Abstract
A Veterans Affairs long term care facility on Long Island New York was confronted with a COVID-19 outbreak in late March to Mid-April 2020. Faced with a dwindling supply of PPE, the Infection Control team distributed supplies saved for a possible Ebola outbreak. A COVID unit was created within the nursing home facilitating the geographic isolation of cases; universal testing of residents and employees allowed for the implementation of proper quarantine measures. It was a multidisciplinary team approach led by the Infection Control team that successfully contained this outbreak.
Key Words: Long term care facilities, Coronavirus, COVID-19
Since the first cases reported in December 2019, infection with the severe acute respiratory coronavirus 2 (SARS CoV-2) has become a worldwide pandemic. The disease caused by this novel virus, the Corona Virus Disease-2019 (COVID-19) has overwhelmed health care systems globally. The World Health Organization declared COVID-19 a pandemic on March 11, 2020.1 The pandemic was first recognized in the United States in Washington State, involving an outbreak in a skilled nursing facility; currently confirmed cases exceed 7 million.2 , 3 Eventually, multiple outbreaks caused by the novel coronavirus quickly emerged in long-term care and assisted living facilities worldwide. Elderly patients are particularly vulnerable and susceptible to adverse clinical outcomes of COVID-19 including respiratory failure and death.4 According to the Nursing Home COVID-19 Public File from data reported by nursing homes to the Centers for Disease Control and Prevention's (CDC) National Healthcare Safety Network, of 207,315 cases in the US nursing homes, 51,700 deaths (25%), have been reported.5 In a review of over 9,000 nursing homes from the United States, COVID-19 outbreaks were more related to facility size, location––with Massachusetts and New Jersey having the greatest number of affected facilities—and greater percentage of African American residents.6 The last mentioned fact mirrors a critical health disparity seen in this pandemic at large, namely African Americans contracting SARS CoV-2 at higher rates and are more likely to die.7 Although nationally the virus spreads like wildfire in nursing homes (among residents and working staff), the Department of Veterans Affairs (VA) reported lower COVID-19 rates in their affiliated nursing homes in a US Congressional hearing.8 We discuss here our experience in facing a COVID-19 outbreak in our VA affiliated nursing homes.
METHODS
Northport Veterans Affairs Medical Center provides hospital-based acute medical and psychiatric care for US Veterans, as well as long-term/extended nursing home and residential mental health program care. The nursing homes are structured as community living centers (CLCs), with a 139 total bed capacity. There are 4 CLCs, CLC1-4, which include mental health (CLC3) and hospice care units (CLC4). The COVID-19 outbreak involved CLC 1 and CLC2, both totaling 80 beds. Veterans are eligible to live in the CLCs if they have service-connected disability (70% or more)–a disability assigned to them by the Department of VA based on injury or illness that incurred, or, was aggravated during active military service. CLC 1 and 2 units admit Veterans with complex underlying medical issues that can include immobility due to stroke and cardiovascular disease, continuous oxygen requirement due to chronic obstructive lung disease, end stage renal disease on dialysis, complex wound care, chemotherapy, prolonged intravenous antibiotic therapy, and other rehabilitation needs.
We performed an observational single-site study to achieve an epidemiological understanding of the COVID-19 outbreak in our facility between March 24 to April 18, 2020. We reviewed the electronic medical records of the infected CLC residents, and we obtained the following data: age, demographic information, medical history, presenting symptoms and vitals, radiographic and laboratory data, and bed location of the patients in the CLCs at the time of COVID-19 diagnosis. Antibiotic choices and outcomes were recorded. Dichotomous and categorical variables were compared using chi square test or Fisher's exact test. All data during the outbreak were collected as part of the facility's healthcare epidemiology process by our infection control/infectious diseases specialists, in an effort to control the outbreak. We also describe the implemented preventive measures and their impact on containing the outbreak. The study was approved by the Institutional Review Board.
