Abstract
Objectives
Cervical cancer, the most common female cancer after breast cancer, is typically treated using radiotherapy. However, pelvic radiotherapy can cause irreversible damage to the vagina, seriously affecting patients’ quality of life. In this study, protein scaffolds loaded with rat adipose-derived mesenchymal stem cells (ADSCs) were implanted into irradiated tissue to assess their healing potential.
Methods
We established a rat model of radiation-induced vaginal injury. Complexes (consisting of protein scaffolds loaded with ADSCs) were implanted into injury sites. Histological analysis were used to assess regeneration of the vaginal epithelium. RNA sequencing was used to study the therapeutic mechanism of the complexes.
Results
The complexes promoted vaginal epithelial cell regeneration, vaginal tissue repair and improved vaginal stenosis and contracture. Compared with rats transplanted with ADSCs, rats transplanted with complexes achieved better therapeutic effects.
Conclusions
Protein scaffold-ADSC complexes had a beneficial therapeutic effect on radiation-induced vaginal injury in rats and may serve as the basis of a novel therapeutic approach for radiation dermatitis.
Keywords: Adipose-derived mesenchymal stem cells, biological materials, vaginal radiation injury, animal model, cervical cancer, cytokeratin, mucin 1
Introduction
Cervical cancer is the fourth most common cancer affecting women worldwide, with 528,000 new cases diagnosed every year.1 First-line treatment for patients with cervical cancer depends on a variety of factors, including the patient’s age, health, and desire to have children, as well as tumor stage and characteristics.2 Although the standard therapy for cervical cancer is surgery, radiotherapy is necessary to treat advanced-stage tumors and prevent their recurrence.3 Unfortunately, radiotherapy has many side effects, with vaginal stenosis being the most common and important. One study suggested that radiotherapy for treatment of invasive cervical cancer was associated with increased sexual dysfunction, a less-active sexual life and dyspareunia.4 Furthermore, 45.9% of women treated for gynecological cancer develop vaginal stenosis with associated dyspareunia,5 which can impair their sexual function and quality of life.6,7 The relative survival rates for women with stage I to III tumors of the cervix have increased in recent years.8 With the survival rates for locally advanced cervical cancer improving, research focus has shifted to treatment of radiation-induced pelvic injuries. However, studies in this regard are still scarce and their results have been heterogeneous, especially regarding repair of vaginal injury. Recently, some evidence has provided support for the use of vaginal lubricants following radiotherapy to prevent vaginal stenosis.9 The American Cancer Society recommends intercourse or the use of a vaginal dilator three times a week for the prevention of stenosis.10 Dinicola et al.2 reported that low-molecular weight hyaluronic acid was effective in treating radiation-damaged tissue and played a key role in all steps of the healing process. However, these methods treat symptoms only and have not shown efficacy in alleviating patient discomfort.11,12 In fact, no studies have attempted the repair of radiation-induced vaginal injury using complexes composed of adipose-derived mesenchymal stem cells (ADSCs) and protein scaffolds.
Stem cell therapy holds tremendous promise for the repair and/or regeneration of damaged tissue.13 ADSCs are multipotent adult stem cells. Adipose tissue represents an abundant, poorly immunogenic, stably proliferative, and low-injury tissue. The clinical applicability of ADSCs has been demonstrated in several clinical trials.14 Our previous study demonstrated that ADSCs could improve the regenerative capability of the ovary in a mouse model of ovarian injury.14
Intra-articular mesenchymal stem cell (MSC) implantation is usually performed using biocompatible hydrogels to promote local cell attachment within a microenvironment resembling the extracellular matrix and to avoid unwanted cell loss.15 An optimal balance between physical and biological properties of the material carrying cells is required for clinical translation and additional issues regarding isolation and manipulation of cells need to be addressed.16,17 A collagen–hydroxyapatite scaffold was able to commit human MSCs toward osteogenic differentiation in vitro.18 In the present study, ADSCs were complexed with three-dimensional (3D) protein scaffolds for transplantation into the vaginas of irradiated rats. The aim of the study was to evaluate the efficacy of these complexes in regenerating vaginal epithelial cells and improving symptoms of radiation-induced vaginal injury.
Materials and methods
Extraction and culture of ADSCs
Animals were anesthetized by intraperitoneal injection with pentobarbital (30 mg/kg) and placed on a heated table to maintain body temperature at 37°C. After anesthesia, rats were euthanized by asphyxiation with CO2 (displacement 20% of chamber volume/minute). After animals lost spontaneous breathing, they were observed for the following signs for 2 more minutes to verify death: lack of pulse, corneal reflex and response to firm toe pinch; inability to hear respiratory sounds and heartbeat using a stethoscope; and graying of the mucous membranes. ADSC were excised from the inguinal pads of two 6-week-year-old male Wistar rats (provided by the experimental animal center of PLA Medical College) and cut into fine pieces. During extraction, capillaries on the surface of ADSCs were removed. The tissue was placed into a 50-mL tube containing phosphate-buffered saline (PBS, Hyclone, Logan, UT, USA) and centrifuged at 300 ×g for 10 minutes. The adipose tissue was transferred from the upper phase to a fresh tube and digested with 0.075% collagenase I (Invitrogen, Carlsbad, CA, USA) for 1 hour at 37°C with shaking. The same volume of growth medium was added to terminate the digestion, then the sample was filtered through a 200-mesh screen. The digested material was centrifuged at 300 ×g for 5 minutes in 5 mL of PBS. The supernatant was removed and the pellet was resuspended in 8 mL of DME/F12 medium (Hyclone) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin-streptomycin (Hyclone). The cell suspension was placed in a 10 cm culture dish. The culture dish was incubated at 37°C under a humidified atmosphere containing 5% CO2 to allow the formation of ADSC colonies, which were then propagated. After 24 hours, half of the medium was exchanged. The medium in each culture dish was changed once every 2 days until primary cell confluence reached about 80% to 90%. The cells were trypsinized and sub-cultured. Third and fourth passage cells were harvested and frozen for subsequent experiments.
Characterization of ADSCs
Expression of cell surface markers on ADSCs was analyzed by flow cytometry. Expression of markers including CD29 (Abcam, Cambridge, UK), CD44 (Abcam), CD45 (Abcam) and CD90 (Abcam) was evaluated.
