Abstract
Objective
The purpose of this study was to investigate the effect of gonadotropin dose and endometrial thickness (EMT) on pregnancy outcome in patients undergoing intrauterine insemination (IUI).
Methods
We retrospectively analyzed data from 361 patients with unexplained infertility or polycystic ovarian syndrome (PCOS) who underwent 930 IUI cycles treated with gonadotropins. Then, we measured the effects of gonadotropins and EMT on the clinical pregnancy rate. Finally, we assessed the association of various doses of gonadotropins on EMT.
Results
The dose of gonadotropins given and thickness of the endometrium were higher in the pregnancy group than in the nonpregnancy group (636.0 vs. 600.0 IU for gonadotropin dose; 9.15 vs. 8.70 mm for EMT). Clinical pregnancy rates were significantly improved by increasing the dose of gonadotropins (9.1%, <450 IU; 16.2%, 450–599 IU; 18.6%, 600–749 IU, and 17.3%, ≥750 IU), or by increased EMT (0%, <5.0 mm; 12.2%, 5.0–6.9 mm; 15.5%, 7.0–14.0 mm; and 33.3%, >14.0 mm).
Conclusion
Increasing the dose of gonadotropins to stimulate one follicle to develop may benefit endometrial proliferation and improve IUI outcomes.
Keywords: Clinical pregnancy, endometrial thickness, gonadotropins, intrauterine insemination, receiver operating characteristic, infertility
Introduction
Adequate development of the endometrium is necessary for the successful implantation of an embryo. Studies have shown that endometrial thickness (EMT) on the day of human chorionic gonadotropin (hCG) administration is positively associated with pregnancy outcome.1–3 Therefore, EMT is routinely assessed by vaginal ultrasound in intrauterine insemination (IUI) as well as in in vitro fertilization (IVF) treatments to assess the extent of endometrial development.4 However, some studies have shown no evidence of a relationship between EMT and pregnancy outcome.5–7 This inconsistent relationship between EMT and pregnancy might be due to different stimulation protocols because endometrial development has been linked to stimulation protocol.1,7–9 Clomiphene citrate (CC), which acts as a competitive inhibitor of estradiol (E2) binding receptors,10 reduces EMT,9,11–13 whereas gonadotropins neither alter estrogen receptors nor reduce EMT in the same way.
Numerous studies have attempted to identify a cut-off value for thin EMT below which pregnancy rates are reduced2,14 but this remains unknown. In addition, gonadotropins, as one option for ovarian stimulation via E2 production, stimulate not only follicular growth but also endometrial development. However, how the dose of gonadotropins affects EMT and clinical pregnancy rate (CPR) is unclear.
In this study, we used gonadotropins to stimulate the ovaries of patients with unexplained infertility or polycystic ovarian syndrome (PCOS) with the following aims: (1) to evaluate the effects of the total dose of gonadotropins and EMT on the day of hCG administration on IUI outcomes; and (2) to investigate the relationship between the dose of gonadotropins and EMT.
Material and methods
Ethical approval
All procedures involving human participants were performed in accordance with the recommendations of the institutional review board of Nanning Maternal and Child Health Care Hospital and with the 1964 Declaration of Helsinki. This study was approved by the Nanning Maternal and Child Health Care Hospital (No. SZ 20190808-2). Patient consent was not required because the study was conducted retrospectively.