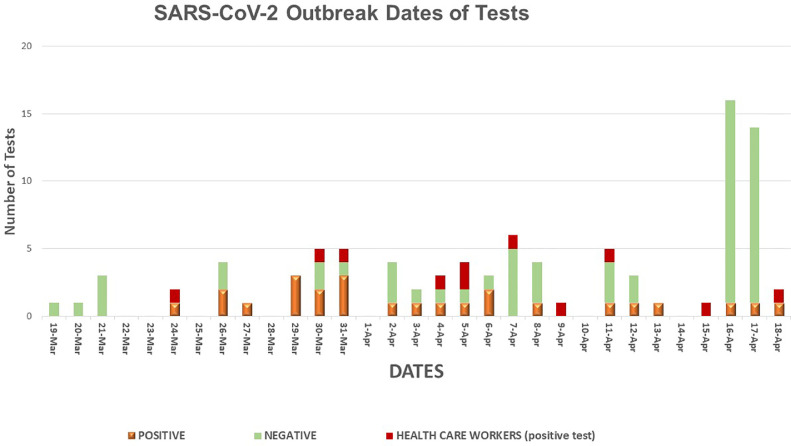
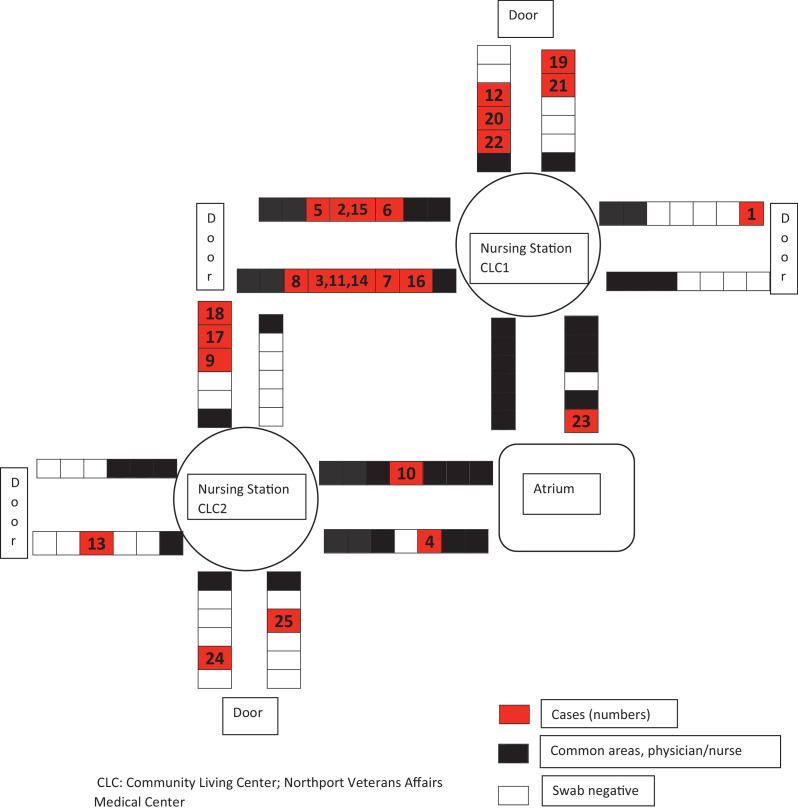
Every CLC resident who either developed a temperature of >99° F with or without symptoms of respiratory illness and tested positive for SARS-CoV-2 by nasopharyngeal or sputum reverse transcription polymerase chain reaction (RT-PCR), or was asymptomatic and screened positive by RT-PCR, was defined as a case of COVID-19. Health care workers were defined as probable COVID-19 cases if they had respiratory symptoms during the outbreak period, whereas definite cases required a positive RT-PCR. In the beginning our PT-PCR tests were performed at the VA Public Health Reference Laboratory in Palo Alto California (utilizing the CDC's RT-PCR test) and the turnaround time was 3-4 days. By March 30th we started utilizing the rapid RT-PCR Cepheid, Sunnyvale, CA. The turnaround time was 1 hour. The first identified cases were a CLC resident and a health care provider (HCP) and were diagnosed on the same day, March 24th. Figure 1 shows the tests performed by date; positive and negative results for residents and positive results for HCP are included. Figure 2 illustrates the CLC 1 and CLC 2 outline of beds indicating positive cases in sequence of detection. Initial spread of the virus was fast, affecting residents and health care workers.
Fig 1.
SARS-CoV-2 outbreak dates of tests.
Fig 2.
Outline of CLCs and COVID-19 cases.
The infection control team in our organization, consists of 4 facility-wide infection control nurses; a nurse manager, a multidrug resistant organisms expert and 2 infection control preventionists. Understandably, during this period, their primary focus was halting and containing the SARS-CoV-2 outbreak.
INFECTION CONTROL MEASURES
The Department of Veterans Affairs Deputy Under Secretary for Health for Operations and Management sent a Memorandum on March 17th providing updated guidance for CLCs, which we followed throughout the outbreak. Initially and in preparation for a possible outbreak we restricted all visitations to the CLCs, except for compassionate care situations, such as an actively dying Veteran. Infection control preventionists educated the residents and staff on the new Coronavirus. Guidance to screen all residents for signs/symptoms of COVID19 daily was implemented. All staff entering the CLCs had temperature checks and were also screened for signs and symptoms of COVID 19.