Assessment of ADSC multipotentiality
The original growth medium was exchanged for differentiation medium to induce ADSC differentiation into adipocytes and osteocytes as a test for their non-committed, multipotential phenotype. For adipocyte differentiation, when third or fourth passage cells reached 80% to 90% confluency, pre-adipocytes can be induced to differentiate. The differentiation media consisted of DME/F12 basal medium supplemented with 10% FBS, 1 μmol/L dexamethasone (Cyagen, Santa Clara, CA, USA), 10 μmol/L penicillin–streptomycin, and 0.5 mmol/L isobutylmethylxanthine (Cyagen). The maintenance medium was composed of DME/F12 basal medium supplemented with 10% FBS and 10 μmol/L penicillin-streptomycin. The original medium was aspirated and the same volume of differentiation medium was added. The culture dish was placed in the incubator. After 3 days, the differentiation media was replaced with maintenance medium for 24 hours. This cycle was repeated until mature adipocytes were obtained (typically three or four cycles). The accumulation of neutral lipids can be detected by staining cells with 0.5% Oil Red-O (Cyagen). For osteogenic differentiation, the differentiation media consisted of DME/F12 basal medium supplemented with 10% FBS, 1 μmol/L dexamethasone, 10 μmol/L penicillin-streptomycin, 50 μmol/L ascorbate (Hyclone), and 10 mmol/L β-glycerophosphate (Cyagen). The media was replaced every 3 days. In general, the induction time was 2 to 4 weeks. Alizarin Red (Cyagen) was used as the indicator of osteogenesis.
Culture of ADSCs within a 3D protein scaffold
We chose a 3D protein scaffold (Tantti Laboratory, Inc., Taoyuan, Taiwan) known as Bio-Scaffold, which is a three-dimensional network structure with micron-sized holes made of type I collagen. The 6-mm cell scaffolds (Tantti) were placed in a 96-well plate, soaked and cleaned three times with PBS for 5 to 10 minutes. Ten microliters of cell suspension (5 × 105 cells/mL) were implanted into the scaffold. The cell suspension was absorbed from the side of the scaffold and released to the top. This operation was repeated 6 to 10 times to increase the efficiency of cell inoculation. The cell scaffolds were placed in the incubator. After 60 minutes, fresh medium was added to the cell scaffold in the 96-well plate and it was placed in the incubator overnight. The next morning, the scaffolds were moved to new petri dishes. Growth medium was added again and culture was continued with regular media changes. One week later, the scaffold was observed for cell growth.
Development of rat model of radiation-induced vaginal injury
Female Wistar rats were maintained under specific pathogen-free conditions in a conventional facility at the Animal Experiment Center of PLA General Hospital. All experimental procedures involving laboratory animals were approved by the Institutional Review Board of PUMCH, Beijing, China (ethical code: XHDW-2015-0029) and conformed to the Guidance and Suggestions for the Care and Use of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China, 2006).
Thirty 7-week-old female rats (200–250 g) were randomly divided into three groups (groups 1–3, 10 rats per group). The rats were anesthetized by intraperitoneal injection with chloral hydrate (10%, 300 mg/kg). No signs of peritonitis were observed in rats following chloral hydrate injection. Anesthetized rats were treated by irradiation with gamma-rays. The pelvic area was irradiated and the rest of the body was covered by a lead board. We followed the QUANTEC principle for the design of animal irradiation gradients.19 Groups 1, 2, and 3 were given 30 Gy, 25 Gy, and 20 Gy radiation doses, respectively. For doses less than 25 Gy, injuries recovered spontaneously after about 1 week. For doses greater than 25 Gy, rat mortality was significantly increased. Thus, we choose 25 Gy as the radiation dose for subsequent experiments. After rats recovered from anesthesia, no significant discomfort was observed and normal movement, ingestion and excretion resumed. The rats were taken back to the animal laboratory and fed regularly. Two estrous cycles later, the rats were euthanized and the vaginal tissue was separated from the pelvis. The method for euthanasia and verification of death was the same as above. The tissue was soaked in 4% paraformaldehyde. Changes in the vaginal epithelium were observed by hematoxylin and eosin (HE) staining and immunohistochemistry to determine the dose of radiation that caused permanent damage to vaginal tissue without reaching a lethal dose. The primary antibodies were against the MUC1 antibody (Abcam, 1:200 dilution), NF-κB (Abcam, 1:300 dilution), proliferating cell nuclear antigen (PCNA; Abcam, 1:100 dilution), cytokeratin 20 (CK20; Abcam, 1:300 dilution), and interferon (IFN)-γ (Abcam, 1:100 dilution). The secondary antibodies were goat anti-rabbit IgG (Santa Cruz Biotechnology, Dallas, TX, USA; 1:3000 dilution) and goat anti-mouse IgG (Santa Cruz Biotechnology, 1:3000 dilution). We selected ten slices for each stain from each rat for subsequent measures. Photos were taken using pathological section panoramic scanner (3DHISTECH) and an inverted microscope. The thickness of the vaginal epithelium was computed following HE staining and mean density following immunohistochemistry using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Treatment of rats
Twelve 7- to 8-week-old female Wistar rats (200–250 g) were anesthetized by intraperitoneal injection with pentobarbital (30 mg/kg). As the experimental group, ten rats were randomly selected for gamma ray irradiation. The site of irradiation was the pelvic basin, and the rest of body was covered by a lead plate. The irradiation dose was 25 Gy. Two rats made up the blank control group with no irradiation. All rats were taken to the animal feeding room after awakening from anesthesia. The animals were fed regularly and their behavior was observed. After one estrous cycle, ten rats in the experimental group were randomly divided into groups A, B, C, D and E (two rats per group) and two rats remained in the blank control group F. After anesthesia with 3% pentobarbital, the skin near the vulva was sterilized with 75% alcohol and 0.5% iodophor was used to sterilize the vagina. The following treatments were performed: Group A, 500 µL of PBS were pipetted into the vaginas of rats; Group B, three wetted biological scaffolds were carefully clamped with toothless tweezers and placed in the vaginas of rats; Group C, 500 µL of rat ADSCs (third generation, 5 × 106 cells/mL) were aspirated with a pipette; Group D, three complexes of rat ADSCs and 3D protein scaffolds were carefully extracted with toothless tweezers and placed in the vaginas of the rat; Group E, no treatment following irradiation; and Group F: blank control. For experimental treatments, all 12 rats were placed in a position such that their heads were below their buttocks, which was helpful for planting experimental materials or cells into the vaginas of the rats.
All rats were sent back to the animal feeding room after waking from anesthesia for routine feeding. After two estrous cycles, all rats were sacrificed as described above. The vaginal tissues were dissected and specimens were soaked in 4% paraformaldehyde prior to HE staining and immunohistochemistry. Specimens were placed in a cryopreservation tube and stored at −80°C for subsequent western blotting experiments. The primary antibodies were against MUC1 (1:800 dilution), NF-κB (1:1000 dilution), PCNA (1:500 dilution), CK20 (1:1000 dilution) and IFN-γ (1:500 dilution). The secondary antibodies were goat anti-rabbit IgG (1:3000 dilution) and goat anti-mouse IgG (1:3000 dilution). The grayscale value of the strip was read using Quantity One v.4.6.2 software.