Patients
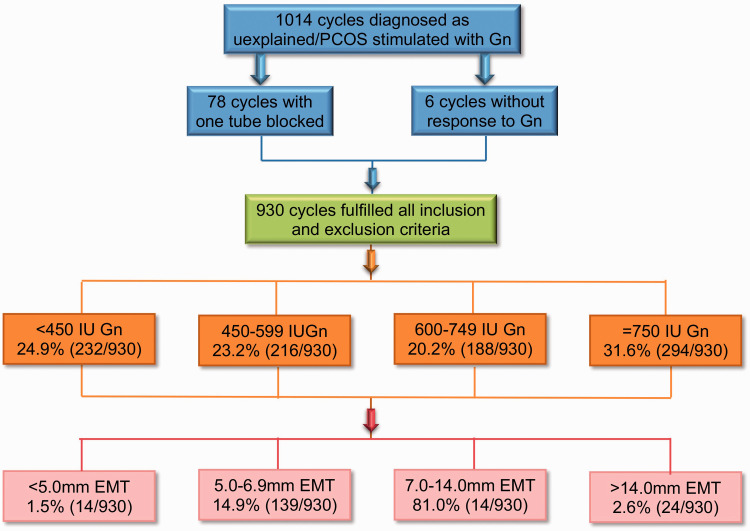
We retrospectively analyzed all IUI cycles treated with gonadotropins during the period from January 2015 to June 2019. To limit the bias that exists in a retrospective study, the inclusion criteria were couples who were diagnosed with unexplained infertility or PCOS. The women were under 40 years old with two patent fallopian tubes and normal endometrial ultrasound imaging. Their male partners had over 5 × 106 spermatozoa/mL after washing. Couples who had undergone IVF treatment were excluded. Before applying gonadotropins as ovarian stimulation, patients with unexplained infertility underwent two or three attempts of IUI without stimulation. All of these cycles were also excluded. A total of 1014 cycles in patients diagnosed with unexplained infertility or PCOS stimulated with gonadotropins met the inclusion criteria (Figure 1). Seventy-eight cycles with one tube blocked and six cycles without response to gonadotropins were removed from this study.
Figure 1.
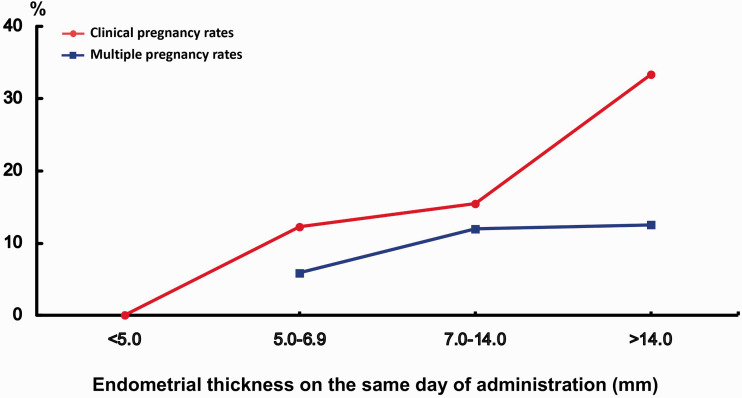
Clinical pregnancy rates and multiple pregnancy rates according to endometrial thickness on the day of human chorionic gonadotropin administration.
The diagnosis of PCOS was made based on the Rotterdam criteria of European Society for Human Reproduction and Embryology (ESHRE)/American Society for Reproductive Medicine (ASRM).15 Couples were diagnosed with unexplained infertility when they had failed to conceive after 12 months of unprotected intercourse without an established cause of infertility such as blocked tubes, anovulation, abnormal uterine cavity, or presence of endometriosis.
Treatments
Administration of gonadotropins started on day 2 or day 3 of the cycle after excluding the presence of an ovarian cyst by ultrasound. The initial stimulating dose of gonadotropins was 75 or 100 IU per day according to the woman’s age, ovarian reserve assessment, and body mass index. Follicle scan was performed by transvaginal ultrasound examination on day 10 of the cycle. The dose was adjusted depending on the development of the follicles. When the diameter of the leading follicle reached 18 mm, ovulation was triggered with 5000 to 10,000 IU of hCG. The number of preovulatory follicles (diameter ≥14 mm) and the EMT were recorded on the day of hCG administration. A single insemination was carried out 36 to 40 hours after ovulation was triggered. Patients with more than three preovulatory follicles were recommended to cancel IUI or switch to IVF.
Semen samples were collected by masturbation in sterile containers after 3 to 7 days of abstinence, processed with a two-layer density gradient (40% and 80%), and then washed with noncapacitating buffer after liquefaction. The volume of the washed semen sample used for insemination was 0.3 to 0.5 mL. The sperm suspension was injected into the uterine cavity with a soft catheter. Patients were advised to keep in the supine position for at least 15 minutes after insemination.
