Abstract
Objective
To compare the therapeutic effects of one-time root canal treatment versus two-time treatment in patients with irreversible pulpitis.
Methods
We classified 130 patients into a one-time treatment group (group A; n = 68) and a two-time treatment group (group B; n = 62) according to the number of patient visits. Clinical efficacy at 6 months and 1 year follow-ups as well as serum levels of interleukin-6, tumor necrosis factor alpha, and high-sensitivity C-reactive protein before and 1 week after endodontic treatment were observed. Additionally, the level of treatment satisfaction between the two groups was compared, and the degree of pain was evaluated using the visual analogue scale.
Result
One week after treatment, serum interleukin-6, tumor necrosis factor alpha, and high-sensitivity C-reactive protein levels were significantly decreased in the two groups; however, levels in group B were significantly lower than those in group A. Additionally, visual analogue scale scores of patients in group A were significantly higher than those of patients in group B.
Conclusion
One-time root canal therapy can effectively improve postoperative pain and the expression of inflammatory factors in the serum of patients undergoing treatment.
Keywords: One-time root canal therapy, pulpitis, IL-6, TNF-α, hs-CRP, visual analog pain scale
Introduction
Pulpitis is the most common inflammatory disease in clinical stomatology.1 In the study by Oliveira et al.,2 more than 57% of the 1,346 patients who received treatment at specialist endodontic clinics at the Federal University of Pernambuco between 2003 and 2010 were diagnosed with endodontic disease. This high incidence rate leads to a serious impact on patients’ quality of life. Pulpitis can be classified as acute or chronic.3 Chronic pulpitis refers to chronic lesions that present in the patient's pulp tissue. Here, the clinical symptoms of affected patients are not obvious, and the patient suffers from tooth pain following the application of external stimuli. Acute pulpitis is caused by acute inflammation in the pulp tissues, mainly induced by deep caries, but can also be brought on by chronic pulpitis. Both acute and chronic pulpitis are caused by bacterial infections, and patients almost uniformly report severe pain at disease onset.4,5 Clinically, general analgesic drugs are not effective for pain management. Furthermore, pulp gangrene can easily occur when effective treatment is not applied in a timely manner, further increasing the severity of pain in affected patients.6
Currently, the clinical treatment of pulpitis primarily involves root canal therapy,7 where infectious regions are cleared by mechanical or chemical means. After disinfection, the root canal is sealed, thereby blocking the connection to the periapical tissues to prevent further disease progression.8 Conventional root canal treatment requires multiple therapeutic applications to completely eradicate the occurrence of bacterial infections and can take a long time.9 Recently, one-time root canal therapy for pulpitis treatment has gradually attracted the attention of clinicians. One-time root canal therapy removes the step of sealing the root canal and reduces the number of required visits, saving both patients’ and doctors’ time. Moreover, one-time root canal therapy can reduce repeated infections caused by drug microleakage into patients during root canal sealing.10 In the study conducted by Xavier et al.,11 it was found that multiple root canal therapy and one-time root canal therapy could both reduce but not completely eliminate bacteria and endotoxins in the root canals of patients. Thus, it remains disputable whether one-time or multiple root canal therapy should be used to treat pulpitis in clinical settings.
In this study, the therapeutic effects of one-time and multiple root canal therapy for irreversible pulpitis were assessed, ultimately offering a reference on the subject for clinicians.
Materials and methods
Clinical data
This retrospective analysis was performed on 130 pulpitis patients who were divided into a one-time treatment group (group A; n = 68) and a two-time treatment group (group B; n = 62) according to the number of visits. Patients older than 18 years, those without malignant tumors, and those who had not previously undergone root canal treatment and/or related endodontic treatment were included in this study. Conversely, patients with incomplete clinical data, those with more than one affected tooth, those who did not complete treatment, and those with other periodontal lesions were excluded. All patients voluntarily signed a consent form, and the study protocols were reviewed by the ethical oversight committee of Stomatological Hospital of Southern Medical University.
Main reagents
ELISA kits targeting interleukin (IL)-6, tumor necrosis factor alpha (TNF-α) (PI330 and PT518, respectively; Beyotime Biotechnology, Shanghai, China), and high-sensitivity C-reactive protein (hs-CRP) (tw035331; Shanghai Tongwei Bio, Shanghai, China) were used to measure serum concentrations of these markers before and after treatment. Dental supplies including K-file, PROTAPER, DENTSPLY X-Smart Endo motor, and dental root canal obturating points were purchased from Dentsply (Tianjin, China; YZN-005-K256,QBB-005-ZJA0413, QBB-005-CJ, and YCY-005-YJJ999.244.015.06.N, respectively).