Throughout the animal experiments, animals were given ad libitum access to food and clean drinking water. The quality of experimental cages and bedding materials all met national standards. Regular cleaning and disinfection of cages as well as sterilizing, dusting and replacement of bedding materials was necessary. The rats could turn, stand, and lie down in their cages. During the experiments, operation areas were clean and quiet. Operations were gentle and rapid to ensure animals lost consciousness quickly. Rat euthanasia was not seen by other experimental rats to reduce the fear and pain.20
RNA sequencing
Rats were divided into three groups: CK, the blank control group; Rad, the irradiation group; and Tre, the complex treated group. Low quality indicated the ratio of low-quality reads to the total number of reads. High quality clean indicated the ratio of the data obtained by removing impurities from the original sequence data to the total number of reads. Each group in this study was above 98%. The vaginal tissues of three groups were selected for RNA extraction. We used oligo(dT) beads to purify mRNA, which was then fragmented and converted into double-stranded cDNA by reverse transcription and DNA repair. A poly A tail was added at the 3ʹ end. Using the specific connection on both ends of the DNA sequencing joint, a sequencing library was constructed after PCR amplification. The Illumina Genome Analyzer was used for high-throughput sequencing.
Statistical analysis
SPSS version 20.0 (IBM, Armonk, NY, USA) was used for statistical analysis and GraphPad Prism 5.0 was used to prepare figures (GraphPad Inc., San Diego, CA, USA). One-way analysis of variance was used to assess differences in measurement data across multiple groups, and this was followed by Tukey’s test. Values of p < 0.05 were considered statistically significant.
Results
ADSC-protein scaffold complexes
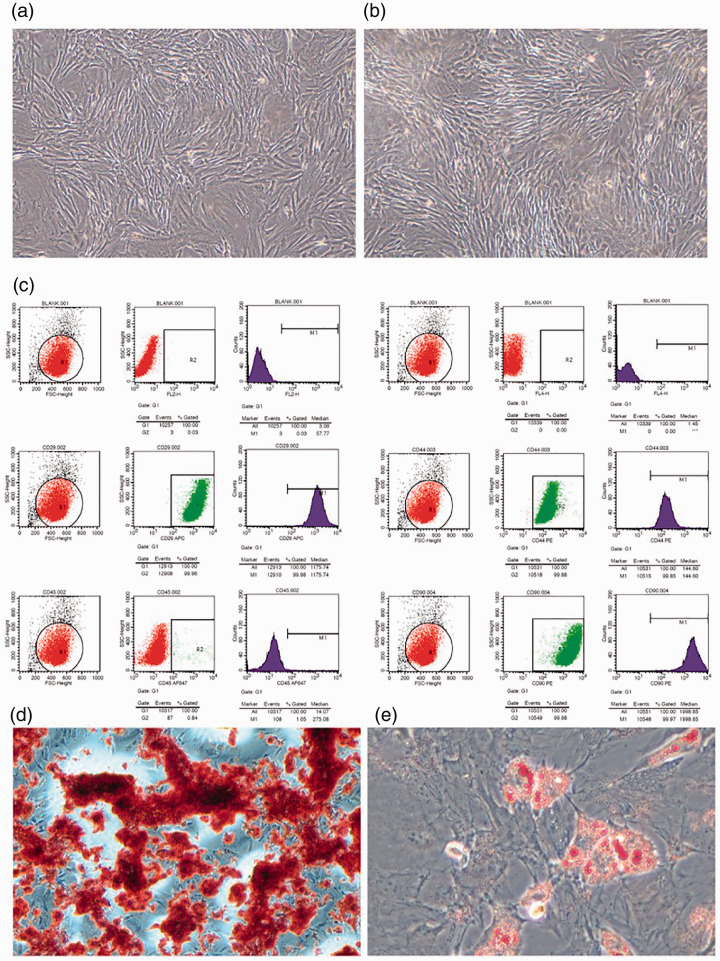
Purified cells were cultured in tissue culture dishes. After 3 days in culture, the number of adherent cells gradually increased. Some cells were spindle shaped, some grew alone, and some formed symmetrical colonies (Figure 1a). After approximately three passages (Figure 1b), flow cytometry was used to assess the expression of ADSC surface markers. More than 99% of cells expressed CD29, CD44, and CD90. In contrast, the negative markers FL2-H, FL4-H, and CD45 were not present (Figure 1c). These results indicated that ADSCs had multipotent differentiation potential, and could differentiate into osteoblasts and adipocytes (Figure 1d, 1e).
Figure 1.
Isolation and identification of ADSCs. (a, b) The ADSCs exhibited typical fibroblastic morphology. (c) Flow cytometry of ADSCs. The cells were positive for CD29, CD44, and CD90 but negative for FL2-H, FL4-H, and CD45. (d, e) ADSCs differentiate into osteoblasts and adipocytes. Panels a and b: magnification 100 times. Panels d and e: magnification 400 times.
ADSC, adipose-derived mesenchymal stem cell.
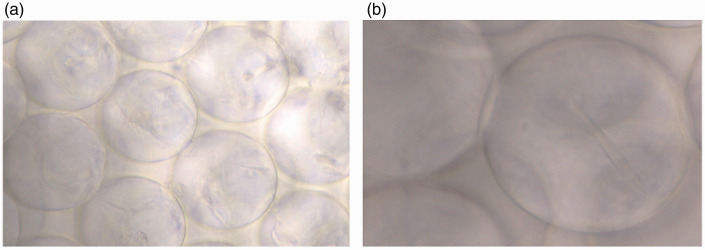
The 3D protein scaffold was cocultured with second-passage ADSCs for 1 week. The complexes were then moved to a 10-cm culture dish to continue the culture. The ADSCs detached from the complexes and continued to grow in the culture dish (Figure 2). The cells could survive normally for at least 1 week within the 3D protein scaffold.
Figure 2.
Spindle cells within the scaffold were faintly visible and the growth state was good and stable. (a) magnification 400 times. The nest structure of collagen can be seen. (b) magnification 1000 times. Spindle cells within the collagen scaffold can be seen.
ADSC-protein scaffold complexes promoted healing of radiation-induced vaginal injury in rats
Rats were randomly selected for irradiation with 25 Gy of gamma rays. After two estrous cycles, vaginal injuries were not repaired.