Luteal support was prescribed routinely in all patients. A serum sample was collected for hCG measurement 14 days after IUI. If the hCG test was positive, detection of a gestational sac by transvaginal ultrasound confirmed the intrauterine pregnancy after 4 weeks of insemination. The main outcome was clinical pregnancy, defined as the presence of a fetal heartbeat.
Statistical analysis
Data were analyzed using SPSS software (version 17; SPSS Inc., Chicago, IL, USA). Normality was determined by the Shapiro-Wilk test. Non-normally distributed continuous data are expressed as medians with interquartile ranges. Non-normally distributed data were analyzed using the Mann–Whitney U test, and categorical data were analyzed by Chi-square test and Fisher’s exact test. The cut-off value for statistical significance was P < 0.05. The association between total dose of gonadotropins and other variables was analyzed by using stepwise multiple linear regression. Multivariate logistic regression analysis with stepwise selection was used to evaluate correlations between variable quantity and occurrence of pregnancy. Odds ratios and 95% confidence intervals (CI) were used for descriptive analysis. The area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the predictive power of gonadotropins and EMT for the occurrence of clinical pregnancy.
Results
A total of 930 cycles (361 couples) were included in the study; the CPR was 15.3%. Among 142 clinical pregnancies, 16 were twin gestations (11.3%). The demographic characteristics of pregnant women compared with nonpregnant women are shown in Table 1. Pregnant women were younger than nonpregnant women (32.0 vs. 33.0 years; P = 0.008), required a higher dose of gonadotropins (636.0 vs. 600.0 IU; P = 0.008), and had a thicker endometrium (9.15 vs. 8.70 mm; P = 0.039) on the day of hCG administration. Both groups had similar levels of follicle-stimulating hormone, luteal hormone, and E2; similar durations of infertility; and similar numbers of preovulatory follicles. There was no significant difference in CPR between patients with unexplained infertility and PCOS (13.9% vs. 17.9%).
Table 1.
Demographic data related to pregnant and nonpregnant patients.
| Characteristic | Clinical pregnancy(n = 142) | Nonpregnancy(n = 788) | P-value |
|---|---|---|---|
| Female age (years) | 32.0 (29.8–35.0) | 33.0 (31.0–36.0) | 0.008 |
| FSH (IU/L) | 6.90 (5.90–8.00) | 7.00 (5.95–8.30) | 0.378 |
| LH (IU/L) | 4.40 (3.40–6.25) | 4.40 (3.30–6.00) | 0.544 |
| E2 (pmol/mL) | 154.5 (109.3–186.8) | 137.0 (103.0–181.0) | 0.185 |
| Type of infertility | |||
| Primary | 73 (13.4%) | 471 (86.6%) | 0.063 |
| Secondary | 69 (17.9%) | 317 (82.1%) | |
| Cause of infertility | |||
| Unexplained | 79 (13.9%) | 491 (86.1%) | 0.133 |
| PCOS | 63 (17.5%) | 297 (82.5%) | |
| Duration of infertility (years) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.956 |
| Total dose of gonadotropins (IU) | 636.0 (450.0–856.3) | 600.0 (412.5–784.0) | 0.008 |
| No. of preovulatory follicles | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.126 |
| EMT (mm) | 9.15 (7.50–10.63) | 8.70 (7.40–10.00) | 0.039 |
Results are expressed as median with interquartile range or number (percentile).
FSH, follicle-stimulating hormone; LH, luteal hormone; E2, estradiol; PCOS, polycystic ovarian syndrome; EMT, endometrial thickness.