After the first case was identified, abiding by the above-mentioned guidance, we initially implemented social distancing and isolation practices. All resident group activities and communal dining were permanently halted, floating of staff between units minimized as feasibly as possible, new admissions to the units were stopped. Residents were requested to stay in their rooms; if exiting their room, they were educated to wear a mask and follow proper respiratory etiquette. Hand hygiene was also enforced, as well as education on proper use of personal protective equipment (PPE) by the infection preventionists on their daily environmental rounds. Symptomatic employees including asymptomatic who were screened positive were requested to self-isolate at home. Symptomatic residents with fever or respiratory symptoms (including cough, dyspnea) were placed in a single room, the doors were to remain closed and geriatricians were requested to stop nebulized treatments. Alternatively, they could use Meter Dose Inhaler's, if that was feasible, as per CDC recommendations. As RT-PCR results were initially delayed, these residents were considered “patients under investigation” and droplet/contact precautions with proper PPE was required to enter their rooms, focusing on containing and even stopping the spread of SARS-CoV 2.
Increased demand and utilization of the appropriate PPE can quickly deplete resources and pose operational dilemmas to health care facilities. As part of contingency strategies in resource utilization, the infection control team inventoried the facility's stored supplies. The team, distributed PPE originally saved for an Ebola outbreak to the health care teams in the emergency room and dedicated COVID units. These included whole body suits, head and neck coverings, booties/shoe coverings, and N95 respirators. This initiative from the infection control team helped in providing solidarity and improving the treatment experience for our colleagues working in the nursing homes.
On April 2nd the Center for Medicare and Medicaid Services recommended screening of all nursing home residents and cohorting of staffing teams for infected and uninfected patients.9 By April 3rd with the help of the Engineering department, a geographic and staff cohort was constructed and a dedicated COVID unit was created inside the CLC1. This COVID unit had newly placed double doors that remained closed, an anteroom, and donning and doffing areas. The rapid RT-PCR test was eventually used, which contributed to early identification and relocation of COVID 19 cases into this unit. Neighboring asymptomatic residents were tested first, and by April 17th all residents of CLC 1 and 2 along with health care members were screened for SARS-CoV-2 in accordance with the CDC's American Testing Guidance for Nursing Homes.10 Universal masking and temperature checks were required for every employee entering the CLCs throughout this period. Withdrawal of COVID-19 isolation precautions required resolution of symptoms, and two sequential negative nasopharyngeal RT-PCR tests which were obtained after 14 days from diagnosis. If the RT-PCR test was positive, a repeat test was obtained in 72 hours.
RESULTS
We were able to halt the spread of the disease over a 3-week period. The first patient was identified in CLC1. He developed fever and cough on March 24th, the test was obtained the same day and resulted positive on March 27th; he was then transferred to the VA Medical Center in our campus. The fourth patient had tested negative on March 20th but was hospitalized on March 23rd for febrile illness and was diagnosed with Klebsiella pneumonia bacteremia. During his admission he became hypoxic, was retested and was positive on March 27th. Overall, only 25 out of 80 (31%) CLC residents were tested positive for SARS-CoV-2 infection. The initial spread was rapid with the first 12 cases being diagnosed over a period of 8 days, by March 31st. Our measures including the creation of a dedicated COVID unit, the use of rapid RT-PCR test, and universal testing lead to a decline in cases and finally to a full resolution of the outbreak by April 18th. Of the total 25 patients, 5 remained asymptomatic, 9 were hospitalized, 6 died. The mortality rate was 24%. No coinfection with influenza or other respiratory viruses was identified. Table 1 summarizes the demographic characteristics, medical history and laboratory findings of the patients. The median age was 74 years, with no difference in age between recovered and diseased, 73 vs 77, P: .105. Simplified acute physiology score II was higher in the deceased group (P: .001) and so were D-dimer (admission and peak levels), C-reactive protein, lactate dehydrogenase, and peak ferritin and procalcitonin levels. See Table 1. During this outbreak, 11 HCPs were diagnosed with COVID 19 and all recovered. Among the patients who recovered, 13 had persistent positive nasopharyngeal RT-PCR for average 32 days (range 19-52 days) since diagnosis. Seven out of these 13 were tested for SARS-CoV-2 IgG antibody (Abbott, Lake Forrest, IL) and were all positive.
Table 1.