Histological analysis
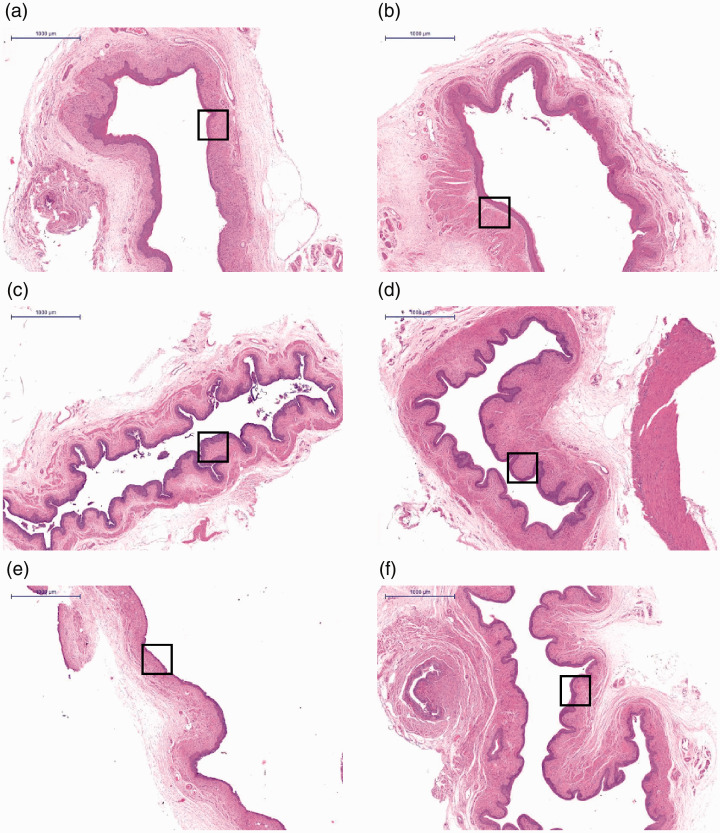
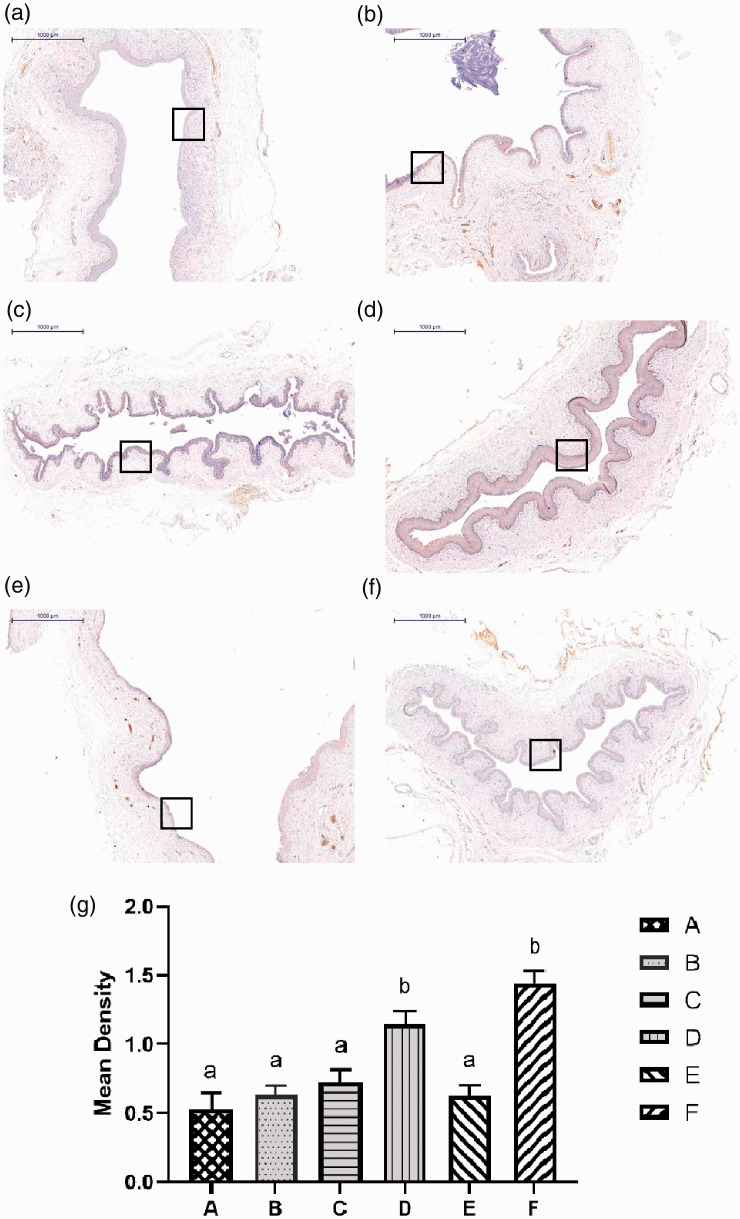
HE staining was used to observe histological structures of the regenerating vaginal epithelium. After 2 weeks, epithelial disruption and disorganization of the subepithelial fibromuscular tissue were observed in groups A, B and E. By contrast, the epithelial layers of rats in groups C, D and F treated with complexes and with ADSCs alone had a normal appearance (Figure 3c, 3d and 3f). The vaginal epithelial thickness of the complex-treated group was significantly increased and was similar to that of the negative control group (Table 1). These data showed that vaginal injuries in the complex- and ADSCs-treated groups had partially healed by week 2. However, injuries in the other groups did not heal during the observation period.
Figure 3.
Hematoxylin and eosin staining of the vaginal epithelium. (a) Injection of PBS into the vaginal injury site following irradiation; (b) injection of protein scaffold into the vaginal injury site following irradiation; (c) injection of ADSCs into the vaginal injury site following irradiation; (d) Injection of complex into the vaginal injury site following irradiation; (e) untreated rats following irradiation; (f) blank control group. N=5 rats per group. Scale bar = 1000 μm. Squares indicate the studied areas.
ADSC, adipose-derived mesenchymal stem cell.
Table 1.
Vaginal epithelial thickness in the different groups of rats.
| Group | Epithelial thickness (µm) | CK20 (OD) | IFN-γ (OD) | MUC1 (OD) | NF-κB (OD) | PCNA (OD) |
|---|---|---|---|---|---|---|
| A | 39.57±4.01 | 2.53±0.75 | 9.71±0.93 | 0.53±0.12 | 1.22±0.13 | 1.14±0.41 |
| B | 39.7±5.29 | 2.17±0.65 | 8.28±1.3 | 0.63±0.06 | 1.06±0.34 | 1.19±0.18 |
| C | 48.26±4.19 | 2.96±1.37 | 10.46±2.71 | 0.72±0.09 | 1.25±0.09 | 1.34±0.42 |
| D | 87.41±5.16 | 4.29±0.11 | 18.16±4.23 | 1.15±0.09 | 3.71±0.57 | 2.69±0.68 |
| E | 20±1.41 | 2.74±0.25 | 6.06±3.8 | 0.63±0.07 | 1.75±0.29 | 1.29±0.41 |
| F | 65.52±4.36 | 5.14±0.56 | 19.16±0.43 | 1.44±0.09 | 6.12±0.38 | 3.56±0.65 |
| F-statistic | 29.493 | 2.297 | 5.188 | 13.528 | 25.686 | 3.894 |
| P-value | <0.001 | 0.142 | 0.020 | 0.001 | <0.001 | 0.037 |
OD, optical density.