Treatment programs
Patients in both groups were treated with root canals. Patients in the two groups underwent routine X-ray imaging prior to treatment to analyze the number of root canals requiring endodontic treatment. In each patient, the shape of the root tip and the length of the root canal were observed. Patients were anesthetized using articaine epinephrine. Next, a rubber dam was placed, the pith was opened to remove the coronal pith, and the radicular pulp was extracted. The root canal was prepared using a ProTaper motorized nickel–titanium root canal expansion system. Each root canal was flushed with 5.25% sodium hypochlorite for each sputum replacement that occurred during the preparation process. For patients in group A, root canals were pre-sealed with calcium hydroxide and zinc hydroxide and filled again 1 week after treatment. For patients in group B, the trial point test was performed after root canal preparation. When the correct fill-in was identified, the gutta-percha point was removed, and repeated rinsing of the root canal was performed to achieve disinfection with subsequent drying. After each patient's root canal was dried, a cement base and AH Plus paste for lateral compaction filling were applied.
Expression of IL-6, TNF-α, and hs-CRP in the serum of patients
Both previous to and at 1 week after endodontic treatment, 5 mL of venous blood was acquired and centrifuged at 111.8 ×g for 10 minutes, and then serum was collected for subsequent experiments. ELISA detection was conducted according to the manufacturer’s instructions as follows: the collected serum was separately added to 50 µL of various concentrations of standard solution in blank microwells, and 50 µL of antibody was added to 50 µL of distilled water in the blank control wells. Additionally, 40 µL of the sample was added to the remaining microwells and 10 µL of biotin-labeled antibody was added thereafter. Subsequently, the plate was incubated at 37°C for 30 minutes. When washing the plate, the washing liquid in each well was guaranteed to be full without overflowing and left to stand for 30 seconds before being discarded. Next, the plate was patted dry five times. Next, 50 µL of the enzyme standard solution was added to each well, and the plate was incubated at 37°C for 1 hour. The plate was then washed five times and thoroughly patted dry with absorbent paper after the final wash. Horseradish peroxidase (100 µL/well) was then added to the sealing plate and incubated at 37°C for 15 minutes in the dark. Next, 100 µL/well of the chromogenic substrate 3,3′,5,5′-Tetramethylbenzidine was added, and the plate was incubated for 20 minutes at room temperature in the dark. Finally, 50 µL/well of the stop solution was added, and detection was performed using a microplate reader within 15 minutes. The maximum absorption wavelength at 450 nm was determined using an enzyme marker within 15 minutes.
Observation indices
The main observation index was the clinical efficacy of root canals as observed at 6 months and 1 year after treatment. These efficacy findings are summarized in Table 1. Changes in the serum levels of IL-6, TNF-α, and hs-CRP before and after endodontic treatment were also observed.
Table 1.
Evaluation of clinical efficacy in patients.
| Classification | Standard |
|---|---|
| Success | Patients had no symptoms or abnormal signs, normal occlusal force, complete occlusal relationship, partial sealing of the root canal filling, and disappearance of periapical lesions. |
| Failure | Patients had no symptoms or abnormal signs. The occlusal lesions were slightly uncomfortable or could not be chewed on normally. X-ray revealed that there was no abnormal projection area before periodontal ligament widening. |
The secondary observation index was the satisfaction with treatment in the two groups, which was calculated as follows: satisfaction = (number of very satisfied patients + number of satisfied patients)/total number of patients × 100%. The visual analogue scale (VAS) was used to evaluate the degree of pain at 1 week after endodontic treatment.
Statistical methods
In this study, data were statistically analyzed using SPSS v20.0 software (IBM Corp., Armonk, NY, USA) and visualized using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). Measurable data are reported as the usage rate (%) using the χ2 test. Measurement data are expressed as mean ± standard deviation. An independent-samples t-test was used to analyze differences between the two groups. Changes before and after treatment were analyzed using a paired t-test, which was indicated by t. P-values < 0.05 were used to indicate statistically significant differences.
Results
Clinical data
A total of 68 patients were included in group A, including 34 men and 34 women, with an age range of 22 to 65 years and an average age of 39.5 ± 4.9 years. With respect to the affected teeth, eight cases involved anterior teeth, 20 involved premolars, and 40 involved molars. Additionally, 26 cases presented with living pulp and 42 presented with dead pulp. Furthermore, 12 patients had hypertension, seven had heart disease, and 13 had diabetes. Fifty-five patients lived in cities and 13 lived in villages. There were 40 patients with a smoking history and five with a history of alcohol abuse.
Group B comprised 62 patients, including 35 men and 27 women, with an age range of 25 to 68 years and an average age of 41.2 ± 5.1 years. The position of the affected teeth included the anterior teeth in 12 cases, premolars in 18 cases, and molars in 32 cases. Twenty-seven cases presented with living pulp and 35 cases presented with dead pulp. Eight patients had a history of hypertension, 12 had heart disease, and 16 had diabetes. Fifty patients lived in cities and 12 lived in villages, and there were 35 individuals with a history of smoking and three with a history of alcohol abuse. Clinical data of the two groups were not statistically different (Table 2).
Table 2.