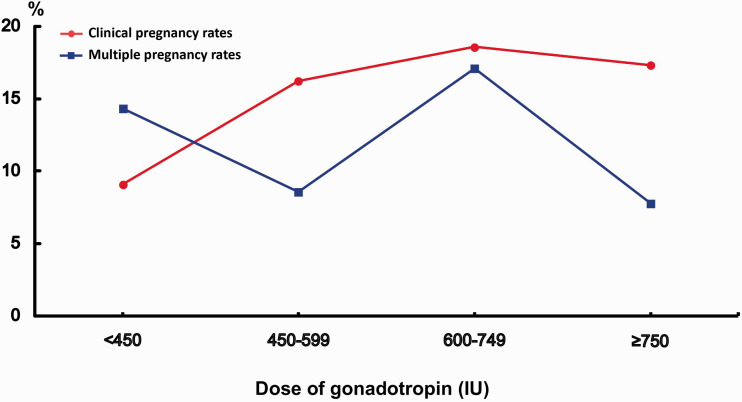
To examine the effect of the total dose of gonadotropins on IUI outcome, we divided the dose of gonadotropins into four groups based on the distribution of dose of gonadotropins in our data: <450 IU, 450 to 599 IU, 600 to 749 IU, and ≥750 IU. As shown in Figure 2, the CPR was significantly higher in the higher-dose groups (16.2% in 450–599 IU; 18.6% in 600–749 IU; and 17.3% in ≥750 IU; P < 0.05) compared with the lowest dose group (9.1% in <450 IU). However, there was no relationship between the dose of gonadotropins and multiple pregnancies.
Figure 2.
Flowchart of study population.
PCOS, polycystic ovarian syndrome; Gn, gonadotropin; EMT, endometrial thickness.
EMT on the day of hCG administration ranged from 2.9 to 17.0 mm. To investigate which level of EMT would be detrimental for pregnancy in IUI cycles, patients were divided into four groups based on EMT16,17: (1) <5.0 mm, (2) 5.0 to 6.9 mm, (3) 7.0 to 14.0 mm, and (4) >14 mm; the first two groups were considered to have thin EMT, and the fourth group was considered to have thick EMT. CPR and multiple pregnancy rates in the four groups of patients are shown in Figure 3. No pregnancies occurred when EMT was <5.0 mm. CPR increased with a thicker EMT (12.2% in the 5.0–6.9 mm group, and 15.5% in the 7.0–14.0 mm group) (P = 0.031), with the highest CPR (33.3%) being observed in the group with EMT >14 mm. CPR appeared to be positively associated with EMT on the day of hCG administration (χ2 = 9.611, P = 0.022). No significant difference was observed for multiple pregnancies within groups.
Figure 3.
Relationship of dose of gonadotropin to clinical pregnancy rate and multiple pregnancy rate.
The demographic characteristics of patients in the four gonadotropin dose groups are shown in Table 2. When ovaries were stimulated with higher doses of gonadotropins, EMT increased significantly (8.00 mm in <450 IU, 8.70 mm in 450–599 IU, 9.00 mm in 600–749 IU, and 9.10 mm in ≥750 IU; P < 0.01). Stepwise multiple linear regression analysis was used to examine the association between dose of gonadotropins and EMT. In this model, the total dose of gonadotropins was taken as the dependent variable, and parameters having a P-value <0.10 in Table 2 were entered as independent variables: E2, type of infertility, cause of infertility, EMT, and the number of preovulatory follicles. The analysis revealed that the total dose of gonadotropins depended on the type and cause of infertility and influenced the development of preovulatory follicles and the endometrium (Table 3).
Table 2.
Demographic data according to dose of gonadotropins administered.
