Abstract
Progress against tuberculosis (TB) requires faster-acting drugs. Mycobacterium tuberculosis (Mtb) is the leading cause of death by an infectious disease and its treatment is challenging and lengthy. Mtb is remarkably successful, in part, due to its ability to become dormant in response to host immune pressures. The DosRST two-component regulatory system is induced by hypoxia, nitric oxide and carbon monoxide and remodels Mtb physiology to promote nonreplicating persistence (NRP). NRP bacteria are thought to play a role in the long course of TB treatment. Therefore, inhibitors of DosRST-dependent adaptation may function to kill this reservoir of persisters and potentially shorten therapy. This review examines the function of DosRST, newly discovered compounds that inhibit DosRST signaling and considers future development of DosRST inhibitors as adjunct therapies.
Keywords: : antivirulence therapies, drug discovery and development, drug tolerance, tuberculosis, two-component regulatory systems
As part of pathogenesis, Mycobacterium tuberculosis (Mtb) must navigate a variety of obstacles presented by the immune system, including survival inside stressful environments such as the macrophage and granuloma [1,2]. Stresses associated with the macrophage and granuloma environments, such as hypoxia, acidic pH, or nutrient limitation, can promote Mtb drug tolerance and nonreplicating persistence (NRP), adaptive physiologies that play a role in the driving the long course of tuberculosis (TB) treatment. Therefore, it is possible that interfering with Mtb environmental adaptations may function to limit the reservoir of drug-tolerant bacilli and potentially shorten the course of therapy.
Environmental cues, both in vitro and inside the host, modulate Mtb gene expression to promote adaptation and survival [3]. Two-component regulatory systems (TCS) are one of the mechanisms used by Mtb to detect changes in the environment and modulate gene expression [4]. The TCS is composed of a sensor histidine kinase that detects an environmental cue and a DNA binding response regulator that modulates gene expression [5]. In most cases, upon detecting a cue, the histidine kinase autophosphorylates and then transfers the phosphate to the response regulator, which can then dimerize, bind DNA and induce or repress gene expression [5]. Mtb has 11 paired TCS, of which two are essential in vitro (MtrAB and PrrAB) [6–8] and several orphaned sensor kinases and response regulators. There has been interest in targeting TCS as new antivirulence therapies, as disrupting environmental sensing may sensitize pathogens to clearance by the immune system [9,10]. Indeed, several Mtb TCS are required for virulence in macrophages or animal infection models including DosRS, MprAB, PhoPR, PrrAB and SenX3-RegX3 [11–17]. Small molecules could inhibit TCS selectively by multiple mechanisms, including interference with detection of the environmental cue, inhibition of histidine kinase activity, blocking phosphotransfer to the response regulator, or inhibiting response regulator dimerization or DNA binding. In this review, we will discuss the potential to target the DosRST TCS signaling pathway, and consider the function of the targeted proteins, their role in pathogenesis and NRP, newly discovered small molecules targeting DosRST and approaches for the further development of this potentially new class of TB therapeutic.
The DosRST two-component regulatory pathway
DosRS/DevRS (henceforth referred to as DosRS) was initially discovered to be associated with Mycobacterial spp. virulence and survival during hypoxia [18–20], where DosS is a sensor histidine kinase and DosR is a response regulator (Figure 1). Another sensor kinase, DosT, also promotes sensing of hypoxia and nitric oxide (NO), along with DosRS [21]. DosS and DosT autophosphorylate in response to hypoxia, NO and carbon monoxide (CO), and directly interact with and phosphorylate DosR [21–30]. Phospho-DosR then directly binds a conserved DNA motif and regulates a core regulon of approximately 50 genes [31–33]. DosS also possesses phosphatase activity that is active under aerobic conditions and dephosphorylates DosR to limit expression [34]. Alternatively, spontaneous dephosphorylation of phospho-DosR may also play a role in signal dampening [30].
Figure 1. . Schematic for the DosRST signaling pathway, with examples of where small molecules and peptides interfere with DosRST signaling.