Analysis of the proliferation marker PCNA
To determine whether the proliferative capacity of epithelial cells was modified in the irradiated vagina, immunohistochemical analysis of PCNA was performed. PCNA expression in cells can be used as an indicator of cell proliferation.21 PCNA was highly expressed in the vaginal epithelium of treated and negative control rats. There were differences in PCNA staining in the subepithelial vaginal muscularis layer in the wound area between treated and untreated animals (Figure 4a–f).
Figure 4.
Analysis of the proliferation marker PCNA. (a) Injection of PBS into the vaginal injury site following irradiation; (b) injection of protein scaffold into the vaginal injury site following irradiation; (c) injection of ADSCs into the vaginal injury site following irradiation; (d) Injection of complex into the vaginal injury site following irradiation; (e) untreated rats following irradiation; (f) blank control group. N=5 rats per group. Scale bar = 1000 μm. (g) The average optical density of each group. There was no statistical difference between groups marked with the same letter. Higher power magnifications of the square area are shown in (d, f). The expression of PCNA in groups D and F was significantly increased.
PCNA, proliferating cell nuclear antigen; ADSC, adipose-derived mesenchymal stem cell.
The average optical densities of the model group rats were significantly lower than those of complex-treated and negative control rats. There was no significant difference between the complex-treated and negative control groups (Figure 4g).
Investigation of the therapeutic mechanism of ADSCs using RNA sequencing
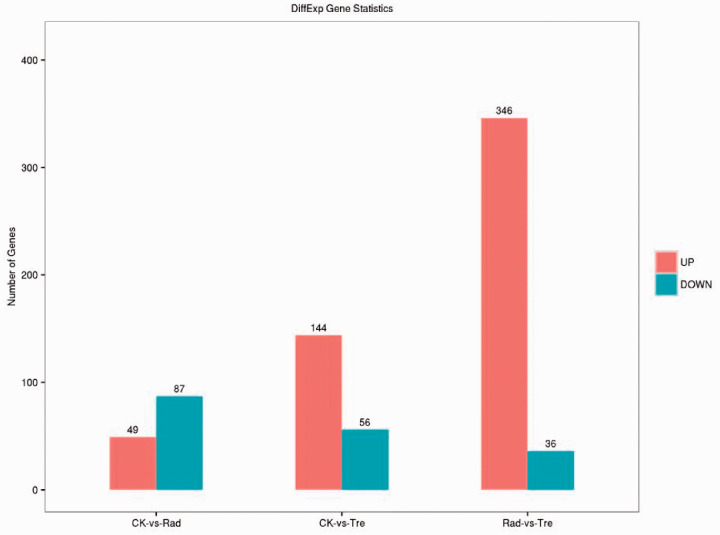
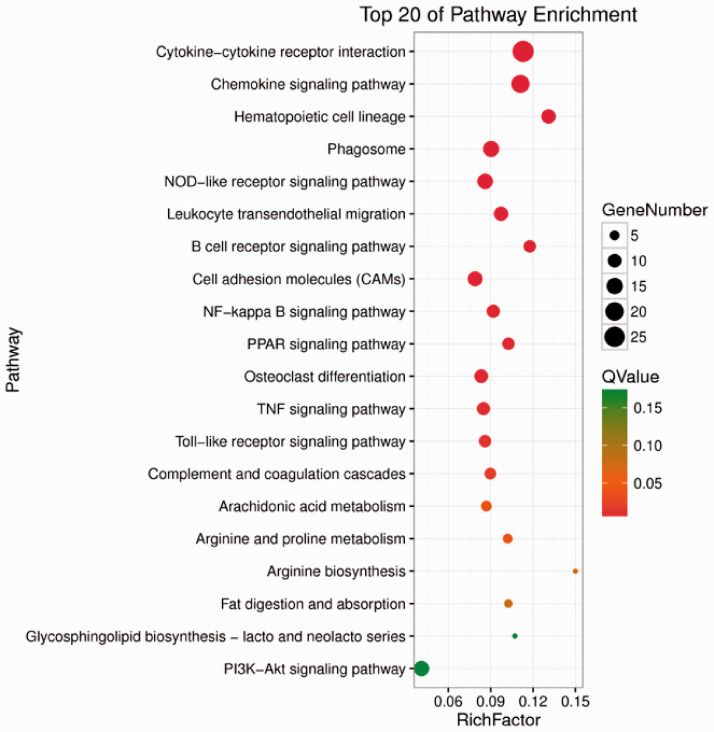
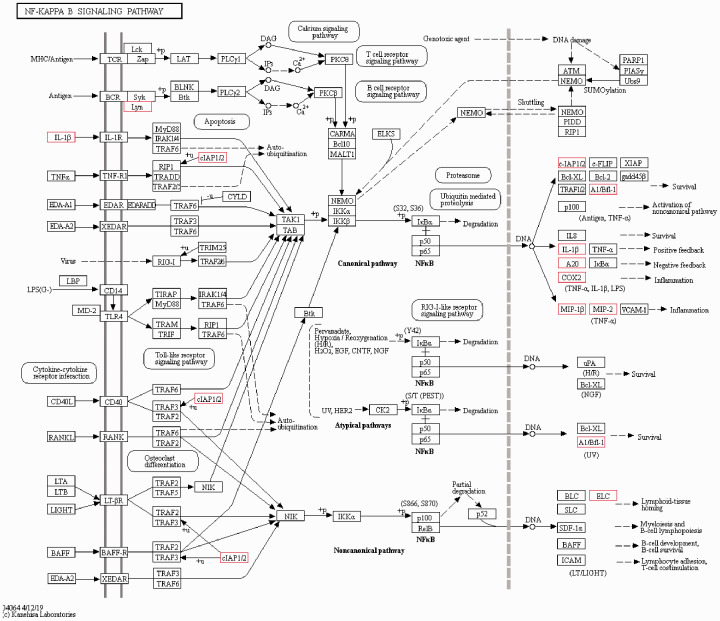
Rats were divided into three groups: CK, the blank control group; Rad, the irradiation group; and Tre, the complex-treated group. Low quality indicated the ratio of low-quality reads to the total number of reads. High quality clean indicated the ratio of the data obtained by removing impurities from the original sequence data to the total number of reads. Each group in this study was above 98% (Figure 5, 6). The Illumina Genome Analyzer was used for high-throughput sequencing. Eleven genes in the NF-κB pathway were found to have significant changes (Figure 7, 8). The NF-κB pathway is an important signaling pathway involved in tissue damage repair.22 ADSCs may exert their repair effect on damaged tissues by activating the expression of this pathway. Molecular regulation of the early stages of the immune response and of various stages of the inflammatory response, perhaps involving colony-stimulating factors as well as anti-inflammatory and apoptosis-related molecules and tumor necrosis factor receptor-related factors, could affect tissue damage and repair processes.