| Characteristic | Total dose of gonadotropins |
P-value | |||
|---|---|---|---|---|---|
| <450 IU (n = 232) | 450–599 IU (n = 216) | 600–749 IU (n = 188) | ≥750 IU (n = 294) | ||
| Female age (years) | 32.5 (30.0–35.0) | 34.0 (31.0–36.0) | 33.0 (31.0–36.0) | 33.0 (30.0–36.0) | 0.124 |
| FSH (IU/L) | 7.10 (6.10–8.00) | 6.90 (5.85–8.55) | 7.10 (6.10–8.25) | 6.80 (5.80–8.40) | 0.772 |
| LH (IU/L) | 4.40 (3.50–5.80) | 4.40 (3.30–5.75) | 4.50 (3.30–6.10) | 4.20 (3.30–6.90) | 0.882 |
| E2 (pmol/mL) | 146.0 (108.0–182.5) | 151.0 (106.0–181.8) | 132.5 (107.3–177.0) | 125.5 (84.0–180.3) | 0.001 |
| Type of infertility | |||||
| Primary | 148 (63.8%) | 135 (62.5%) | 108 (57.4%) | 153 (52.0%) | 0.026 |
| Secondary | 84 (36.2%) | 81 (37.5%) | 80 (42.6%) | 141 (48.0%) | |
| Cause of infertility | |||||
| Unexplained | 150 (64.7%) | 142 (65.7%) | 125 (66.5%) | 153 (52.0%) | 0.001 |
| PCOS | 82 (35.3%) | 74 (34.3%) | 63 (33.5%) | 141 (48.0%) | |
| Duration of infertility (years) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.325 |
| No. of preovulatory follicles | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.004 |
| EMT (mm) | 8.00 (7.00–9.30) | 8.70 (7.13–10.00) | 9.00 (7.70–10.28) | 9.10 (8.00–10.83) | <0.001 |
Results are expressed as median with interquartile range or number (percentile). FSH, follicle-stimulating hormone; LH, luteal hormone; E2, estradiol; PCOS, polycystic ovarian syndrome; EMT, endometrial thickness.
Table 3.
Variables associated with dose of gonadotropins analyzed by multivariate linear regression.
| Variable | Coefficient | Standard error | P-value |
|---|---|---|---|
| Endometrial thickness | 27.500 | 5.838 | <0.001 |
| Preovulatory follicles | 46.531 | 20.424 | 0.023 |
| Cause of infertility | −135.156 | 26.795 | <0.001 |
| Type of infertility | 52.822 | 26.144 | 0.044 |
| Constant | 502.673 | 79.797 | <0.001 |
Multivariate stepwise logistic regression was conducted to determine the potential predictive variables that influenced CPR. Results showed that the age of the woman and the type of infertility were significantly associated with CPR (Table 4). Removal of the gonadotropins parameter from the model in step 2 of the analysis showed that gonadotropin was not an independent variable that affected CPR; neither was EMT in the final model.
Table 4.
Variables associated with clinical pregnancy analyzed by multivariate logistic regression.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Female age | 1.069 | 1.019–1.120 | 0.006 |
| Endometrial thickness | 0.931 | 0.860–1.007 | 0.075 |
| Type of infertility | 0.667 | 0.462–0.962 | 0.030 |
| Constant | 2.198 | 0.394 |
OR, odds ratio; 95% CI, confidence interval.
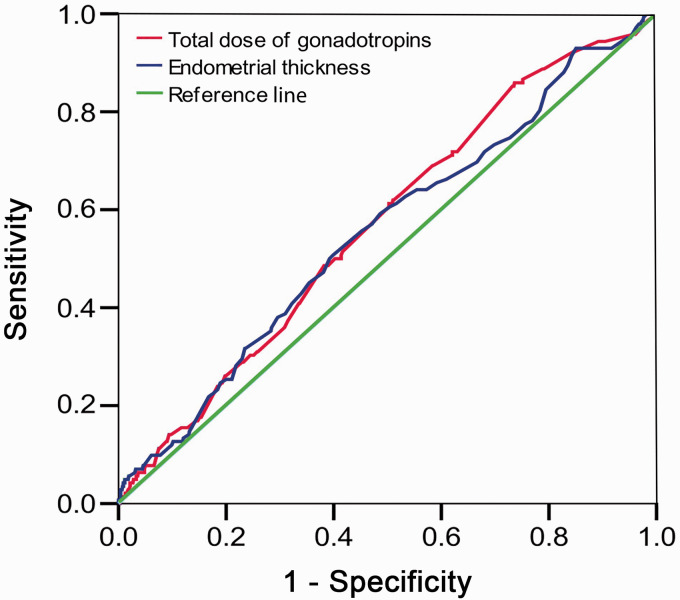
Results of the ROC analysis, using CPR as a dependent variable and total dose of gonadotropins and EMT on the day of hCG administration as predictors, are shown in Figure 4. The AUCs were 0.57 (95% CI: 0.52–0.62) for gonadotropins and 0.55 (95% CI: 0.50–0.61) for EMT, suggesting that both gonadotropins and EMT are poor predictive variables for CPR.
