Abstract
Objective
Patients with non-ST elevation acute coronary syndrome (NSTE-ACS) benefit from coronary intervention, but the optimal timing for an invasive strategy is not well defined. This study aimed to determine whether an early invasive strategy (<12 hours) is superior to a delayed invasive strategy.
Methods
Twelve studies of nine randomized, controlled trials of 8586 patients were included.
Results
There were no significant differences in all-cause death (risk ratio [95% confidence interval]) (0.90, [0.77–1.06), re-myocardial infarction (re-MI) (0.95 [0.70–1.29]), major bleeding (0.97 [0.77–1.23]), and refractory ischemia (0.74 [0.53–1.05]) when we compared use of early and delayed invasive strategies. Furthermore, analysis of the effect of the chosen strategy on high-risk patients showed that the rate of composite death or re-MI was significantly decreased in patients with either a Global Registry of Acute Coronary Events (GRACE) risk score >140 or with elevated troponin levels (risk ratio 0.82 [0.72–0.92]; risk ratio 0.84 [0.76–0.93], respectively).
Conclusions
This meta-analysis shows that an early angiographic strategy does not improve clinical outcome in patients with NSTE-ACS. An early invasive strategy might reduce the rate of composite death or re-MI in high-risk patients with GRACE risk scores >140 or elevated cardiac markers.
Keywords: Invasive strategy, non-ST elevation acute coronary syndrome, early intervention, coronary angiography, myocardial infarction, composite death
Introduction
Coronary revascularization improves clinical outcomes in patients with non-ST elevation acute coronary syndrome (NSTE-ACS). The American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guidelines suggest that patients with NSTE-ACS should undergo coronary angiography within 2 hours when patients meet a high-risk condition. Coronary angiography should be performed within 24 hours in patients who are initially stabilized, especially in high- and intermediate-high-risk patients.1 However, the optional timing of intervention for NSTE-ACS is controversial.
Several randomized, controlled trials (RCTs) and meta-analyses showed that an early invasive strategy (<24 hours) did not significantly improve the risk of all-cause death or recurrent myocardial infarction (re-MI).2–4 In contrast, one meta-analysis reported that an early invasive strategy improved the clinical outcome.5 In the TIMACS trial,2 coronary interventions were performed either within 24 hours (median time after randomization: 14 hours) or 36 hours after randomization in patients with acute coronary syndrome (ACS). In this trial, the primary outcome (death, MI, or stroke) was similar between patients with early and delayed intervention, but a significant beneficial effect on the secondary endpoint of refractory ischemia (RI) was found in the early intervention group. However, whether this benefit was associated with the intervention time, which was conducted within 14 hours in patents with ACS, is unknown. Furthermore, recently, some investigators examined whether an early (<12 hours) intervention strategy is superior to a delayed invasive strategy in patients with NSTE-ACS. Deharo et al. found that high-risk patients (Global Registry of Acute Coronary Events [GRACE] risk score >140) with non-ST elevation myocardial infarction (NSTEMI) who underwent coronary angiography within 12 hours had a reduced risk of death and re-MI compared with patients who underwent intervention within 12 to 24 or >24 hours.6
To date, there is no definite conclusion regarding coronary angiography within 12 hours versus a delayed invasive strategy for NSTE-ACS. Regardless of admission time, the first 12 hours after admission allows most patients to be scheduled during the day, which could be more reasonable. Therefore, we conducted this meta-analysis to investigate whether coronary angiography performed within 12 hours post-MI improves clinical outcomes in high- to moderate-risk patients with NSTE-ACS.
Methods
Data sources and search parameters
We searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials for appropriate studies that were performed from 1990 to 24 April 2019. The search terms included invasive strategy, invasive coronary angiography, early coronary intervention, delayed coronary intervention, acute coronary syndrome, non-ST elevation myocardial infarction, unstable angina, non-ST elevation acute coronary syndrome, NSTE-ACS, and NSTEMI. Review articles, editorials, and meta-analyses were also considered to assess potential information for this study. We did not include unpublished research. Data selection was performed by two investigators independently. There were no restrictions on language, study period, or sample size.