Figure 5.
In this experiment, rats were divided into three groups: CK, the blank control group; Rad, the irradiation group; and Tre, the complex treatment group. Low quality indicated the ratio of low-quality reads to the total number of reads. High quality clean indicated the ratio of the data obtained by removing impurities from the original sequence data to the total number of reads. Each group in this study was above 98%.
Figure 6.
Orange represents up-regulated genes, blue represents down-regulated genes. Some genes were up- or down-regulated in the control group compared with the complex treatment group.
Figure 7.
The NF-κB pathway was enriched for some genes whose expression was up-regulated. The NF-κB pathway is an important signaling pathway involved in tissue damage repair. The diameter represents the number of differentially expressed genes; the wider the diameter, the greater the number. Red represents statistical significance.
Figure 8.
Differentially expressed genes within the NF-κB pathway are marked with a red frame. These include Lyn, IL-1, cIAP1/2, A1/Bfl-1, A20, COX2, MIP-1, MIP-2, ELC, and A20 (zinc finger protein). This figure was selected from the KEGG website (numbered map04064).
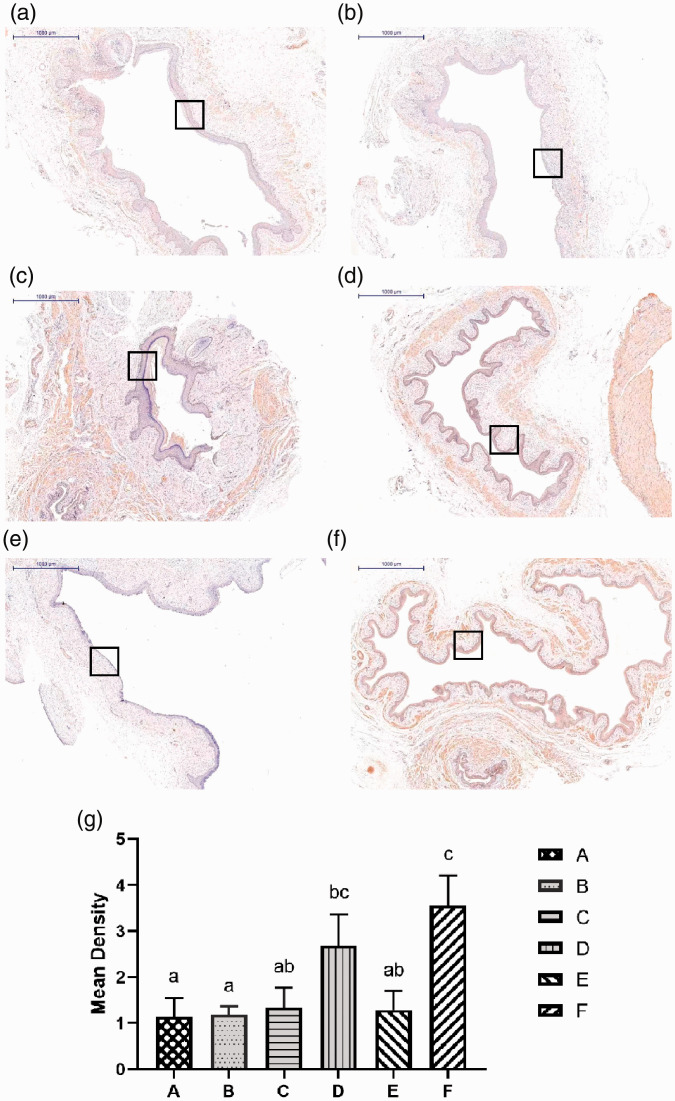
Analysis of MUC1 expression
The mucin MUC1 is a membrane-bound glycoprotein expressed on the apical surfaces of most mucosal epithelial cells.23 The expression of MUC1 is tissue specific. The protein participates in essential functions, such as protection and lubrication of epithelial cells, maintenance of epithelial layers, cellular adhesion, differentiation, and immunity.24 Analysis of MUC1 expression in the healed vaginal epithelium was performed. We found that there were significant differences between treated and untreated rats (Figure 9a–f). The results showed that model group rats had significantly lower MUC1 expression than complex-treated and negative control rats (p < 0.01). In contrast, MUC1 had significantly higher expression in complex-treated and the negative control rats than in other groups (p < 0.01). There was no difference in MUC1 expression between complex-treated and negative control rats (Figure 9g).
Figure 9.
Analysis of MUC1 expression. (a) Injection of PBS into the vaginal injury site following irradiation; (b) injection of protein scaffold into the vaginal injury site following irradiation; (c) injection of ADSCs into the vaginal injury site following irradiation; (d) Injection of complex into the vaginal injury site following irradiation; (e) untreated rats following irradiation; (f) blank control group. N=5 rats per group. Scale bar = 1000 μm. (g) The average optical density of each group. There was no statistical difference between groups marked with the same letter. Squares indicate the studied areas. Higher power magnifications of the square area are shown in (d, f). The expression of MUC1 in groups D and F was significantly increased.
PBS, phosphate-buffered saline.
Western blot assessment protein expression in each group
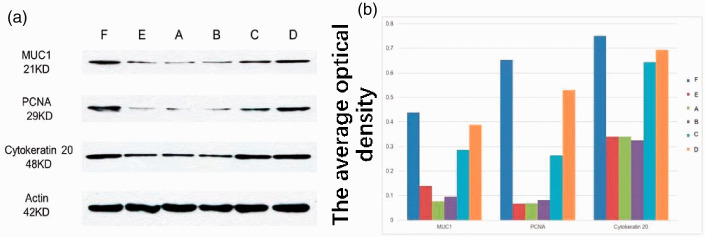
Western blotting showed that levels of MUC1 and PCNA expression were significantly higher in complex-treated and negative control rats than in the untreated rats (p < 0.01; Figure 10). There was no significant difference in CK20 expression between groups. These results showed that the complex significantly promoted repair of vaginal injury.
Figure 10.
Protein expression in different groups. A. (a) Injection of PBS into the vaginal injury site following irradiation; (b) injection of protein scaffold into the vaginal injury site following irradiation; (c) injection of ADSCs into the vaginal injury site following irradiation; (d) Injection of complex into the vaginal injury site following irradiation; (e) untreated rats following irradiation; (f) blank control group. N=5 rats per group. B. The average optical density of each group.
PBS, phosphate-buffered saline; ADSC, adipose-derived mesenchymal stem cell.