Clinical data of the two patient groups [n (%)].
| Factor | Group A (n = 68) | Group B (n = 62) | χ2/t value | P value |
|---|---|---|---|---|
| Sex | 0.542 | 0.462 | ||
| Male | 34 (50.00) | 35 (56.45) | ||
| Female | 34 (50.00) | 27 (43.55) | ||
| Age (years) | 39.5 ± 4.9 | 41.2 ± 5.1 | 1.938 | 0.055 |
| BMI (kg/m2) | 23.44 ± 1.84 | 23.15 ± 2.01 | 0.859 | 0.392 |
| Tooth position | 1.520 | 0.468 | ||
| Front teeth | 8 (11.77) | 12 (19.35) | ||
| Dentes premolares | 20 (29.41) | 18 (29.04) | ||
| Grinding of one's teeth in sleep | 40 (58.82) | 32 (51.61) | ||
| Pulp condition | 0.379 | 0.538 | ||
| Vital pulp | 26 (38.24) | 27 (43.55) | ||
| Dead pulp | 42 (61.76) | 35 (56.45) | ||
| History of hypertension | 0.560 | 0.454 | ||
| Yes | 12 (17.65) | 8 (12.90) | ||
| No | 56 (82.35) | 54 (87.10) | ||
| Heart disease history | 2.134 | 0.144 | ||
| Yes | 7 (10.29) | 12 (19.35) | ||
| No | 61 (89.71) | 50 (80.65) | ||
| Diabetes history | 0.837 | 0.360 | ||
| Yes | 13 (19.12) | 16 (25.81) | ||
| No | 55 (80.88) | 46 (74.19) | ||
| Domicile | 0.001 | 0.973 | ||
| City | 55 (80.88) | 50 (80.65) | ||
| Village | 13 (19.12) | 12 (19.35) | ||
| Smoking history | 0.075 | 0.785 | ||
| Yes | 40 (58.82) | 35 (56.45) | ||
| No | 28 (41.18) | 27 (43.55) | ||
| History of alcoholism | 0.355 | 0.551 | ||
| Yes | 5 (7.35) | 3 (4.84) | ||
| No | 63 (92.65) | 59 (95.16) |
Note: BMI, Body mass index.
Comparison of clinical efficacy between the two groups
All patients underwent X-ray imaging 2 years after surgery. Success was indicated when X-ray images revealed that the dark area in the bone around the root had disappeared and when the patient reported no discomfort and presented with a normal bite. A response was indicated when X-ray images revealed that the dark area in the bone around the root was reduced and the patient was asymptomatic, with a normal bite. Conversely, failure was indicated when X-ray images revealed that the dark area in the bone around the root had increased in size and the patient demonstrated poor occlusion accompanied by pain and discomfort.
Next, the clinical efficacy of root canal therapy was compared between the two groups. In group A, 64 and four patients demonstrated successful and failed treatment, respectively, at 6 months. In group B, 56 and six patients demonstrated successful and failed treatment, respectively, at 6 months. Thus, the success rates of treatment in the two groups were 94.12% and 90.32%, respectively. Statistical analysis revealed no significant difference in the success rate between the two groups at 6 months. Additionally, the clinical efficacy of the two groups was compared at 1 year after treatment: in group A, 63 patients presented success and five presented failure, while in group B, 53 patients presented success and 9 presented failure. The success rates of treatment in the two groups were 92.65% and 85.48% at 1 year post-treatment, respectively. Statistical analysis revealed no significant difference in treatment success rates between the two groups at 1 year (Tables 3 and 4).
Table 3.
Comparison of clinical efficacy between the two groups at 6 months post-treatment [n (%)].
| Group | Success | Failure | χ2 value | P value |
|---|---|---|---|---|
| Group A (n = 68) | 64 (94.12) | 4 (5.88) | 0.658 | 0.417 |
| Group B (n = 62) | 56 (90.32) | 6 (9.68) |
Table 4.
Comparison of clinical efficacy between the two groups at 1 year post-treatment [n (%)].
| Group | Success | Failure | χ2 value | P value |
|---|---|---|---|---|
| Group A (n = 68) | 63 (92.65) | 5 (7.35) | 1.732 | 0.188 |
| Group B (n = 62) | 53 (85.48) | 9 (14.52) |
Serum IL-6, TNF-α, and hs-CRP levels in the two groups before and after treatment
Serum levels of IL-6, TNF-α, and hs-CRP in the two groups before treatment were compared, and no statistical difference was found. At 1 week after treatment, the levels of IL-6, TNF-α, and hs-CRP in the two groups were significantly decreased compared with pre-treatment levels, and there was a statistical difference between groups A and B. Specifically, the IL-6, TNF-α, and hs-CRP levels in group A at 1 week after treatment were significantly higher than those in group B. Furthermore, the changes in IL-6, TNF-α, and hs-CRP levels in group B before and after treatment were more significant than those in group A. There was a statistical difference in this regard between the two groups (P < 0.05) (Tables 5 and 6 and Figures 1 and 2).
Table 5.