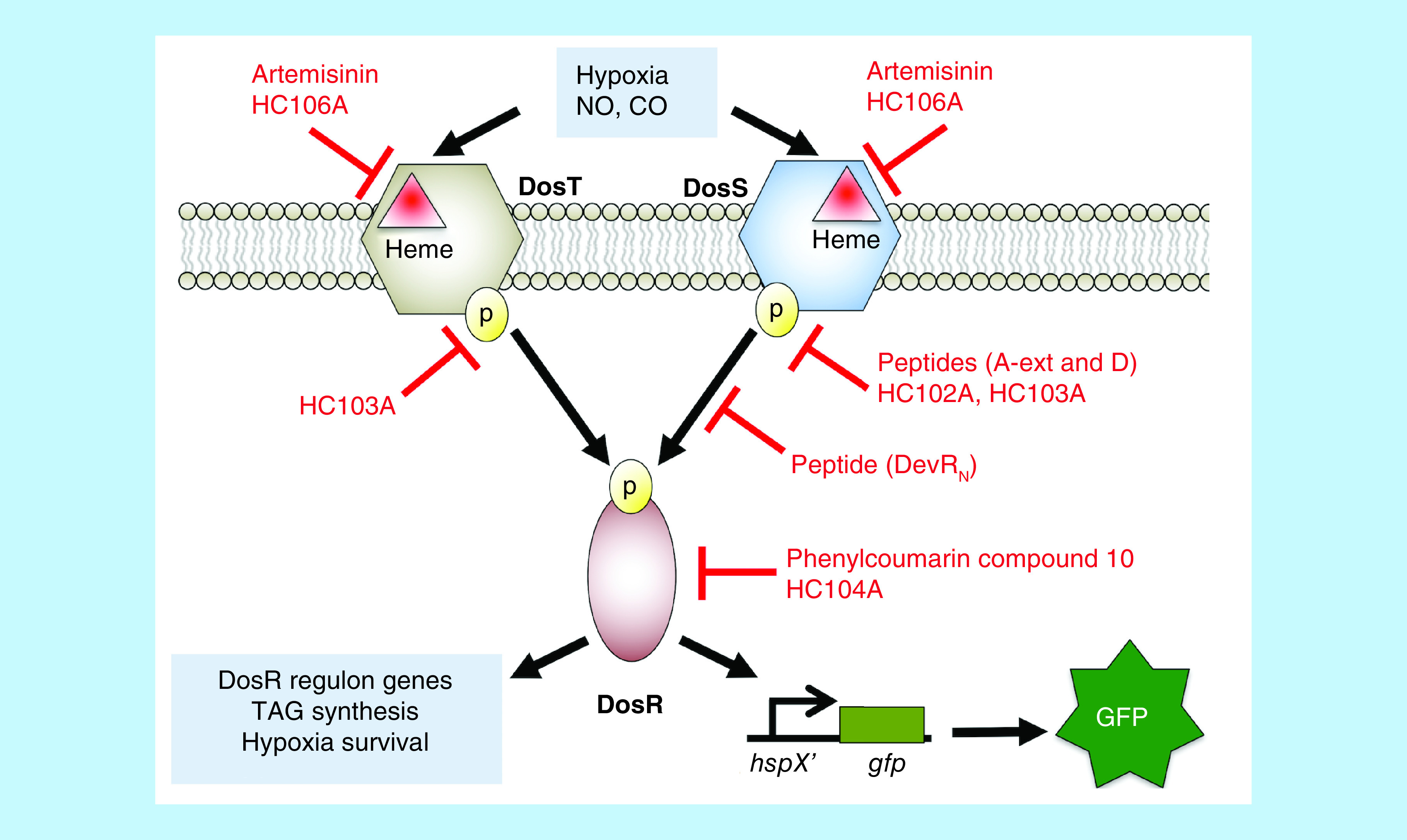
Artemisinin and HC106A target DosST heme to inhibit the sensing domain. Peptides A-ext and D, and small molecules HC102A and HC103A inhibit histidine kinase autophosphorylation. Peptide DevRN inhibits phosphotransfer from DosS to DosR. Phenylcoumarin compound 10 and HC104A inhibit DosR DNA-binding. These compounds inhibit expression of DosR-regulated genes and inhibit survival during hypoxia, with the exception of HC104A. Compounds HC101A–HC106 were identified using a reporter strain where the DosR-regulated promoter, hspX, was cloned upstream of green fluorescent protein (GFP). Whole cell screening of a library of >540,000 compounds for inhibitors of hypoxia-inducible GFP fluorescence was conducted to discover DosRST inhibitors.