Discussion
Cervical cancer is a global public health problem, and is the fourth most common cancer affecting women worldwide.25 Although radiotherapy is the standard treatment for locally advanced cervical cancer, it causes side effects such as radiation cystitis, radiation proctitis, and vaginal stenosis and contracture.26 Radiation-induced vaginal injury is the most common complication following radiotherapy of cervical cancer.27 During treatment, the applicator directly contacts the vaginal wall, causing radiation to damage its blood vessels and resulting in local blood circulation disorders, vaginal mucosal edema, necrosis, shedding, and ulcers. Radiation-induced oxidative stress is a consequence of the rapid generation of reactive oxygen species from radiolysis of cellular water and oxidative damage to biomolecules like proteins, lipids, and DNA.28 In addition, the tumor tissue becomes necrotic and detached after radiotherapy. If the tumor tissue is not removed in time, it will accumulate in the vagina, causing and aggravating bacterial infections. In addition, vaginal reactions to radiation occur, such as radiation vaginitis, vaginal adhesions, and atresia.29 With the long-term survival of patients with cervical cancer improving, maximizing their quality of life following radiotherapy has become increasingly important. Therefore, finding ways to prevent the occurrence of vaginal injuries following radiotherapy has become a clinical goal. However, there are only a few reports regarding the use of lubricants or estrogen for healing of vagina injuries.30
Radiation therapy is one of the most important treatment modalities for cervical, breast and esophageal cancer.31 Despite advances in radiation techniques enabling dose delivery and distribution directly to the tumor mass with less toxicity to surrounding healthy tissue, radiation-induced vaginal injury still occurs and remains a dose-limiting issue.32 Different methods of pelvic floor reconstruction have been proposed to avoid these radiation complications, but there is no consensus on the best technique.33 Recently, stem cell-based tissue engineering, which is under study for genitourinary tract reconstruction, has been proposed for pelvic organ prolapse treatment.34 Because there is no accepted animal model of vaginal radiation injury, we established a rodent model of vaginal radiation injury that can be used to study the process of healing.
Potential therapeutic effects of ADSC transplantation in models of radiation-induced injury were suggested to involve paracrine and/or anti-inflammatory mechanisms.35MSCs can promote the healing of damaged tissues. This beneficial effect mainly arises from nutrition and secretion of various growth factors that promote angiogenesis.36 We chose ADSCs combined with a Bio-Scaffold to heal radiation-induced vaginal injuries. As a novel source of MSCs, adipose tissue is abundant, poorly immunogenic, fast growing, minimally invasive, safe for autologous transplantation, free of ethical problems, and superior to other sources.37 MSCs were transplanted into suburethral tissues of different animal models and vaginal dilation models.38,39 However, previous studies of stem cell transplantation into vaginal tissues yielded unclear results and the survival rate of transplanted cells was very low.35 The development of tissue engineering has provided a new way to solve this problem.40
A collagen hydroxyapatite scaffold was able to commit human MSCs toward osteogenic differentiation in vitro and in vivo.41 In our model, the Bio-Scaffold was composed of type I collagen. The Bio-Scaffold showed high biocompatibility, supporting host cell proliferation and differentiation and promoting formation of the extracellular matrix on scaffold surfaces and pores.42
The ideal scaffold requires mechanical strength and also needs to have good biocompatibility. It should also accommodate a certain density of ADSCs and promote cell growth and reproduction.43 The protein scaffold, which has a uniform 3D cross-sectional pore structure, has been found to have good histocompatibility in bone tissue engineering.44 To the best of our knowledge, the present study was the first to apply this material to the treatment of vaginal injury. After culture of ADSCs within the scaffold, cells were found to have spread throughout the scaffold; some cells were immersed and could survive for at least 1 week. This suggested that the new scaffold had good histocompatibility and could adapt to the growth and proliferation of ADSCs. ADSCs can stably survive within biological scaffold and are not easily spilled into other tissues. The ADSCs within the scaffold can be continuously renewed through paracrine and anti-inflammatory effects, and the transplanted cells can even differentiate into host tissue.45
In this study, we constructed an animal model of post-irradiation vaginal injury and demonstrated the beneficial effects of ADSC-protein scaffold complexes in healing the injured vaginal epithelium. Untreated animals had delayed injury healing, with some not healing at all, as well as prolonged expression of PCNA and MUC1. We used RNA sequencing to explore the mechanism underlying the therapeutic effect of ADSCs and found that ADSCs may mediate repair by activating expression of the NF-κB signaling pathway. NF-κB activation plays a major role in the transcriptional control of acute and chronic inflammation,46 and the complex may induce anti-inflammatory effects through this pathway. These findings suggest that ADSCs alone as well as the complex could effectively treat radiation-induced vaginal injury.
The therapeutic effect of the ADSC-protein scaffold complex was better than that of the ADSCs alone. After complex treatment, the thickness of the vaginal epithelium reached that of negative control group rats. Therefore, therapy with the ADSC-protein scaffold complex may be useful for patients suffering from vaginal injury following radiotherapy. More research is needed to evaluate the efficacy and safety of the complex in treating tissue injury. Future studies could consider longer observation periods to determine whether some damage is not repaired.
Conclusions
This study provides an important reference for clinical application of protein scaffolds combined with ADSCs to treat post-irradiation vaginal injury. Furthermore, it provides important methods for preparing animal models of radiation-induced vaginal injury.
Acknowledgments
The authors thank Yao Yuanqing (Department of Obstetrics and Gynecology, The First Medical Center, Chinese PLA General Hospital) for help with the experiments. We also thank everyone in the Department of Obstetrics and Gynecology, The First Medical Center, Chinese PLA General Hospital, for their scientific advice and encouragement.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (81601262).