Serum IL-6, TNF-α, and hs-CRP levels before and after treatment in the two groups.
| Group |
IL-6 (pg/mL) |
TNF-α (mg/g) |
hs-CRP (µg/L) |
|||
|---|---|---|---|---|---|---|
| Pretherapy | 1 week after treatment | Pretherapy | 1 week after treatment | Pretherapy | 1 week after treatment | |
| Group A (n = 68) | 146.05 ± 12.05 | 55.79 ± 8.28 | 6.37 ± 2.67 | 5.19 ± 1.98 | 2.26 ± 0.46 | 1.76 ± 0.27 |
| Group B (n = 62) | 146.63 ± 13.84 | 30.38 ± 5.01 | 6.28 ± 1.29 | 3.07 ± 0.94 | 2.32 ± 0.43 | 1.22 ± 0.10 |
| t value | 0.255 | 20.920 | 0.241 | 7.677 | 0.766 | 14.843 |
| P value | 0.799 | < 0.001 | 0.810 | < 0.001 | 0.445 | < 0.001 |
Note: IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; hs-CRP, high-sensitivity C-reactive protein.
Figure 1.
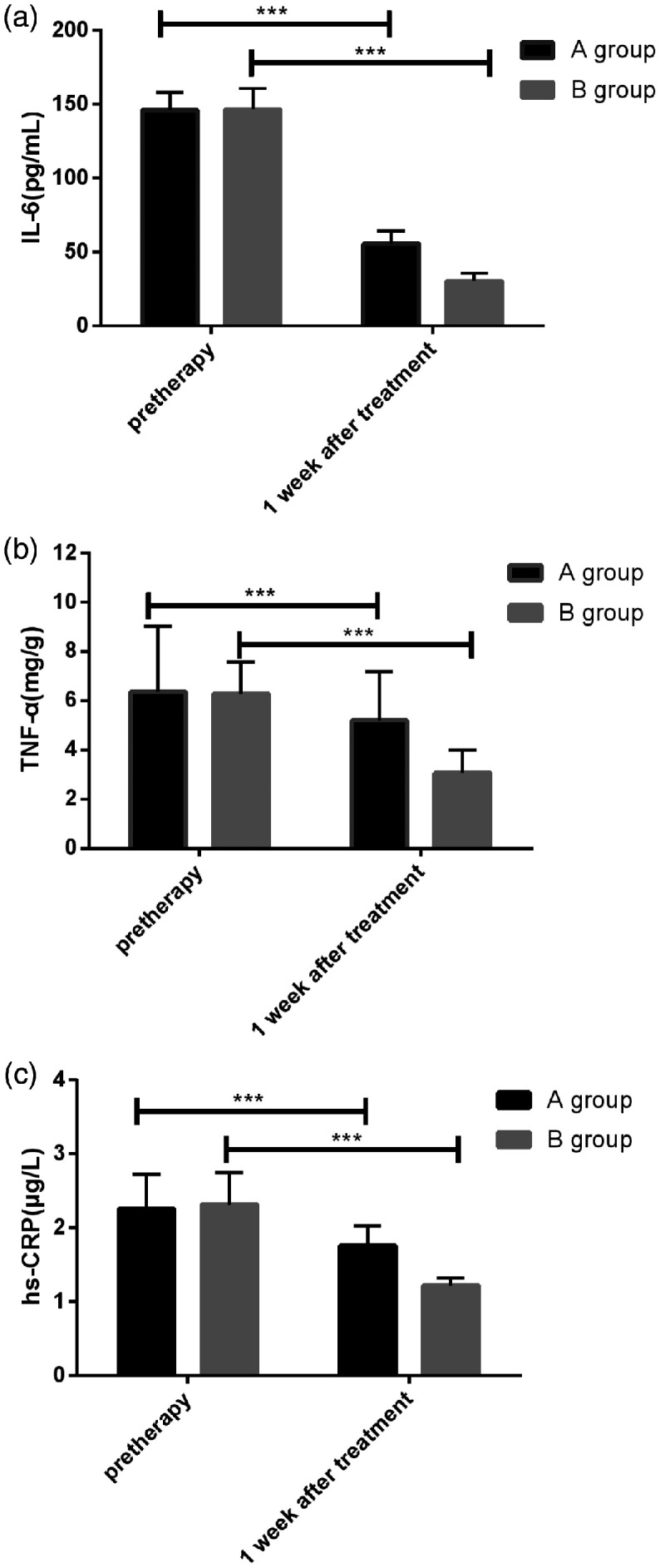
Serum IL-6, TNF-α, and hs-CRP levels before and after treatment in the two groups. (a) Comparison of serum IL-6 levels in patients of the two groups before treatment showed no statistical difference (P < 0.05), whereas IL-6 levels in patients of the two groups were significantly reduced at 1 week after treatment (P < 0.05). IL-6, interleukin-6. (b) Comparison of serum TNF-α levels in patients of the two groups before treatment showed no statistical difference (P < 0.05), whereas TNF-α levels in patients of the two groups were significantly reduced at 1 week after treatment (P < 0.05). TNF-α, tumor necrosis factor alpha. (c) Comparison of serum hs-CRP levels in patients of the two groups before treatment showed no statistical difference (P < 0.05), whereas hs-CRP levels in patients of the two groups were significantly reduced at 1 week after treatment (P < 0.05). * indicates differences between the two groups (***P < 0.001). hs-CRP, high-sensitivity C-reactive protein.