Study selection, data extraction, and quality assessment
We included RCTs that met the following criteria: (1) enrolled patients had NSTE-ACS; (2) each trial compared an early invasive strategy with a delayed invasive strategy, where an early invasive strategy was defined as coronary intervention performed within 12 hours after enrollment and a delayed invasive strategy was defined as intervention performed on the next working day after enrollment or at least 12 hours after hospitalization; and (3) clinical follow-up must have occurred at least 30 days after the intervention. For all clinical events, we used the longest available follow-up period for each trial. The quality of RCTs was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias for RCTs.7
Endpoints and definitions
The primary endpoint was all-cause death. Secondary endpoints were re-MI, recurrent or refractory ischemia (RI), and major bleeding. If the trials reported refractory angina (RA) instead of RI, RA was used for the secondary endpoint analysis.
Data synthesis and analysis
The included data were combined to estimate the pooled risk ratio (RR) of an early invasive strategy versus a delayed invasive strategy as the comparator treatment. Subgroup analyses were performed to evaluate 1) the rate of death and re-MI in the two invasive groups at 30 days and at long-term follow-up (>1 year), and 2) the rate of composite death or re-MI in the early and delayed invasive strategies for high-risk patients. Statistical analysis was performed using Stata software version 12.0 (Stata Corp, College Station, TX, USA). The RR with 95% confidence intervals (CIs) are shown as the summary statistic. We used Q and I2 statistics to analyze heterogeneity among the included trials. The Q statistic indicated heterogeneity when P values were <0.10, whereas I2 ≤ 50% indicated that the magnitude of heterogeneity was moderate. If I2 was >50% or P was <0.10, a random-effects model was adopted. We also performed a sensitivity analysis by sequentially excluding each study if I2 was >50% or P was <0.10, and computed a meta-analysis. Results were considered statistically significant at P ≤ 0.05.
Results
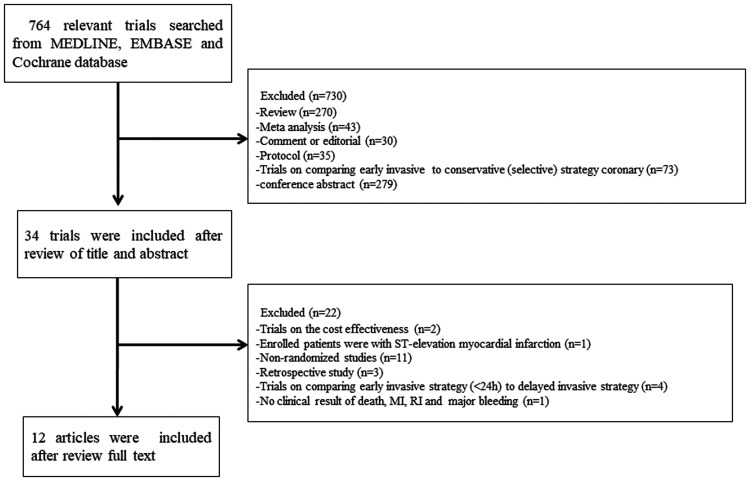
A total of 764 relevant trials were found using our search parameters. Finally, 12 studies of 9 trials that satisfied our selection criteria were included, involving a total of 8586 patients (Figure 1).6,8–18 Three trials, namely, OPTIMA, ELISA-3, and RIDDLE-NSTEMI, were updated with long-term follow-up clinical outcomes at 5, 2, and 3 years, respectively.11,12,14–17 Studies in which coronary intervention was performed 12 hours or later after hospitalization or there was randomization in the early invasive strategy were not included. Of those trials, there were 3907 patients in the early invasive treatment group and 4679 patients in the delayed group. Details of the trials are summarized in Tables 1 and 2. LIPSIA-NSTEMI,13 RIDDLE-NSTEMI16,17 and TAO6 included patients with NSTEMI, and the other six trials included NSTE-ACS. The median time of intervention (2.48 hours in the early invasive strategy and 47.19 hours in the delayed invasive strategy) was available in all trials, except in TAO.6 The clinical follow-up period ranged from 30 days to 5 years. However, most trials with long-term clinical follow-up reported only rates of death and re-MI.