CO: Carbon monoxide; GFP: Green fluorescent protein; NO: Nitric oxide; TAG: Triacylglycerol.
DosS and DosT have approximately 60% amino acid similarity and share structural homology, including a N-terminal GAF domain, a histidine kinase domain and a C-terminal ATP-binding domain [35,36]. In both DosS and DosT, sensing of environmental cues is dependent on a heme embedded in a GAF domain. Crystal structures show that the heme is exposed to the environment through a channel that allows gases such as oxygen, NO or CO to interact with the heme [37–40]. Ligand–heme interactions cause conformational changes in the GAF domain pocket and changes in quaternary structure that are thought to be associated with modulating kinase or phosphatase activity [34,37–39,41]. The specific sensing functions of DosS and DosT remain an area of active investigation. DosS is thought to function as a redox and oxygen sensor, based on biochemical studies of kinase activation, whereas, DosT is proposed to sense hypoxia [23,37]. However, a more recent study shows that DosS has a slow auto-oxidation rate and functions in the reduced ferrous state, supporting that DosS may primarily function as an oxygen sensor [42]. Both kinases sense ligands via the heme, where hypoxic conditions convert oxidized heme in DosS to the ferrous form, and O2-bound heme in DosT to the deoxy form, activating the kinases [23,38,40,41,43,44].
There also exists alternative regulatory control and functions of DosRST, beyond the interactions between DosS/T and DosR in a direct signaling pathway. For example, cross-talk exists between sensor kinases and response regulators from different TCS. In biochemical studies, PhoR interacts with DosR [26], and transcriptional profiles and network analysis support cross-talk between these pathways [32,45,46]. PhoPR responds to acidic pH and its regulation is associated with maintenance of redox homeostasis [47,48], supporting a potential link between balancing redox poise during pH- and hypoxia-dependent adaptation [49,50]. DosS interacts with the response regulator NarL [26] and DosR and NarL coregulate Mtb gene expression [51], linking DosR signaling with nitrate metabolism, an electron acceptor under hypoxia. PknH also phosphorylates DosR to enhance DNA binding [52]. It is also possible that a response regulator can be controlled in the absence of the histidine kinases. For example, in response to acetyl-phosphate or growth in acetate containing medium, DosR is stimulated under aerobic conditions, independent of DosS or DosT [53]. Additionally, acetylation of DosR also control its function, with DosR lysine deacetylation associated with enhanced DNA binding and DosR regulon gene expression [54]. Thus, when designing inhibitors of TCS, one must keep in mind that modulating a sensor kinase or response regulator may have activities that are more complicated than simply targeting a canonical cognate TCS signaling pathway.
The DosRST regulon is induced by a variety of in vitro and in vivo environments. Mtb grown in hypoxia or treated with NO, CO or vitamin C exhibits robust induction of the DosRST pathway. Understanding DosRST induction in animal models is complicated by the fact that the pathway is induced by multiple signals and that many mouse models do not generate hypoxic granulomas in response to Mtb infection. In C57Bl/6 mice, which do not generate hypoxic granulomas, the DosR pathway is strongly induced, and its induction is associated with inducible nitric oxide synthase (iNOS) expression, M1 macrophage polarization and is dependent on interferon-gamma [55,56]. These findings support that induction in C57Bl/6 mice is driven by pro-inflammatory environments and likely NO or CO as the signals. C3HeB/FeJ ‘Kramnik’ mice generate hypoxic granulomas and DosR is observed to be strongly induced in these hypoxic granulomas [57,58]. The DosRST pathway is also strongly induced in granulomas of nonhuman primates and humans [59]. Interestingly, the pathway is also found to be induced by Mtb isolated from human sputum, suggesting that prior to transmission from a necrotic granuloma the pathway is induced and remains so in sputum [60]. Thus, there is substantial evidence supporting that DosRST is induced in animal models and during human infection, supporting an important role for the pathway in pathogenesis.