Table 6.
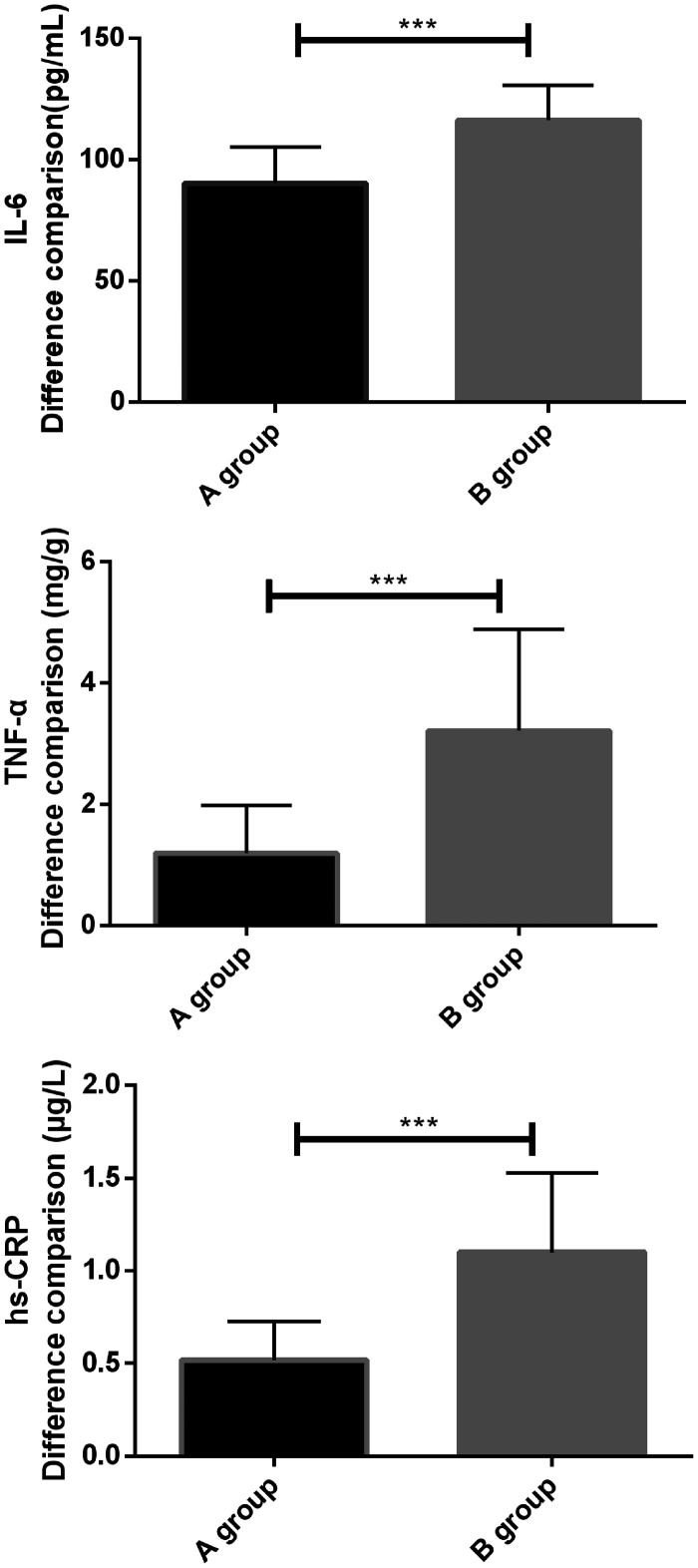
Comparison of the difference between IL-6, TNF-α, and hs-CRP levels during treatment between the two groups.
| Group | IL-6 (pg/mL) | TNF-α (mg/g) | hs-CRP (µg/L) |
|---|---|---|---|
| Group A (n = 68) | 90.26 ± 14.90 | 1.19 ± 0.79 | 0.52 ± 0.21 |
| Group B (n = 62) | 116.25 ± 14.59 | 3.21 ± 1.68 | 1.10 ± 0.43 |
| t value | 10.032 | 8.897 | 9.905 |
| P value | < 0.001 | < 0.001 | < 0.001 |
Note: IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; hs-CRP, high-sensitivity C-reactive protein.
Figure 2.