ORCID iD: Ling Yu https://orcid.org/0000-0002-8781-3033
References
- 1.Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Curr Opin Obstet Gynecol 2016; 28: 4–10. [DOI] [PubMed] [Google Scholar]
- 2.Dinicola S, Pasta V, Costantino D, et al. Hyaluronic acid and vitamins are effective in reducing vaginal atrophy in women receiving radiotherapy. Minerva Ginecol 2015; 67: 523–531. [PubMed] [Google Scholar]
- 3.Greimel ER, Winter R, Kapp KS, et al. Quality of life and sexual functioning after cervical cancer treatment: A long-term follow-up study. Psychooncology 2009; 18: 476–482. [DOI] [PubMed] [Google Scholar]
- 4.Javier MG, Sastre-Garau X. Uterine cervix carcinoma: recent biological data and update for improving follow-up and treatment. Isr Med Assoc J 2012; 14: 700–704. [PubMed] [Google Scholar]
- 5.Vaz AF, Conde DM, Costa-Paiva L, et al. Quality of life and adverse events after radiotherapy in gynecologic cancer survivors: A cohort study. Arch Gynecol Obstet 2011; 284: 1523–1531. [DOI] [PubMed] [Google Scholar]
- 6.Grion RC, Baccaro LF, Vaz AF, et al. Sexual function and quality of life in women with cervical cancer before radiotherapy: A pilot study. Arch Gynecol Obstet 2016; 293: 879–886. [DOI] [PubMed] [Google Scholar]
- 7.Corrêa CS, Leite IC, Andrade AP, et al. Sexual function of women surviving cervical cancer. Arch Gynecol Obstet 2016; 293: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Chen L, Tergas AI, et al. Population-level trends in relative survival for cervical cancer. Am J Obstet Gynecol 2015; 213: 670.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candy B, Jones L, Vickerstaff V, et al. Interventions for sexual dysfunction following treatments for cancer in women. Cochrane Database Syst Rev 2016; 2: CD005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician 2010; 82: 381–388, 394. [PubMed] [Google Scholar]
- 11.Chan JL, Letourneau J, Salem W, et al. Sexual satisfaction and quality of life in survivors of localized cervical and ovarian cancers following fertility-sparing surgery. Gynecol Oncol 2015; 139: 141–147. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Yang X, Dai Y, et al. Survey of cervical cancer survivors regarding quality of life and sexual function. J Cancer Res Ther 2016; 12: 938–944. [DOI] [PubMed] [Google Scholar]
- 13.Platt JL, Cascalho M. New and old technologies for organ replacement. Curr Opin Organ Transplant 2013; 18: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M, Wang S, Li Y, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther 2013; 4: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 2011; 17: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prousky J. The treatment of pulmonary diseases and respiratory-related conditions with inhaled (nebulized or aerosolized) glutathione. Evid Based Complement Alternat Med 2008; 5: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoch AI, Leach JK. Concise review: Optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med 2015; 4: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzoni E, D'Agostino A, Iaquinta MR, et al. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl Med 2020; 9: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanic S, Mayadev JS. Tolerance of the small bowel to therapeutic irradiation: A focus on late toxicity in patients receiving para-aortic nodal irradiation for gynecologic malignancies. Int J Gynecol Cancer 2013; 23: 592–597. [DOI] [PubMed] [Google Scholar]
- 20.March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25: 544–551. [DOI] [PubMed] [Google Scholar]
- 21.Takeno S, Hamamura N, Tatsukawa T, et al. [Proliferating cell nuclear antigen (PCNA) immunolocalization in human nasal epithelium with chronic sinusitis detected by confocal laser scanning microscopy]. Nihon Jibiinkoka Gakkai Kaiho 1996; 99: 1119–1125. [DOI] [PubMed] [Google Scholar]
- 22.Abdel FSM, Saif-Elnasr M, Soliman AF. Platelet-rich plasma as a potential therapeutic approach against lead nitrate- and/or gamma radiation-induced hepatotoxicity. Environ Sci Pollut Res Int 2018; 25: 34460–34471. [DOI] [PubMed] [Google Scholar]
- 23.Kato K, Lillehoj EP, Lu W, et al. MUC1: The first respiratory mucin with an anti-inflammatory function. J Clin Med 2017; 6: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds IS, Fichtner M, McNamara DA, et al. Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer Metastasis Rev 2019; 38: 237–257. [DOI] [PubMed] [Google Scholar]
- 25.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 26.Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev 2014; 9: CD007291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusumoto S, Fujiwara H, Sagawa M, et al. Successful radiotherapy for endometrial serous carcinoma with local repeated recurrence. Int Cancer Conf J 2018; 7: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haton C, François A, Vandamme M, et al. Imbalance of the antioxidant network of mouse small intestinal mucosa after radiation exposure. Radiat Res 2007; 167: 445–453. [DOI] [PubMed] [Google Scholar]
- 29.Said RS, Badr AM, Nada AS, et al. Sodium selenite treatment restores long-lasting ovarian damage induced by irradiation in rats: impact on oxidative stress and apoptosis. Reprod Toxicol 2014; 43: 85–93. [DOI] [PubMed] [Google Scholar]
- 30.Shveiky D, Iglesia CB, Sarkar DS, et al. Age-associated impairments in tissue strength and immune response in a rat vaginal injury model. Int Urogynecol J 2020; 31: 1435–1441. [DOI] [PubMed] [Google Scholar]
- 31.Zhang K, Yang S, Zhu Y, et al. Protection against acute radiation-induced lung injury: A novel role for the anti-angiogenic agent Endostar. Mol Med Rep 2012; 6: 309–315. [DOI] [PubMed] [Google Scholar]
- 32.Zanoni M, Cortesi M, Zamagni A, et al. The role of mesenchymal stem cells in radiation-induced lung fibrosis. Int J Mol Sci 2019; 20: 3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carboni F, Federici O, Giofre' M, et al. Empty pelvis syndrome: the use of breast prosthesis in the prevention of complications. Colorectal Dis 2019; 21: 1321–1325. [DOI] [PubMed] [Google Scholar]
- 34.Ding J, Han Q, Deng M, et al. Induction of human umbilical cord mesenchymal stem cells into tissue-forming cells in a murine model: Implications for pelvic floor reconstruction. Cell Tissue Res 2018; 372: 535–547. [DOI] [PubMed] [Google Scholar]
- 35.Sadeghi Z, Isariyawongse J, Kavran M, et al. Mesenchymal stem cell therapy in a rat model of birth-trauma injury: Functional improvements and biodistribution. Int Urogynecol J 2016; 27: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronckaers A, Hilkens P, Martens W, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther 2014; 143: 181–196. [DOI] [PubMed] [Google Scholar]
- 37.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007; 100: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo F, Ninotta G, Schenone M, et al. Advances in stem cell therapy for male stress urinary incontinence. Expert Opin Biol Ther 2019; 19: 293–300. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S, Zhang K, Atala A, et al. Stem cell therapy for treatment of stress urinary incontinence: The current status and challenges. Stem Cells Int 2016; 2016: 7060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med 2011; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabrese G, Giuffrida R, Fabbi C, et al. Collagen-hydroxyapatite scaffolds induce human adipose derived stem cells osteogenic differentiation in vitro. PLoS One 2016; 11: e0151181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabrese G, Forte S, Gulino R, et al. Combination of collagen-based scaffold and bioactive factors induces adipose-derived mesenchymal stem cells chondrogenic differentiation in vitro. Front Physiol 2017; 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Kemp V, De Graaf P, Fledderus JO, et al. Tissue engineering for human urethral reconstruction: Systematic review of recent literature. PLoS One 2015; 10: e0118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trottier V, Marceau-Fortier G, Germain L, et al. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008; 26: 2713–2723. [DOI] [PubMed] [Google Scholar]
- 45.Jin M, Chen Y, Zhou Y, et al. Transplantation of bone marrow-derived mesenchymal stem cells expressing elastin alleviates pelvic floor dysfunction. Stem Cell Res Ther 2016; 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun F, Shi J, Chen S, et al. Lazaroid U-74389G inhibits the osteoblastic differentiation of IL-1β-induced aortic valve interstitial cells through glucocorticoid receptor and inhibition of NF-κB pathway. J Steroid Biochem Mol Biol 2015; 152: 114–123. [DOI] [PubMed] [Google Scholar]