Role of DosRST in NRP & pathogenesis
In response to environmental stresses, such as hypoxia, NO, acid stress or starvation, Mtb arrests its growth and establishes a state of NRP [61]. NRP bacteria can remain viable for months or years and become highly tolerant to many antibiotics, including the first line antibiotic isoniazid (INH) [62]. DosRS is not required for survival under aerobic conditions in vitro, however dosRS mutants have survival defects during prolonged hypoxia or in response to hypoxic shock [18,63–65]. DosS and DosT appear to have distinct roles during adaptation in vitro, where DosT is required for induction of the pathway during early stages of hypoxic adaptation and DosS being required for continued expression of the DosR regulon during prolonged hypoxia [66]. Killing of a dosR mutant during hypoxia is associated with its inability to maintain intrabacterial pH-homeostasis [50]. The requirement for DosRST to maintain drug tolerance is less clear. Bartek et al. did not observed changes of drug tolerance in a ΔdosR mutant, in vitro using a model of gradual hypoxia or in vivo in C57Bl/6 mice [67]. However, under low iron and hypoxia, Baek et al., observed that a mutant in the dosR-regulated gene, tgs1, has significantly reduced drug tolerance both in vitro and in vivo [68]. Given that tgs1 expression is almost completely lost in a dosR mutant [65,69], a functional DosRST pathway is associated with drug tolerance. Thus, DosRST is required for Mtb to establish and maintain NRP in response to hypoxia and its chemical inhibition, based on these genetic studies, is predicted to reduce survival during NRP and potentially reduce drug tolerance.
When considering DosRST as a potential drug target, it is important to consider the impact of its disruption on pathogenesis in animals. A dosR mutant had no impact on virulence of Mtb in acute or chronic infection of C57Bl/6 mice, supporting the pathway is not required for virulence in a model that does not generate hypoxic granulomas [70]. In contrast, several independent studies show that mutants in the DosRST pathway have attenuated virulence in animal models that generate well-formed hypoxic granulomas, such as rabbits, C3HeB/FeJ mice and cynomolgus macaques. In rabbits infected with a dosRS mutant, a severe growth defect was observed [71], where 8 weeks post infection there was a approximately 100-fold reduction of CFUs in lungs infected with the dosRS mutant as compared with the control [71] and significantly fewer and smaller lesions in the lungs (113 tubercles in wild-type-infected rabbits, versus 27 in dosRS mutant [71]). In C3HeB/FeJ mice, the role of DosRST is more complicated, where only the ΔdosS mutant had a virulence defect and both ΔdosR and ΔdosT mutants had virulence similar to the wild-type [14]. In nonhuman primates, considered the best animal model to simulate human disease, infection with wild-type Mtb or ΔdosR mutant complemented strain caused death of >50% of the macaques by day 150 [13]. In contrast, when infected with single mutant strains of ΔdosR, ΔdosS or ΔdosT, all of the macaques survived. Bacterial burdens of all three mutants, relative to the wild-type or complemented strain, were significantly reduced in the bronchoalveolar lavage and lung tissue and the mutants were undetectable in the bronchial lymph node tissue. Like in rabbits, the mutants also generated fewer well organized granulomas in nonhuman primates. Overall, the findings from rabbits and nonhuman primates provide strong evidence that genetic disruption of DosRST results in attenuated virulence and limited formation of necrotic granulomas. These phenotypes are attractive for a compound that could function to shorten the course of therapy. By limiting granuloma formation, it could improve penetration of standard of care antibiotics, and also limit the generation of hypoxic environments, that drive Mtb into a drug-tolerant persistence.
Molecules targeting the DosRST pathway
Targeting TCS has been an active area of research to translate studies of bacterial pathogenesis into new classes of antivirulence antibiotics [9,10,72]. A variety of approaches have been employed to discover TCS inhibitors, including whole cell reporter-based high-throughput screens (HTS) [45,64,65,73]; phenotypic HTS targeting TCS-dependent responses [74]; target-based biochemical assays focused on inhibition of bacterial histidine kinase activity by small molecules [75–77] or response regulator mimetic peptides [78,79]; or inhibition of response regulator DNA binding [80,81]. These approaches have generated several new compounds that are in preclinical development as novel antivirulence therapies.
