patients with critical COVID-19 requiring invasive mechanical ventilation (IMV). All patients showed improvement in symptoms and decrease of inflammatory markers especially CRP (p = 0.03). 10 patients were liberated from IMV after a median of 5.5 (3–36) days, post sequential therapy. Day 7 and Day 28 mortality was 21.4% and 28.6% respectively. The median duration ICU and hospital LOS were 12 (5−42) days and 18 (12−47) days respectively. No patient developed transfusion-associated complications, but three patients developed secondary bacterial sepsis within 14 days of therapy, and one died.
This case series demonstrated the sequential use of TPE followed by CP transfusion as a therapeutic option in critical COVID-19.
Introduction
The immune system plays a critical part in pathogenesis of severe coronavirus diseases 2019 (COVID-19) (Vabre et al., 2020). Proposed mechanisms include host immune dysregulation causing excessive cytokines release and/or defective B-cell response with ineffective neutralizing antibodies (Vabre et al., 2020). The success story of corticosteroids in severe COVID-19 signals towards hyperinflammation and immunomodulation (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). Convalescent plasma for viral neutralization had been tried with varying success in COVID-19 (Li et al., 2020, Liu et al., 2020). Therapeutic plasma exchange (TPE) to remove inflammatory cytokines also had been tried in severe COVID-19 in smaller case series (Khamis et al., 2020, Shi et al., 2020, Zhang et al., 2020). We used a sequential therapy with TPE followed by CP transfusion in 14 severe COVID-19 patients who were on invasive mechanical ventilation (IMV) to evaluate its effectiveness and safety.
Study design
This prospective case-cohort study was conducted in two tertiary care hospitals of Dubai from April 1 until July 30, 2020 after approval from Dubai Scientific and Research Ethics Committee (DSREC-04/2020_7). 14 adult (aged ≥18 years) patients with critical COVID-19 (WHO severity classification of COVID-19: ARDS, sepsis and septic shock) requiring IMV and confirmed reverse transcriptase–polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus were enrolled in the study. Pregnant women and patients with suspected or confirmed pulmonary embolism were excluded from the study. Patients received a single session of TPE with a total of 30−40 ml/kg bodyweight (BW) of plasma exchange. Vascular access for TPE was secured through double lumen hemodialysis catheter in either the right internal jugular or femoral vein. Vascular access was removed 48 h after completion of TPE. Fresh frozen plasma (FFP) of a normal donor was used for replacement. All 14 patients were transfused 500 ml of ABO-compatible CP, eight hours after TPE. CP was obtained from patients (mean age, 38.1 years) who had previously recovered from COVID-19, demonstrated by two negative RT-PCR results, 24−48 h apart. The neutralizing antibody titer (IgG) for acceptance of plasma was measured using Abbott Architect CoV-2 IgG kit. This kit detects IgG immunoglobulins antibodies against nucleocapsid (N) protein of SARS-CoV-2 using High Throughput chemiluminescent microparticle immunoassay (CMIA). Result of the assay is interpreted as positive (ratio ≥1.4) or negative (ratio <1.4). The test has 100% sensitivity and 99.6% specificity according to the United States Food and Drug administration (US-FDA) (FDA, 2020).
The enoxaparin at 1 mg/kg subcutaneous every 12 h and injection methylprednisolone at 1 mg/kg/day was used in all patients. The clinical and laboratory data on baseline demographics, co-morbidities, symptoms, interval between symptoms to sequential therapy, and negative RT-PCR were collected. The outcomes measured were day 7 and day 28 mortality, length of stay (LOS) on IMV, intensive care unit (ICU) or hospital and time to tracheal extubation after therapy. PaO2/FiO2 ratio and inflammatory markers (C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH) and d-dimer) were also collected before, at 12 h, at 24 h and until seven days after sequential therapy. All patients were observed for any immediate complications during TPE or CP and any secondary infection up until 14 days after sequential therapy.
Mean [±standard deviation (SD)] and median (range) were used for continuous variables. Student's unpaired t-test was used for comparison of variables before and after sequential therapy.
Results and discussion
To our knowledge this is the first case series on sequential use of TPE and CP for the management of critical COVID-19.