Comparison of differences in serum IL-6, TNF-α, and hs-CRP levels before and after treatment in the two groups. (a) By comparing the difference in IL-6 levels between the two groups before and after treatment, the difference in group B was found to be significantly higher than that of group A (P < 0.05). IL-6, interleukin-6. (b) By comparing the difference in TNF-α levels between the two groups before and after treatment, the difference in group B was found to be significantly higher than that of group A (P < 0.05). TNF-α, tumor necrosis factor alpha. (c) By comparing the difference in hs-CRP levels between the two groups before and after treatment, the difference in group B was found to be significantly higher than that of group A (P < 0.05). *indicates differences between the two groups (***P < 0.001). hs-CRP, high-sensitivity C-reactive protein.
Comparison of VAS scores between the two groups
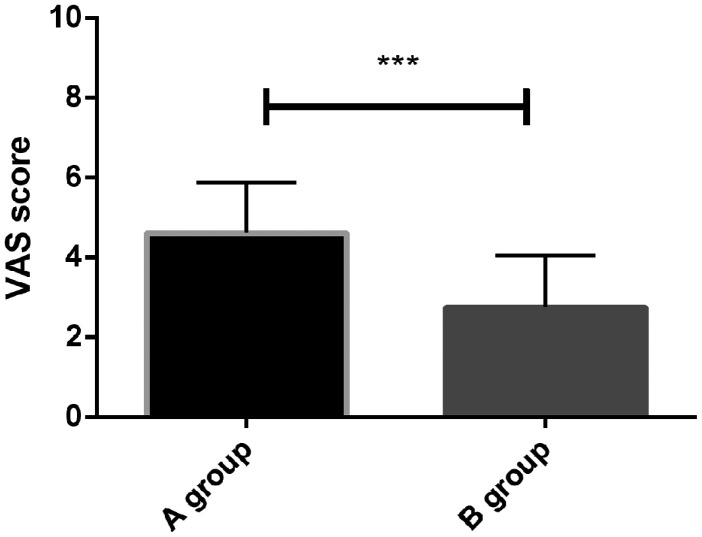
VAS scores of the two groups at 1 week post-treatment were compared. The VAS scores of patients in group A (4.62 ± 1.25 points) were higher than those in group B (2.75 ± 1.30 points), representing a significant difference between the two groups (t = 8.359; P = 0.001) (Figure 3).
Figure 3.
Comparison of VAS scores between the two treatment groups.
The VAS scores of patients in group A were significantly higher than those of patients in group B at 1 week after endodontic treatment (P < 0.05). *indicates differences between the two groups (***P < 0.001). VAS, visual analogue scale.
Patient satisfaction survey
We conducted a survey of patient satisfaction and found that in group A, 38 patients were very satisfied, 25 were satisfied, and five were generally satisfied, while in group B, 33 patients were very satisfied, 23 were satisfied, and six were generally satisfied. There was no significant difference in patient satisfaction between the two groups (χ2 = 0.226; P = 0.634).
Discussion
Pulpitis is one of the most common diseases in clinical stomatology. In a study by Prirokh et al.,12 pulpitis was found to occur most commonly in individuals over 45-years-old. Another investigation indicated that the proportion of patients with pulp and periapical periodontitis was up to 41%, based on data of 215,073 dental emergency patients.13 Clinical manifestations of pulpitis include redness, swelling, bleeding, and abnormal gingival color. Depending on the nature of the disease, it can be diagnosed as either acute pulpitis or chronic pulpitis. Chronic pulpitis develops gradually. Under normal circumstances, patients will not feel pain but may experience different degrees of pain if receiving external stimulation. In contrast, acute pulpitis develops rapidly. In this context, severe tooth pain can occur when the patient is not exposed to external stimuli and may cause pain in the head and face, which may lead to challenges during diagnosis and treatment.14,15
Currently, the clinical management of pulpitis primarily involves root canal therapy. The main purpose of this approach is to thoroughly clean the infected dental pulp while at the same time cleaning the dentin and removing toxic decomposition products of the teeth. The infected teeth are rinsed, disinfected, and any spaces are filled in through the root canal to effectively prevent further bacterial invasion.16 Conventionally, clinical root canal therapy has primarily been performed two or more times. Patients undergoing treatment are required to make multiple visits, which is inconvenient and may increase the likelihood of infection (due to the multiple treatments).17 With the development of medical standards, one-time root canal therapy has been gradually recognized as a clinical option, offering the benefits of short treatment time and low incidence of infection due to not undergoing multiple treatments.18 However, there are some controversies regarding use of the two-treatment methods in the clinic. Therefore, this study sought to provide a reference for clinicians by comparing the clinical efficacy of one-time root canal versus multiple root canal treatments.