Figure 1.
Study selection process.
MI, myocardial infarction; RI, refractory ischemia.
Table 1.
Demographic data and medical history of the included studies.
| Trial name (year) | No. of patients | Average age (years) | Follow-up (months) |
Medical history, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM | Hypertension | Hyperlipidemia | Smoking | Prior MI | Prior PCI | Prior CABG | ||||
| ELISA (2003) | 109/111 | 63/65 | 1 | 15/14 | 45/38.7 | 38.5/37.8 | 36.7/32.4 | 17.4/12.6 | 14.7/14.4 | 11/7.2 |
| ISAR-COOL (2003) | 203/207 | 70/70 | 12 | 31.4/26.1 | 85.7/87 | 64.5/71.5 | 24.1/18.4 | 21.7/25.1 | 20.7/23.2 | 9.9/13.5 |
| ABOARD (2009) | 175/177 | 65/65 | 1 | 22/32 | 66/61 | NR | 32/33.9 | 16.6/18.6 | 24.6/30.5 | 5.1/6.8 |
| OPTIMA (2009, 2016) | 73/69 | 63/62 | 60 | 19/20 | 61/33 | 57.6/32 | 33.9/39 | 21/26 | 27/19 | 11/1 |
| LIPSIA-NSTEMI (2012) | 200/200 | 68/70 | 6 | 39/43 | 82/82 | 40/42 | 29/25 | 18/24 | 16/16 | 5/8 |
| ELISA-3 (2013, 2017) | 269/265 | 72.1/71.8 | 24 | 23.8/20.4 | 54.3/58.1 | NR | 21.2/26.4 | 17.8/19.6 | 18.2/20.8 | 13.8/12.1 |
| RIDDLE-NSTEMI (2016, 2018) | 162/161 | 60.5/63 | 36 | 21.6/32.3 | 65.4/72 | 74.7/73.9 | 51.9/38.5 | 19.1/21.1 | 10.5/9.3 | 4.9/7.5 |
| TAO (2017) | 1648/1003 | 70.7/70.5 | 6 | 34/31.2 | 80.1/78.7 | 52.7/57.2 | 19.7/20.2 | 22.5/23/4 | 100/100 | 9.5/10.5 |
| VERDICT (2018) | 1075/1072 | 63.6/63.6 | 51.6 | 14.7/16.1 | 50.5/53.9 | NR | 31.8/30.1 | 17.3/17/4 | 14/15.2 | 5.3/5.3 |
Data are reported for early invasive/delayed invasive strategies.
DM, diabetes mellitus; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; NR, not reported.
Table 2.
Clinical characteristics of the included studies.
| Trial name (year) |
Invasive strategy |
Median time to angiogram (hours) | Troponin positive, % | GRACE risk score >140, % | Clinical outcome | |
|---|---|---|---|---|---|---|
| Early | Delayed | |||||
| ELISA (2003) | Within 12 hours | 24–48 hours after | 6/50 | 61/50 | NR | Death, MI, major bleeding, re-PCI, RI |
| ISAR-COOL (2003) | Within 6 hours | 72 hours after | 2.4/86 | 66/68 | NR | Death, MI, major bleeding, RI |
| ABOARD (2009) | Immediate | Next working day | 1.2/21 | 75.4/72.9 | NR | Peak troponin I levels, death, MI, or UR |
| OPTIMA (2009, 2016) | Immediate | 24–48 hours after | 0.5/25 | 47/45 | NR | Death, MI, major bleeding, re-PCI |
| LIPSIA-NSTEMI (2012) | Immediate | Next working day | 1.1/18.3 | 100/100 | 42/48 | Peak CK-MB activity, death, MI, RI, |
| ELISA-3 (2013, 2017) | Within 12 hours | No sooner than 48 hours | 2.6/54.9 | 78/79 | 40.5/43.0 | Death, MI, RI, major bleeding |
| RIDDLE-NSTEMI (2016, 2018) | No later than 2 hours | Within 72 hours | 1.4/61 | 100/100 | 34.6/41.6 | Death, MI, RI, major bleeding |
| TAO (2017) | First ECG <12 hours | First ECG ≥24 hours | NR | 90.2/90 | 100/100 | Death, MI, ST, unplanned revascularization |
| VERDICT (2018) | Within 12 hours | Within 48–72 hours | 4.7/61.6 | 81.2/79.2 | 49.3/48.7 | Death, MI, RI, repeat coronary revascularization, CA, bleeding, stroke |
Data are reported for early invasive/delayed invasive strategies.