The median age was 51 (range 28−65) years; 78.6% were male (Table 1 ). The patients were obese with a median body mass index (BMI) of 32.5 (24−41) kg/m2. Co-morbidities were present in 10 (71.4%) patients, with hypertension (64.2%) being most common. All patients were on IMV with a mean PaO2/FiO2 ratio of 138.89 (±41.90) mm of Hg before sequential therapy. The therapy was administered 9 (6−21) days from the onset of symptoms. An improvement in symptoms (resolution of fever) and decrease in inflammatory markers (CRP, LDH, d-dimer and ferritin) were observed in all 14 patients with significant decrease in CRP (p = 0.03) (Table 1). Day 7 and Day 28 mortality were 21.4% and 28.6% respectively. The time from sequential therapy to negative RT-PCR was 8 (3−18) days. 10 patients were liberated from IMV with a median duration of 8 (6–36) days and 5.5 (3–36) days post sequential therapy. Median ICU and hospital LOS were 12 (5−42) days and 18 (12−47) days respectively. Five (35.7%) patients had acute kidney injury, and two patients require renal replacement therapy, both of whom died.
Table 1.
Demographics, Inflammatory markers, treatment and outcomes measured in 14 patients.
| Proportion- Mean(±SD), Median(range) | |
|---|---|
| Demographics | |
| Age (years) | Mean-49.14 (±12.50), Median-51.5 (28−65) |
| Gender | Male-11(78.6%), Female-3(21.4%) |
| BMI (kg/m2) | Mean-29.66 (±4.99), Median 32.5 (24−41) |
| Comorbidities | 10(57.1%) |
| Interval between symptoms onset to sequential therapy (days) | Mean = 16.93 (±9.40), Median 9 (6−21) |
| Treatment | |
| Anti-viral agent | HCQ -35.7%, Favipiravir- 42.9%, Lopinavir/ritonavir-71% |
| Outcomes | |
| Day 7 Mortality Day 28 mortality |
3 (21.4%) 4 (28.6%) |
| Complications (Hypotension) | 3 (21.4%) |
| Interval between sequential therapy and negative RT-PCR for SARS-CoV-2 (days) | Mean-10.33 (±5.20), Median 8 (3−18) |
| Interval between sequential therapy and removal of IMV (days) | Mean -18.63 (±17.85), Median 6 (2−46) |
| ICU-Length of stay (days) | Mean = 26.43 (±17.77), Median 12 (5−42) |
| IMV-Length of stay (days) | Mean = 28.86 (±18.45), Median 6.5 (5−36) |
| Hospital- Length of stay (days) | Mean = 35.64 (±16.98), Median 18 (12−47) |
| Variables | Before therapy, Mean ± SD | 7 days after therapy Mean ± SD | p value |
|---|---|---|---|
| Body temperature (ºC) | 37.24 ± 0.92 | 37.16 ± 0.77 | 0.81 |
| PaO2/Fio2 ratio (mm of Hg) | 138.89 ± 41.90 | 224.78 ± 136.35 | 0.08 |
| Ferritin (ng/mL) | 1416.25 ± 1150.62 | 1051.42 ± 740.96 | 0.22 |
| D-dimer (μg/mL) | 4.20 ± 5.46 | 4.21 ± 5.93 | 0.51 |
| LDH (U/L) | 462.73 ± 178.66 | 402.45 ± 149.05 | 0.23 |
| CRP (μg/mL) | 86.74 ± 79.86 | 30.56 ± 30.73 | 0.03 |
| Lymphocyte count (x109/L) | 0.70 ± 0.54 | 1.04 ± 0.49 | 0.27 |
p value less than 0.05 is significant.
BMI- body mass index, CRP- C-reactive protein, HCQ-hydroxychloroquine, ICU-intensive care unit, IMV-Invasive mechanical ventilation, LDH- lactate dehydrogenase, RT-PCR-reverse transcriptase polymerase chain reaction, SARS-CoV-2-severe acute respiratory syndrome coronavirus 2, SD-standard deviation.