Figure 4.
Receiver operating characteristic (ROC) curve of total dose of gonadotropins and endometrial thickness (EMT) assessed on the day of human chorionic gonadotropin administration in relation to achievement of clinical pregnancy. Areas under the ROC curve are 0.57, 95% confidence interval (CI): 0.52–0.62 and 0.55, 95% CI: 0.50–0.61, for total dose of gonadotropin dose and EMT, respectively.
Discussion
The relationships between gonadotropin dose, EMT, and IUI outcome in this population of patients with unexplained infertility or PCOS are not clear. To examine the effect of EMT on IUI outcomes, we classified patients into four groups based on EMT. We found that EMT of the clinical pregnancy group was significantly higher than that of the nonpregnancy group (9.15 mm vs. 8.70 mm; P < 0.05). We also found a positive relationship between EMT on the day of hCG administration and CPR in gonadotropin-stimulated IUI cycles when EMT reached 5 mm, with the highest CPR occurring in the group with EMT >14 mm. Furthermore, our results showed that the total dose of gonadotropin used as ovarian stimulation was positively related to CPR as well as EMT. Our findings suggest that increasing the dose of gonadotropins to trigger one follicle to develop would benefit endometrial proliferation and improve IUI outcomes. Cycles of IUI should not be canceled even if EMT is >14 mm.
Some studies did not find the differences between pregnancy and nonpregnancy groups, irrespective of ovarian stimulation with gonadotropins,17 CC,5 or combined ovarian stimulation8 in IUI cycles; these findings were confirmed by Weiss et al. in a recent review.7 Two possible explanations for the failure to find such a discrepancy in EMT are as follows. First, it is not appropriate to compare the relationships between heterogeneous EMT and pregnancy outcomes across different stimulation protocols because the endometrium responds differently to different stimulation protocols. The thickness of the endometrium is greater in gonadotropin-stimulated cycles than in CC-stimulated cycles.7,9 Second, a statistical difference may not be found by simply comparing EMT between pregnancy and nonpregnancy groups when there is a nonlinear correlation between pregnancy and EMT, especially because poor pregnancies occur at both the low and high extremes of EMT.17
Thin endometrium is thought to be associated with low success rates.8,17 It is thought that, with thin endometrium, embryos would be too close to the spiral arteries, and the higher vascularity and high oxygen concentration would make embryo implantation difficult.18 However, to date, there is no consensus on the definition of “thin” endometrium; a cut-off value of 6 to 10 mm has been used in most studies.2,14,17,19,20 Interestingly, a study by Kovacs et al. showed significant differences in pregnancy rates in groups below and above an EMT cut-off when the cut-off was from 10 to 13 mm, whereas no difference was found for EMT cut-offs >13 mm or <10 mm, suggesting that discrepant findings in different studies may be due to different cut-off values.2 In this study, we did not observe pregnancies when EMT was <5 mm, which agrees with the finding by Friedler et al. in an IVF study.4 However, because 12.1% CPR (in the 5.0 to 6.9 mm EMT group) is considered accepted in IUI treatment, the practice of canceling cycles when EMT is <7 mm is not recommended. In contrast to the clear association of thin endometrium with a low chance of pregnancy, the effect of thickened endometrium on pregnancy is conflicting. Weissman et al. reported significantly lower implantation and pregnancy rates among women with EMT >14 mm on the day of hCG administration,21 whereas other authors have reported no adverse effect on implantation, pregnancy, or miscarriage rates when the EMT was >14 mm.16,17,20,22 In contrast to those results, our study showed that the highest CPR was achieved when EMT was >14 mm (the highest was 17 mm). Interestingly, our results showed similarities to studies on fresh IVF cycles: pregnant women have significantly higher EMT than nonpregnant women,2,23–26 and pregnancy rates improve as EMT increases.2,23,24,26 These similarities are not surprising because ovarian stimulation with gonadotropins, as applied in this study, is commonly used in IVF treatment.