Demographics, laboratory findings, outcomes of COVID-19 CLC cohort
| COVID-19 recoverd N = 19 | COVID-19 deceased N = 6 | P value | |
|---|---|---|---|
| Median age years(range) | 77 (68-90) | 73 (48-87) | 0.105 |
| Men | 18 | 6 | 1.000 |
| Caucasian | 19 | 5 | 0.240 |
| Cough, # | 7 | 5 | 0.073 |
| Dyspnea, # | 1 | 4 | 0.005 |
| Median Temp °F (range) | 102 (99.4-104) | 102.3 (97.5-104.6) | 0.338 |
| Median SBP mmHg, (range) | 99 (93-112) | 102 (77-154) | 0.246 |
| Median Heart Rate bpm, (range) | 81 (81-153) | 102 (65 –122) | 0.036 |
| Median Respiratory rate breaths/min | 18 (19-29) | 24 (15-26) | 0.003 |
| BMI (range) | 26 (13-29) | 26 (19-41) | 0.591 |
| Oxygen saturation on Room Air, % | 92 (84-96) | 86 (70-92) | 0.018 |
| Diabetes, # | 6 | 3 | 0.630 |
| Hypertension, # | 12 | 4 | 1.000 |
| CHF, # | 3 | 1 | 1.000 |
| COPD, # | 5 | 2 | 1.000 |
| CAD, # | 5 | 3 | 0.344 |
| Hemodialysis, # | 1 | 1 | 0.430 |
| Number hospitalized | 6 | 3 | 0.630 |
| # of influenza tests, viral panels, result | 12 and 3 respectively, NEG | 6 and 3 respectively, NEG | |
| Hydroxychloroquine, # | 11 | 3 | 1.000 |
| Azithromycin, # | 4 | 2 | 0.606 |
| Doxycycline, # | 6 | 3 | 0.600 |
| Beta lactam antibiotics, # | 5 | 3 | 0.340 |
| Steroids given, # | 0 | 1 | 0.240 |
| History of ACEI/ARB use | 4 | 1 | 1.000 |
| Median SAPS II score | 32 | 47 | 0.0009 |
| Median D-dimer on admission ng/mL | 399 (150-2226) | 1525 (497-3039) | 0.019 |
| Median PEAK D-Dimer | 465 (150-2339) | 2592 (887-3039) | 0.0005 |
| Median Procalcitonin ng/mL on Admission | 0.1 | 0.25 | 0.042 |
| Median PEAK Procalcitonin | 0.1 | 4.56 | 0.023 |
| Median CRP mg/L on Admission | 24 | 174 | <0.001 |
| Median PEAK CRP | 49 | 188 | 0.003 |
| Median Ferritin on Admission ng/mL | 136 (19-1500) | 385 (249-1500) | 0.158 |
| Median PEAK Ferritin | 216 (21-1500) | 664 (385-1500) | 0.047 |
| Median White Blood Cells/mm3 | 5.3 (3.5-19.6) | 6.4 (4.3–25.1) | 0.230 |
| Median Absolute Lymphocytes | 1.2 (0.7-10.6) | 0.7 (0.5–1.0) | 0.312 |
| Median Serum Creatinine mg/dL | 1.1 (0.7-6.6) | 1.8 (0.8-6.3) | 0.165 |
| Median LDH IU/L | 150 (89-279) | 264 (199-320) | 0.002 |
| blood types: | |||
| O positive | 2 | 2 | |
| O negative | 2 | 0 | |
| A positive | 2 | 2 | |
| A negative | 2 | 0 | |
| B negative | 1 | 0 | |
| AB positive | 4 | 1 | |
| Unknown | 6 | 1 |
ACEI, ace inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive lung disease; CRP, C-reactive protein; LDH, lactate dehydrogenase; NEG, negative; SAPS II, simplified acute physiologic score II; SBP, systolic blood pressure.
DISCUSSION
The median incubation period for a symptomatic SARS-CoV-2 infection is 5 days; symptoms can appear within 12 days of infection.11 This novel coronavirus can be transmitted by an asymptomatic carrier; Arons et al showed that half of the residents in a skilled nursing facility who tested positive for the virus were asymptomatic. This was likely contributing to the transmission, as focusing infection control strategies solely on symptomatic residents failed to prevent transmission.12 By closely reviewing Figure 2, the epidemiologic analysis of this outbreak likely suggests introduction of the virus in the units via an asymptomatic carrier (healthcare worker, family member) which then lead to fast propagation among staff and residents. Transmission between residents was likely aided by sharing of double occupancy rooms, participating in group activities, including community dining, and utilizing similar resources. For example, cases 1 and 4 had been using the dialysis unit on our campus. Notably, a subsequent COVID-19 outbreak took place by end of March in this dialysis unit.