GRACE, Global Registry of Acute Coronary Events; NR, not reported; MI, myocardial infarction; PCI, percutaneous coronary intervention; RI, recurrent or refractory ischemia; UR, urgent revascularization; CK-MB, creatinine kinase-MB; ECG, electrocardiogram; ST, stent thrombosis; CA, cardiac arrest.
Risk of bias
Risks of bias were similar in all enrolled RCTs (Table 3). All studies were conducted in accordance with the intention-to-treat principle. Clinical follow-up was performed for almost all patients and patients lost to follow-up were rare. In the OPTIMA trial,11 methods for random-sequence generation, allocation concealment, and blinding of outcome assessment were unclear.
Table 3.
Risk of bias assessment.
| Trial name | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data and selective reporting |
|---|---|---|---|---|---|
| ELISA (2003) | + | + | + | + | + |
| ISAR-COOL (2003) | + | + | + | + | + |
| ABOARD (2009) | + | + | + | + | + |
| OPTIMA (2009, 2016) | ? | ? | + | ? | + |
| LIPSIA-NSTEMI (2012) | + | + | + | + | + |
| ELISA-3 (2013, 2017) | + | + | + | + | + |
| RIDDLE-NSTEMI (2016, 2018) | + | + | + | + | + |
| TAO (2017) | + | + | + | + | + |
| VERDICT (2018) | + | + | + | + | + |
+, Low risk; ?, unclear risk.
Primary endpoint
All studies described the rate of death. The rate of total death was similar between the early and delayed invasive strategies (RR 0.90 [0.77–1.06]; P = 0.197; Figure 2a).
Figure 2.
Forest plots showing (a) all cause death; (b) recurrent MI; (c) major bleeding; and (d) recurrent or RI.
MI, myocardial infarction; RI, refractory ischemia; RR, risk ratio; CI, confidence interval.
Secondary endpoints
The incidence of re-MI was recorded in all studies. The incidence of re-MI was similar between the early and delayed invasive strategies (RR 0.95 [0.70–1.29]; P = 0.733; Figure 2b). All studies included the number of major bleeding episodes as a clinical outcome. The rate of major bleeding was similar between the two strategies (RR 0.97 [0.77–1.23]; P = 0.799; Figure 2c). The occurrence of RI was reported in all trials, except in OPTIMA11 and TAO.6 ELISA reported RA rather than RI. There was no significant difference between the early and delayed invasive strategies for RI (RR 0.74 [0.53–1.05]; P = 0.088; Figure 2d).
Subgroup analyses
We investigated the risk of composite death or re-MI in patients with a GRACE risk score >140 and elevated troponin levels. Additionally, we compared the risk of death and re-MI as subgroup analyses between the early and delayed invasive strategy groups at 30 days and at a long-term follow-up (>1 year).
Four studies included patients with GRACE risk scores >1406,14–18 and five studies included patients with elevated serum troponin levels.6,13–18 The risks of composite death or re-MI in the early intervention group were significantly decreased in patients with GRACE risk scores >140 (RR 0.82 [0.72–0.92]; P = 0.001) and in those with elevated troponin levels (RR 0.84 [0.76–0.93]; P = 0.001; Figure 3) compared with those in the delayed invasive strategy group.
Figure 3.
Forest plot showing composite all-cause death or recurrent myocardial infarction in high-risk patients.
GRACE, Global Registry of Acute Coronary Events; RR, risk ratio; CI, confidence interval.