Here, the dose of gonadotropins used was significantly higher in the pregnancy group than in the nonpregnancy group. Better pregnancy outcomes were achieved when the total dose of gonadotropins was >450 IU; the pregnancy rate was lower at lesser doses of gonadotropins. Multiple linear regression analysis revealed that the dose of gonadotropins was associated with EMT and preovulatory follicle number. However, we detected no significant difference in follicle growth between the pregnancy group and the nonpregnancy group in the condition of controlling ovarian stimulation aimed at development of one follicle. This implies that gonadotropins influenced CPR by developing the endometrium, not by increasing the follicle number. The beneficial effect of increasing the dose of gonadotropins on endometrial proliferation could occur through regulation of endometrial gene expression.27,28 Unlike the detrimental effect of supraphysiologic levels of gonadotropins on endometrial development in IVF,29 increasing the dose of gonadotropins to stimulate one follicle may benefit endometrial proliferation.
The average multiple pregnancy rate in the current study was 11.3%, consisting of 15.3% in women with PCOS and 7.6% in couples with unexplained infertility (data not shown). The higher multiple pregnancy rate in patients with PCOS requires physicians to pay more attention to these patients when choosing gonadotropins as an ovarian stimulation protocol.
By multivariable logistic regression analysis, neither gonadotropins nor EMT was found to be an independent variable affecting CPR after adjusting for female age and other potential confounders. Our ROC curve revealed that neither gonadotropins nor EMT on the day of hCG administration could predict pregnancy in gonadotropin-stimulated IUI cycles, which is in agreement with previous studies in IUI cycles5,17 and IVF cycles.2,24,26 We acknowledge the retrospective nature of this study as a limitation; however, we eliminated the bias of the effects of other variables on CPR by recruiting patients from the population of unexplained infertility and PCOS who were younger than 40 years and had undergone gonadotropin-stimulated IUI cycles. The small group sizes for EMT <5 mm and >14 mm indicate the need for larger sample sizes in future studies.
Conclusions
Clinical pregnancy rate is positively related to the dose of gonadotropins and EMT measured on the day of hCG administration. Increasing EMT is associated with the dose of gonadotropins administered. However, neither gonadotropin dose nor EMT is a good predictor of IUI outcome in gonadotropin-stimulated cycles. These findings suggest appropriate doses of gonadotropins for patients with unexplained infertility and PCOS and reinforce that clinicians should pay attention to endometrial development.
Acknowledgements
The authors thank the staff at the reproductive medicine center in Nanning Maternal and Child Health Care Hospital for their support.
Footnotes
Author contributions: QL, ZD, LPW, and MZ designed the study; QL, ZD, YH, and LR acquired the data; QL, ZD, LHW, YH, LR, SH, LPW, and MZ analyzed and interpreted the data; QL, ZD, LPW, YH, and MZ wrote and revised the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by Guangxi Scholar Grant (30501020001).