Given the evidence that DosRST is induced during infection in animals and humans, is required for persistence in vitro and is necessary for survival in animals that generate hypoxic granulomas, there has been significant interest in generating small molecules targeting this pathway. Initial approaches were focused on targeting the response regulator DosR. Using a virtual screen against a DosR homology model, 11 compounds were identified that were predicted to dock in a large binding pocket. Of these compounds, a phenylcoumarin compound (named “Compound 10”, Figure 2) was shown to selectively kill Mtb under hypoxic conditions (but not aerobic conditions), inhibit expression of DosR-regulated genes and block promoter binding in an electrophoretic mobility shift assay (EMSA) [81] (Figure 1). Another vulnerability is the histidine kinase activity or the phosphotransfer from the sensor kinase to the response regulator. Using phage-display approaches, DosR mimetic peptides were discovered that could bind to DosS and inhibit autokinase activity or phosphotransfer to DosR in in vitro biochemical assays [78,79]. Several peptides were shown to inhibit expression of DosR-regulated genes in Mtb and also inhibit survival during hypoxia (Figure 1). Thus, these targeted biochemical studies identified new molecules targeting histidine kinase autophosphorylation, phosphotransfer or response regulator DNA binding.
Figure 2. . Selected chemical structures of small molecules that inhibit DosRST signaling.
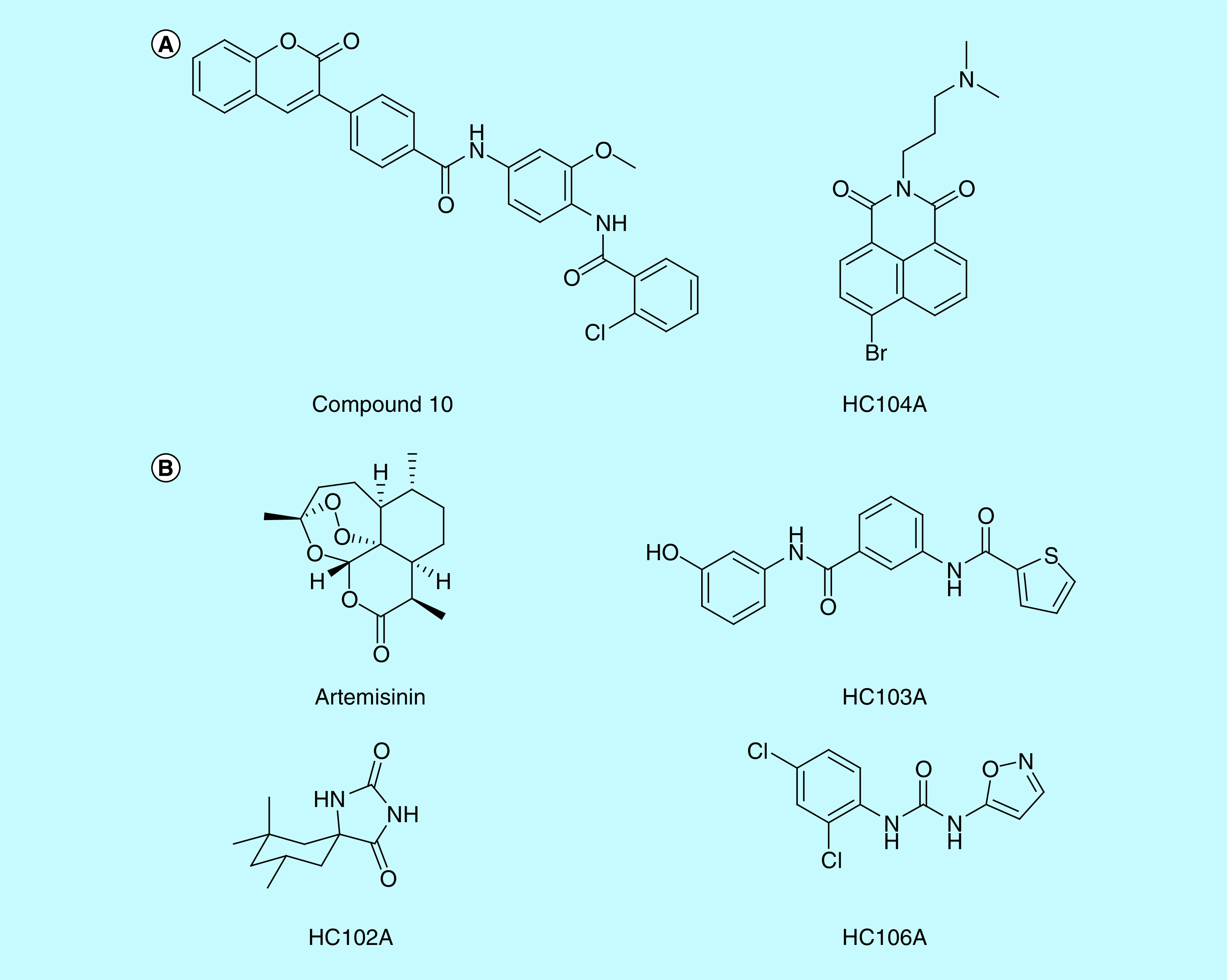
(A) Inhibitors of DosR. Compound 10 and HC104A inhibit DosR binding of promoter DNA. (B) Inhibitors of DosS and DosT. Artemisinin and HC106A inhibit DosS and DosT by interacting with the embedded heme sensor. HC102A and HC103A do not inhibit heme redox, but instead inhibit sensor kinase autophosphorylation.