Seven studies reported 30 days of clinical outcome,6,8–11,14,16 and only four studies reported long-term clinical follow-up,12,15,17,18 with a median follow-up of 3.5 years. The rate of death or re-MI was similar in the two groups, regardless of whether there was a short- or long-term follow-up (death: RR 0.97 [0.71–1.33]; P = 0.858, RR 0.93 [0.76–1.13]; re-MI: RR 0.87 [0.54–1.39]; P = 0.547, RR 0.87 [0.49–1.54], respectively; Figure 4). Sensitivity analysis showed no difference when compared with the results of the main analysis.
Figure 4.
Forest plots for 30 days and long-term follow-up. a) All-cause death and b) recurrent MI
MI, myocardial infarction; RR, risk ratio; CI, confidence interval.
Discussion
Several meta-analyses have shown that a routine invasive strategy reduces ischemic events (death or MI) compared with a selective invasive strategy in patients with NSTE-ACS, regardless of a short- or long-term follow-up.19,20 However, results of several conflicting studies and meta-analyses have shown that an early invasive strategy was not superior to a delayed or routine strategy. Therefore, we performed this meta-analysis to investigate whether coronary angiography performed within 12 hours could improve clinical outcomes. Our meta-analysis showed that an early invasive strategy did not reduce the risk of death, re-MI, RI, or major bleeding. Furthermore, this strategy significantly reduced the risk of composite death or re-MI in patients with high-risk factors, such as a GRACE risk score >140 or elevated troponin levels.
An early invasive strategy is not superior to a delayed invasive strategy
The rates of all-cause death, re-MI, and major bleeding were similar between the two invasive strategies, as well as long-term mortality, in our meta-analysis. Patients with acute coronary syndrome are at risk of death because of persistent coronary occlusion caused by acute thrombosis. Therefore, stabilization of the culprit lesion to prevent growth of thrombus and its complications are major therapeutic strategies in these patients.21 Coronary intervention significantly reduces clinical outcomes of mortality and MI compared with a conservative management strategy.22,23 However, we did not find that an early intervention reduced the mortality rate in patients with NSTE-ACS.
In this meta-analysis, the rate of RI was not significantly reduced with the early invasive strategy, which is different from the finding of a previous study.3 One possible reason for this difference between studies is that the inclusion criteria were different. In this meta-analysis, we evaluated coronary angiography performed within 12 hours as the early invasive strategy and intervention that was performed on the next working day after enrollment or at least 12 hours after hospitalization was the delayed strategy. In contrast, the above-mentioned study investigated the effectiveness of early (<24 hours) and delayed (>24 hours) invasive strategies. In this previous meta-analysis, TIMACS was included,2 which produced a significant reduction of the risk of RI with the early strategy (1% versus 3.27%, P<0.001). Overall, we suggest that an early invasive strategy within 12 hours does not significantly improve the risk of mortality.
An early invasive strategy might be beneficial to high-risk patients
ACC/AHA and ESC guidelines suggest that patients with high-risk factors should have revascularization performed within 24 hours (class I). In TIMACS,2 the risk of composite death, MI, or RI at 6 months was significantly decreased using an early invasive strategy for patients with GRACE risk scores >140, elevated cardiac markers, or ST-segment deviation. A collaborative meta-analysis showed that for predefined subgroup analyses, patients with elevated cardiac biomarkers at baseline, diabetes, a GRACE risk score >140 points, or age >75 years could benefit from early intervention.4 Therefore, in our meta-analysis, we investigated the effect of an early invasive strategy within 12 hours in patients with a GRACE risk score >140 or elevated cardiac troponin levels. Three trials (ELISA-3, RIDDLE-NSTEMI, and VERDICT) reported these variables. TAO included patients with NSTEMI with GRACE risk scores >140. Therefore, we included this definition for the subgroup analysis. We did not analyze the effect of age and ST-T deviation because of the lack of data provided by the studies. We found that the incidence of composite death or re-MI was significantly reduced in patients with GRACE risk scores >140 who received angiography within 12 hours. Recently, the MINAP trial24 showed that an invasive coronary strategy improved survival for intermediate- and high-risk patients with NSTEMI. In the clinical setting, physicians use the GRACE risk score to estimate the ischemic risk for NSTEMI and define patients as high risk when this score is >140. High-risk patients should undergo coronary intervention within 24 hours according to this guideline. However, in TAO, undergoing coronary angiography within 12 hours in high-risk patients with NSTEMI reduced ischemic clinical outcomes compared with intervention performed at 12 to 24 hours or >24 hours, which is supported by our meta-analysis. Additionally, this study showed that patients with elevated cardiac biomarkers benefited from an early invasive strategy (RR 0.84 [0.76–0.93]; P = 0.001). An elevated cardiac biomarker is a parameter of the GRACE risk score, indicating the presence of necrosis of myocytes due to coronary occlusion, and myocardial ischemia of an extended duration can promote necrosis. Therefore, an early invasive strategy could shorten the ischemic duration and reduce necrosis of myocytes, resulting in an improved clinical outcome.