ORCID iD: Liping Wang https://orcid.org/0000-0001-6372-5939
References
- 1.Dickey RP, Olar TT, Taylor SN, et al. Relationship of endometrial thickness and pattern to fecundity in ovulation induction cycles: effect of clomiphene citrate alone and with human menopausal gonadotropin. Fertil Steril 1993; 59: 756–760. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs P, Matyas S, Boda K, et al. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod 2003; 18: 2337–2341. [DOI] [PubMed] [Google Scholar]
- 3.Esmailzadeh S, Faramarzi M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fertil Steril 2007; 88: 432–437. [DOI] [PubMed] [Google Scholar]
- 4.Friedler S, Schenker JG, Herman A, et al. The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Hum Reprod Update 1996; 2: 323–335. [DOI] [PubMed] [Google Scholar]
- 5.Kolibianakis E, Zikopoulos K, Fatemi H, et al. Endometrial thickness cannot predict ongoing pregnancy achievement in cycles stimulated with clomiphene citrate for intrauterine insemination. Reprod Biomed Online 2004; 8: 115–118. [DOI] [PubMed] [Google Scholar]
- 6.Asante A, Coddington CC, Schenck L, et al. Thin endometrial stripe does not affect likelihood of achieving pregnancy in clomiphene citrate/intrauterine insemination cycles. Fertil Steril 2013; 100: 1610–1614.e1. [DOI] [PubMed] [Google Scholar]
- 7.Weiss N, Van Vliet M, Limpens J, et al. Endometrial thickness in women undergoing IUI with ovarian stimulation. How thick is too thin? A systematic review and meta-analysis. Hum Reprod 2017; 32: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 8.De Geyter C, Schmitter M, De Geyter M, et al. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil Steril 2000; 73: 106–113. [DOI] [PubMed] [Google Scholar]
- 9.Bromer JG, Aldad TS, Taylor HS. Defining the proliferative phase endometrial defect. Fertil Steril 2009; 91: 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark J, Peck E, Anderson J. Oestrogen receptors and antagonism of steroid hormone action. Nature 1974; 251: 446–448. [DOI] [PubMed] [Google Scholar]
- 11.Gonen Y, Casper RF. Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod 1990; 5: 670–674. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Ono M, Yoshida Y, et al. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril 1997; 67: 256–260. [DOI] [PubMed] [Google Scholar]
- 13.Gadalla MA, Huang S, Wang R, et al. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018; 51: 64–76. [DOI] [PubMed] [Google Scholar]
- 14.Kasius A, Smit JG, Torrance HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 2014; 20: 530–541. [DOI] [PubMed] [Google Scholar]
- 15.The Rotterdam ESHRE/ASRM‐sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19–25. DOI: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 16.Dietterich C, Check JH, Choe JK, et al. Increased endometrial thickness on the day of human chorionic gonadotropin injection does not adversely affect pregnancy or implantation rates following in vitro fertilization-embryo transfer. Fertil Steril 2002; 77: 781–786. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Ye XY, Chan C. The association between endometrial thickness and pregnancy outcome in gonadotropin-stimulated intrauterine insemination cycles. Reprod Biol Endocrinol 2019; 17: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casper RF. It’s time to pay attention to the endometrium. Fertil Steril 2011; 96: 519–521. [DOI] [PubMed] [Google Scholar]
- 19.Merviel P, Heraud MH, Grenier N, et al. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril 2010; 93: 79–88. [DOI] [PubMed] [Google Scholar]
- 20.Fang R, Cai L, Xiong F, et al. The effect of endometrial thickness on the day of hCG administration on pregnancy outcome in the first fresh IVF/ICSI cycle. Gynecol Endocrinol 2016; 32: 473–476. [DOI] [PubMed] [Google Scholar]
- 21.Weissman A, Gotlieb L, Casper RF. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril 1999; 71: 147–149. [DOI] [PubMed] [Google Scholar]
- 22.Yoeli R, Ashkenazi J, Orvieto R, et al. Significance of increased endometrial thickness in assisted reproduction technology treatments. J Assist Reprod Genet 2004; 21: 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter KS, Bugge KR, Bromer JG, et al. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril 2007; 87: 53–59. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ghamdi A, Coskun S, Al-Hassan S, et al. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol 2008; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momeni M, Rahbar MH, Kovanci E. A meta-analysis of the relationship between endometrial thickness and outcome of in vitro fertilization cycles. J Hum Reprod Sci 2011; 4: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Zhang Q, Wang Y, et al. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod Biomed Online 2014; 29: 291–298. [DOI] [PubMed] [Google Scholar]
- 27.Horcajadas JA, Riesewijk A, Polman J, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod 2004; 11: 195–205. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Lee KF, Ng EH, et al. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril 2008; 90: 2152–2164. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs P, Sajgo A, Kaali SG, et al. Detrimental effects of high-dose gonadotropin on outcome of IVF: making a case for gentle ovarian stimulation strategies. Reprod Sci 2012; 19: 718–724. [DOI] [PubMed] [Google Scholar]