In our experience, after the initial rapid spread, formation of a geographic COVID unit within the CLC 1, assignment of a dedicated clinical team, and use of a rapid RT-PCR test had all been instrumental in aiding this outbreak containment. Dora et al also demonstrated how effective the creation of a COVID unit inside the nursing home can be; cohorting of cases allowed for residents to stay in familiar grounds, enabled the restriction of staff movement between the wards, and overall reduced the potential of viral transmission.13 Universal testing of residents (first aiming close neighboring residents and then every resident) and HCPs was another principal measure to successfully interrupt the outbreak. Eckardt et al demonstrated that a productive structured 14-day interval universal testing in their long-term care facility aided to contain the spread of the virus in a 6-week period.14 Implementing the measures described above we managed to suppress the outbreak within 3 weeks in both CLC 1 and 2. At this writing there are no active COVID-19 cases in our CLCs.
We followed the CDC's proposed test-based strategy to remove COVID-19 precaution/isolation measures. This had the unfortunate effect of keeping residents longer on strict isolation as their RT-PCR tests remained positive, even though they had recovered or remained asymptomatic and had also developed an IgG antibody. The CDC has since updated their guidelines regarding discontinuation of transmission-based precautions, no longer requiring a test-based strategy for asymptomatic or patients with mild disease.15
Prolonged positive SARS-CoV-2 RT-PCRs, up to 5 weeks, have been reported in many studies; the RNA detected in these cases is likely from nonviable virus. A proposed way of how to interpret the PCR test in these cases is to utilize the cycle threshold (Ct) value.16 This value is not reported to the clinician but can be reported upon request. It is part of the RT-PCR test, with a specified threshold for a positive result, and it is inversely related with the viral load. A Ct value of >34 likely denotes non transmissible disease.16 Exploring this potential strategy in more rigorous studies could shorten duration of isolation, conserve tests, reagents, and PPE.
In conclusion, SARS-CoV-2 infection can spread rapidly within skilled nursing facilities and can potentially cause high morbidity and mortality. Swift detection by rapid RT-PCR testing of all asymptomatic carriers (residents and employees via universal testing) and implementation of strict infection control and isolation measures are pivotal in containing and thus eliminating a COVID-19 outbreak. Indeed, the infection control team working closely with the dedicated nursing home staff, are both the unsung heroes of the robust and unyielding defense against a formidable virus.
Acknowledgments
The authors would like to thank our Veterans, and the nursing home staff, the facility leadership, the laboratory staff for their help during this pandemic.
Footnotes
Conflicts of interest: None to report.
References
- 1.Cucinotta D, Venelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;383:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Available at: https://coronavirus.jhu.edu/map.html. Accessed August 12, 2020.
- 4.Perrota F, Corbi G, Mazzeo G, et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making [published correction appears in Aging Clin Exp Res. 2020 Sep;32(9):1909] Aging Clin Exp Res. 2020;32:1599–1608. [Google Scholar]
- 5.Available at: https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg/. Accessed September 8, 2020.
- 6.Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68:1653–1656. doi: 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 8.Spotswood S. VA touts lower CLC COVID-19 rates vs. community nursing homes. 2020. U.S Medicine. 56:15. Available at:https://www.usmedicine.com/late-breaking-news/va-touts-lower-clc-covid-19-rates-vs-community-nursing-homes/. Accessed August 12, 2020.
- 9.Centers for Medicare & Medicaid Services . US Department of Health and Human Services, Centers for Medicare and & Medicaid Services; Washington, DC. Available at: 2020. COVID-19 Long-Term Care Facility Guidance.https://www.cms.gov/files/document/4220-covid-19-long-term-care-facility-guidance.pdf [Google Scholar]
- 10.Centers for Disease Control and Prevention. Testing guidance for nursing homes. Interim SARS-CoV-2 testing guidelines for nursing home residents and healthcare personnel. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html. Accessed August 12, 2020.
- 11.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dora AV, Winnett A, Jatt LP, et al. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans - Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:651–655. doi: 10.15585/mmwr.mm6921e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckardt P, Guran R, Hennmyre J, et al. Hospital affiliated long term care facility COVID-19 containment strategy by using prevalence testing and infection control best practices. Am J Infect Control. 2020;48:1552–15555. doi: 10.1016/j.ajic.2020.06.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim Guidance). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Accessed August 12, 2020
- 16.Tom MR, Mina MJ. To interpret the SARS-COV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020;71:2252-2254. [DOI] [PMC free article] [PubMed]