This meta-analysis has several limitations. First, the time to angiography varied in the early and delayed invasive strategies. The time to angiography ranged from 0.5 to 12.2 hours in the early invasive group and from 6.0 to 106.7 hours in the delayed group. TAO did not report the time to angiography and OPTIMA only reported the median time of intervention from randomization. Second, the sample size was small, especially in our subgroup analysis. Only three trials (ELISA-3, RIDDLE-NSTEMI, and VERDICT) reported risk factors for subgroup analysis and four trials (OPTIMA, ELISA-3, RIDDLE-NSTEMI, and VERDICT) investigated long-term clinical outcomes. In pre-specified analysis, VERDICT enrolled 2147 patients, which accounted for 71.5% of the total number of high-risk patients and contributed to 68.5% of the total number of long-term follow-up patients. However, the design of this trial was strict, and this produced high-quality data from VERDICT. Finally, ACC/AHA and ESC guidelines consider dynamic ST- or T-wave changes as a high-risk criterion. Because of limited data, we did not examine the influence of an early invasive strategy on this parameter.
Conclusions
This meta-analysis shows that an early angiographic strategy within 12 hours does not reduce the risk of death, re-MI, major bleeding, or RI in patients with NSTE-ACS. Furthermore, an early invasive strategy might reduce the rate of composite death or re-MI in high-risk patients, such as those with GRACE risk scores >140 or elevated cardiac markers. More studies are required to investigate an early invasive angiographic strategy within 12 hours.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Xinping Du https://orcid.org/0000-0003-0039-1235
References
- 1.Prejean SP, Din M, Reyes E, et al. Guidelines in review: Comparison of the 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes and the 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. J Nucl Cardiol 2018; 25: 769–776. DOI: 10.1007/s12350-017-1137-z. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360: 2165–2175. DOI: 10.1056/NEJMoa0807986. [DOI] [PubMed] [Google Scholar]
- 3.Bonello L, Laine M, Puymirat E, et al. Timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes and clinical outcomes: an updated meta-analysis. JACC Cardiovasc Interv 2016; 9: 2267–2276. DOI: 10.1016/j.jcin.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Jobs A, Mehta SR, Montalescot G, et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: a meta-analysis of randomised trials. Lancet 2017; 390: 737–746. DOI: 10.1016/S0140-6736(17)31490-3. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhang Z, Xiong X, et al. Immediate/Early vs. delayed invasive strategy for patients with non-ST-segment elevation acute coronary syndromes: a systematic review and meta-analysis. Front Physiol 2017; 8: 952. DOI: 10.3389/fphys.2017.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deharo P, Ducrocq G, Bode C, et al. timing of angiography and outcomes in high-risk patients with non-ST-segment-elevation myocardial infarction managed invasively: insights from the tao trial (treatment of acute coronary syndrome with otamixaban). Circulation 2017; 136: 1895–1907. DOI: 10.1161/CIRCULATIONAHA.117.029779. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. DOI: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van't Hof AW, De Vries ST, Dambrink JH, et al. A comparison of two invasive strategies in patients with non-ST elevation acute coronary syndromes: results of the Early or Late Intervention in unStable Angina (ELISA) pilot study. 2b/3a upstream therapy and acute coronary syndromes. Eur Heart J 2003; 24: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 9.Neumann FJ, Kastrati A, Pogatsa-Murray G, et al. Evaluation of prolonged antithrombotic pretreatment (“cooling-off” strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA 2003; 290: 1593–1599. DOI: 10.1001/jama.290.12.1593. [DOI] [PubMed] [Google Scholar]