In this study, we compared the clinical efficacy of two groups of patients receiving either a single root canal treatment or multiple root canal treatments. It was found that the success rates of the two groups at 6 months after treatment were 94.12% and 90.32%, respectively, while the success rates of the two groups at 1 year after treatment were 92.65% and 85.48%, respectively. No significant differences were found when we compared success rates between the two groups at 6 and 12 months. In a study by Fu et al.,18 450 affected teeth were randomly divided into two groups: multiple root canal therapy and single root canal therapy. They found no difference in the total effective rate of the two groups after 1 week of treatment. Additionally, the total effective rates of patients after 1 year of treatment were 99.11% (one-time treatment group) and 99.56% (multiple treatment group). Furthermore, no difference was found in the total efficacy between the two groups, which is consistent with the results of our study. However, the 1-year total response rate was significantly higher than what is reported in this study, which may have been caused by the small sample size in our study. With the continuous improvements in medical technology and equipment over recent years, an increasing number of studies have shown that the long-term therapeutic effects of root canal therapy are independent of the number of root canal procedures performed.19 In a study by Paredes-Vieyra et al.,20 the 2-year total efficacy of one-time root canal therapy and two-time root canal therapy were analyzed, and no significant difference between the two treatments was found.
The principle disputes surrounding the two methods in clinical treatment are postoperative pain and inflammatory response.21,22 Therefore, we evaluated postoperative pain in both groups. In this study, we used VAS scores to assess pain in patients. VAS is considered an effective and reliable scale for measuring the intensity of pain and discomfort in patients.23 The results showed that the VAS scores of patients in group A were significantly higher than those in group B at 1 week after endodontic treatment, indicating that one-time root canal therapy can effectively alleviate postoperative pain. Next, we measured serum levels of IL-6, TNF-α, and hs-CRP. As an important inflammatory factor, IL-6 has important clinical significance and is involved in multiple inflammatory and immune responses. TNF-α is involved in the acute inflammatory response mechanism in humans; it increases the viscosity of vascular endothelial cells by increasing the number of monocytes, macrophages, and neutrophils, thereby improving vascular permeability and accelerating the migration of white blood cells to kill microorganisms. hs-CRP is the most sensitive marker for the direct reaction of inflammatory responses.24–26 Our results showed that serum levels of IL-6, TNF-α, and hs-CRP in the two groups were decreased after treatment compared with levels before treatment. By comparing the differences of each index before and after treatment between the two groups, we found that the differences of each index before and after treatment in group A were significantly lower than those in group B. This revealed that one-time root canal therapy can effectively reduce patients’ inflammatory response. We speculated that the main reason for this is that one-time root canal therapy requires a short operation time to avoid infection during the procedure. Additionally, AH Plus paste can release a low concentration of formaldehyde, which has a certain antibacterial effect and accelerates patient recovery after endodontic treatment.27 However, patients who undergo two-time root canal therapy need to be treated again at 1 week after initial endodontic treatment. This increases the risk of secondary infection, resulting in significant changes in the expression of inflammatory factors.28 At the end of the study, patient satisfaction after treatment was statistically analyzed, and no significant difference was found between the two groups, indicating that both treatments ultimately had good effects on patient outcomes.
Finally, this study found that there was no significant difference in the long-term clinical efficacy between one-time root canal therapy and two-time root canal therapy. Moreover, in terms of the increased levels of inflammatory factors in patients’ serum, one-time root canal treatment was found to be markedly superior to the two-time root canal treatment, with the former also significantly improving patients’ pain status. However, this study has certain limitations that should be noted. First, the study population in this investigation was small, and it is unclear whether there was bias in any of the statistics. Second, as a retrospective study, this study was prone to recall bias, so we hope to increase the number of samples in future studies and conduct clinical randomized controlled trials to verify the results of this study.
In summary, one-time root canal therapy can effectively improve postoperative pain and the expression of inflammatory factors in the serum of patients. In addition, no significant difference was found in terms of clinical efficacy when one-time root canal therapy was compared with two-time root canal therapy, representing a finding that can be widely promoted in clinical practice.
Acknowledgements
None
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Guangdong Medical Research Foundation (A2018339), and Stomatological Hospital of Southern Medical University Research Training Program (PY2017017).