The host response has a significant role to play in the development of severe COVID-19 (Vabre et al., 2020, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). The innate immune system serves the first line of defense against the invading viral pathogen. Single-stranded RNA viruses like SARS-CoV-2 through pattern recognition receptors (PRR) may trigger a downstream cascade of excessive cytokine release [like tumor necrosis factor (TNF)-α, Interleukin (IL)-1, IL-6, IL-18] and hyperinflammation (Vabre et al., 2020). Immunomodulators like steroids or tocilizumab have been used to control this cytokine storm with variable success (Focosi et al., 2020, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). TPE is postulated to have a role in cytokine storm through cytokine clearance and establishing homeostasis (Khamis et al., 2020, Shi et al., 2020, Zhang et al., 2020). FFP as a replacement fluid for TPE may help in modulating the circulating inflammatory cytokines and hypercoagulable state by replacing the ADAMTS13 enzyme (Focosi et al., 2020). Khamis et al. (2020) in a case series of 31 patients with moderate to severe COVID-19 found that use of TPE was associated with significantly lower day 14 and day 28 mortality.
The prolonged replication and shedding of SARS-CoV-2 virus has been demonstrated in critically ill patients with COVID-19 (Vabre et al., 2020, Focosi et al., 2020). CP from recovered donors can help in inactivation of the virus by neutralizing antibodies (Focosi et al., 2020). The randomized controlled trials (RCTs) using CP in severe COVID-19 showed variable success (Li et al., 2020, Liu et al., 2020). Liu et al. (2020) in a retrospective RCT found that use of CP was associated with reduced oxygen requirement and mortality benefit in non-intubated patients [Hazard ratio (HR) = 0.19]. In another prospective RCT, which was stopped prematurely, day 28 mortality was significantly lower in patients with severe COVID-19, but not for life-threatening disease (Li et al., 2020).
The sequential use of TPE and CP as a therapeutic option in severe COVID-19 by removal of inflammatory cytokines followed by transfusion of neutralizing antibodies had never been explored (Kesici et al., 2020, Focosi et al., 2020).
We used sequential TPE and CP early in the disease course with a median of 9 (6−21) days from symptoms onset. The day 28 mortality in these patients was only 28.6%. Armstrong et al. (2020) in systematic meta-analysis reported combined ICU mortality rate (95% CI) in COVID-19 patients of 41.6% (34.0–49.7%). The mortality in patients requiring IMV was reported at 59% in another review of epidemiological studies in COVID-19 (Tzotzos et al., 2020).
Three patients developed transient hypotension (systolic blood pressure less than 90 mm Hg) which responded to crystalloid fluid boluses. None of the patients developed transfusion-associated complications (like hemolytic transfusion reactions, transfusion-related acute lung injury, or fluid overload). Three patients developed secondary bacterial sepsis, all ventilator associated pneumonia, within 14 days of therapy, and one patient died.
This study has few limitations. Firstly, the number of patients was small with no case-control. Secondly, there may be confounding effects of other therapies like corticosteroids besides TPE and CP transfusion. Finally, the effect of therapy in preventing the need of IMV could not be demonstrated as all patients were already on IMV.
Conclusion
This case series demonstrated the sequential use of TPE followed by CP transfusion as a therapeutic option in critical COVID-19 patients with ARDS. We propose RCTs to further explore this sequential treatment in severe to critical COVID-19.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding sources
None declared.
Ethics approval
This study was conducted after approval from Dubai Scientific and Research Ethics Committee (DSREC-04/2020_7).
References
- Armstrong R.A., Kane A.D., Cook T.M. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- EUA Authorized Serology Test Performance | FDA. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance [Accessed 20 October 2020].
- Focosi D., Anderson A.O., Tang J.W., Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin Microbiol Rev. 2020;33:e00072–20. doi: 10.1128/CMR.00072-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesici S., Yavuz S., Bayrakci B. Get rid of the bad first: therapeutic plasma exchange with convalescent plasma for severe COVID-19. Proc Natl Acad Sci U S A. 2020;117:12526–12527. doi: 10.1073/pnas.2006691117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis F., Al-Zakwani I., Al Hashmi S. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Lin H., Baine M. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;10 doi: 10.1038/s41591-020-1088-9. 1038/s41591-020-1088-1089. [DOI] [PubMed] [Google Scholar]
- Shi H., Zhou C., He P. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int J Antimicrob Agents. 2020;56:105974. doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabre N., Britton G.J., Gruber C. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne J.A.C., Murthy S., Diaz J.V. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020 doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhai H., Ma S., Chen J., Gao Y. Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br J Haematol. 2020 doi: 10.1111/bjh.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]