- 10.Montalescot G, Cayla G, Collet JP, et al. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 2009; 302: 947–954. DOI: 10.1001/jama.2009.1267. [DOI] [PubMed] [Google Scholar]
- 11.Riezebos RK, Ronner E, Ter Bals E, et al. Immediate versus deferred coronary angioplasty in non-ST-segment elevation acute coronary syndromes. Heart 2009; 95: 807–812. DOI: 10.1136/hrt.2008.154815. [DOI] [PubMed] [Google Scholar]
- 12.Oosterwerff EF, Fagel ND, Slagboom T, et al. Impact of percutaneous coronary intervention timing on 5-year outcome in patients with non-ST-segment elevation acute coronary syndromes. The ‘wait a day' approach might be safer. Neth Heart J 2016; 24: 173–180. DOI: 10.1007/s12471-016-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiele H, Rach J, Klein N, et al. Optimal timing of invasive angiography in stable non-ST-elevation myocardial infarction: the Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA-NSTEMI Trial). Eur Heart J 2012; 33: 2035–2043. DOI: 10.1093/eurheartj/ehr418. [DOI] [PubMed] [Google Scholar]
- 14.Badings EA, The SH, Dambrink JH, et al. Early or late intervention in high-risk non-ST-elevation acute coronary syndromes: results of the ELISA-3 trial. EuroIntervention 2013; 9: 54–61. DOI: 10.4244/EIJV9I1A9. [DOI] [PubMed] [Google Scholar]
- 15.Badings EA, Remkes WS, The SH, et al. Two-year outcome after early or late Intervention in non-ST elevation acute coronary syndrome. Open Heart 2017; 4: e000538. DOI: 10.1136/openhrt-2016-000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milosevic A, Vasiljevic-Pokrajcic Z, Milasinovic D, et al. Immediate versus delayed invasive intervention for non-STEMI patients: The RIDDLE-NSTEMI Study. JACC Cardiovasc Interv 2016; 9: 541–549. DOI: 10.1016/j.jcin.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Milasinovic D, Milosevic A, Vasiljevic-Pokrajcic Z, et al. three-year impact of immediate invasive strategy in patients with non-ST-segment elevation myocardial infarction (from the RIDDLE-NSTEMI Study). Am J Cardiol 2018; 122: 54–60. DOI: 10.1016/j.amjcard.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Kofoed KF, Kelbæk H, Riis Hansen P, et al. Early versus standard care invasive examination and treatment of patients with non-ST-segment elevation acute coronary syndrome verdict randomized controlled trial. Circulation 2018; 138: 2741–2750. DOI: 10.1161/CIRCULATIONAHA.118.037152. [DOI] [PubMed] [Google Scholar]
- 19.Bavry AA, Elgendy IY, Mahmoud AN, et al. Routine invasive versus selective invasive strategies for non-ST-elevation acute coronary syndrome: an updated meta-analysis of randomised trials. Eur Heart J 2016; 37: 820. DOI: 10.1093/eurheartj/ehw433. [DOI] [PubMed] [Google Scholar]
- 20.Fox KA, Clayton TC, Damman P, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010; 55: 2435–2445. DOI: 10.1016/j.jacc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. DOI: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344: 1879–1887. DOI: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 23.Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA 2005; 293: 2908–2917. DOI: 10.1001/jama.293.23.2908. [DOI] [PubMed] [Google Scholar]
- 24.Hall M, Bebb OJ, Dondo TB, et al. Guideline-indicated treatments and diagnostics, GRACE risk score, and survival for non-ST elevation myocardial infarction. Eur Heart J 2018; 39: 3798–3806. DOI: 10.1093/eurheartj/ehy517. [DOI] [PMC free article] [PubMed] [Google Scholar]