References
- 1.Mohammadi Z, Abbott PV, Shalavi S, et al. Postoperative pain following treatment of teeth with irreversible pulpitis: a review. N Y State Dent J 2017; 83: 44–53. [PubMed] [Google Scholar]
- 2.de Oliveira BP, Câmara AC, Aguiar CM. Prevalence of endodontic diseases: an epidemiological evaluation in a Brazilian subpopulation. Braz J Oral Sci 2016; 15:119–123. [Google Scholar]
- 3.Liu J, Shi C. Comparative study of pain releasing effect of two approaches to acute pulpitis in the night emergency. Shanghai Kou Qiang Yi Xue 2017; 26: 669–671. [PubMed] [Google Scholar]
- 4.Karthikeson P, Gayathri R, Priya VV. Evaluation of salivary total proteins, albumin, globulin, and A/G ratio among healthy individuals and patients with chronic pulpitis. Drug Invent Today 2018; 10: 966–968. [Google Scholar]
- 5.Nelson-Filho P, Ruviére DB, de Queiroz AM, et al. Comparative molecular analysis of gram-negative bacteria in primary teeth with irreversible pulpitis or periapical pathology. Pediatr Dent 2018; 40: 259–264. [PubMed] [Google Scholar]
- 6.Rôças IN, Lima KC, Assunção IV, et al. Advanced caries microbiota in teeth with irreversible pulpitis. J Endod 2015; 41: 1450–1455. [DOI] [PubMed] [Google Scholar]
- 7.Suneelkumar C, Subha A, Gogala D. Effect of preoperative corticosteroids in patients with symptomatic pulpitis on postoperative pain after single-visit root canal treatment: a systematic review and meta-analysis. J Endod 2018; 44: 1347–1354. [DOI] [PubMed] [Google Scholar]
- 8.Pintor AVB, dos Santos MRM, Ferreira DM, et al. Does smear layer removal influence root canal therapy outcome? A systematic review. J Clin Pediatr Dent 2016; 40: 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Parirokh M, Sadr S, Nakhaee N, et al. Comparison between prescription of regular or on-demand ibuprofen on postoperative pain after single-visit root canal treatment of teeth with irreversible pulpitis. J Endod 2014; 40: 151–154. [DOI] [PubMed] [Google Scholar]
- 10.Rao KN, Kandaswamy R, Umashetty G, et al. Post-Obturation pain following one-visit and two-visit root canal treatment in necrotic anterior teeth. J Int Oral Health 2014; 6: 28. [PMC free article] [PubMed] [Google Scholar]
- 11.Xavier ACC, Martinho FC, Chung A, et al. One-visit versus two-visit root canal treatment: effectiveness in the removal of endotoxins and cultivable bacteria. J Endod 2013; 39: 959–964. [DOI] [PubMed] [Google Scholar]
- 12.Parirokh M, Yosefi MH, Nakhaee N, et al. Effect of bupivacaine on postoperative pain for inferior alveolar nerve block anesthesia after single-visit root canal treatment in teeth with irreversible pulpitis. J Endod 2012; 38: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 13.Allareddy V, Nalliah RP, Haque M, et al. Hospital-based emergency department visits with dental conditions among children in the United States: nationwide epidemiological data. Pediatr Dent 2014; 36: 393–399. [PubMed] [Google Scholar]
- 14.Li JG, Lin JJ, Wang ZL, et al. Melatonin attenuates inflammation of acute pulpitis subjected to dental pulp injury. Am J Transl Res 2015; 7: 66–78. [PMC free article] [PubMed] [Google Scholar]
- 15.Giuroiu CL, Cäruntu ID, Lozneanu L, et al. Dental pulp: correspondences and contradictions between clinical and histological diagnosis. Biomed Res Int 2015; 2015: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Garg A. Incidence of post-operative pain after single visit and multiple visit root canal treatment: a randomized controlled trial. J Conserv Dent 2012; 15: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zandi H, Rodrigues RC, Kristoffersen AK, et al. Antibacterial effectiveness of 2 root canal irrigants in root-filled teeth with infection: a randomized clinical trial. J Endod 2016; 42: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 18.Gaozhong F, Chen S. Comparison of effect of one-time root canal therapy and routine root canal therapy applied in acute pulpitis treatment. Chinese J Prim Med Pharm 2015; 19: 2973–2976. [Google Scholar]
- 19.Kvist T, Molander A, Dahlén G, et al. Microbiological evaluation of one-and two-visit endodontic treatment of teeth with apical periodontitis: a randomized, clinical trial. J Endod 2004; 30: 572–576. [DOI] [PubMed] [Google Scholar]
- 20.Paredes-Vieyra J, Enriquez FJJ. Success rate of single-versus two-visit root canal treatment of teeth with apical periodontitis: a randomized controlled trial. J Endod 2012; 38: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 21.Rechenberg DK, Galicia JC, Peters OA. Biological markers for pulpal inflammation: a systematic review. PLoS One 2016; 11: e0167289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alí A, Olivieri JG, Duran-Sindreu F, et al. Influence of preoperative pain intensity on postoperative pain after root canal treatment: a prospective clinical study. J Dent 2016; 45: 39–42. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Chen Y, Luo Y. Evaluation of the visual analog score (VAS) to assess acute mountain sickness (AMS) in a hypobaric chamber. PLoS One 2014; 9: e113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.María E, Albert Q, Juan H. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 2012; 8: 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramseyer VD, Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol 2013; 304: F1231–F1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doumatey AP, Zhou J, Adeyemo A, et al. High sensitivity C-reactive protein (Hs-CRP) remains highly stable in long-term archived human serum. Clin Biochem 2014; 47: 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezzomo CF, Mariana K, Dapper SP, et al. Influence of radiopaque fillers on physicochemical properties of a model epoxy resin-based root canal sealer. J Appl Oral Sci 2013; 21: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siqueira JF., Jr. Aetiology of root canal treatment failure: why well‐treated teeth can fail. Int Endod J 2010; 34: 1–10. [DOI] [PubMed] [Google Scholar]