Inhibitors of DosRST signaling have also been discovered by a reporter-based, whole-cell phenotypic HTS. In this case, the hspX promoter, which is strongly regulated by DosR, was cloned upstream of green fluorescent protein (GFP) on a replicating plasmid and transformed into Mtb. The reporter strain exhibited strong hypoxia and NO inducible GFP fluorescence [55]. To discover new compounds required for hypoxia-driven adaptation, a >540,000 compound library was screened for inhibitors of hypoxia-inducible reporter fluorescence. Six distinct scaffolds named HC101–HC106 were confirmed as DosRST regulon inhibitors [65]. Mechanism of action studies were initially carried out on three of the inhibitors: artemisinin, HC102A and HC103A (Figure 2). RNAseq transcriptional profiling experiments showed that the compounds inhibit the DosR regulon when compared with a dosR mutant control strain [64,65]. Notably, the triacylglycerol (TAG) synthase gene tgs1 was repressed and treatment with the artemisinin, HC102, HC103 significnatly reduced TAG accumulation. Treatment with these inhibitors was also shown to decrease Mtb survival and reduce Mtb tolerance to INH in the hypoxic shiftdown model of NRP [82]. This observation is consistent with previous studies showing that mutants in tgs1 have reduced tolerance to INH [68,83].
Biochemical studies using recombinant DosS and DosT proteins determined the mechanism of action of artemisinin, HC102 and HC103. Artemisinin acts by oxidizing and alkylating the heme group embedded in DosS and DosT, thus inactivating the sensing module of the proteins [65]. Amino acid substitutions that block artemisinin interactions with the heme, promote resistance to heme oxidation in the biochemical assays, and one allele, dosT (G115L), promotes artemisinin resistance when overexpressed in Mtb. Together, these finidng support artemisinin–heme intereactions are associated with the compounds mechanism of action. HC102A and HC103A did not oxidize or alkylate DosS or DosT heme, therefore, the impact on sensor kinase activity was examined. HC103A directly inhibited the autophosphorylation activity of the DosS and DosT sensor kinases, while HC102A only inhibited DosS autophosphorylation [65]. Thus, artemisinin, HC102A and HC103A inhibit DosRST signaling, persistence and drug tolerance by directly targeting the DosS and DosT sensor kinases, and do so using distinct mechanisms.
Further characterizations were undertaken for HC104 and HC106 (Figure 2). RNA-seq transcriptional profiling showed that HC106 broadly inhibited the DosR regulon [64], with many of the genes being almost undetectable (>400-fold downregulated); in contrast, HC104 only inhibited a small subset of the DosR regulon, including the hspX operon used in the reporter. HC106 inhibited the DosR regulon when stimulated by NO (under aerobic conditions) and in infected macrophages [64], this is in contrast to artemisinin, that did not inhibit NO-driven signaling [65]. HC106 also inhibited NRP associated physiologies including TAG accumulation and survival during hypoxia [64], while HC104 did not inhibit survival. Overexpression of DosS promoted resistance to HC106, consistent with DosRST being the targeted pathway.
Mechanism of actions studies were undertaken for HC104 and HC106 and both were found to function by new mechanisms. HC106A was tested for its ability to modulate DosS heme redox status using a UV-visible spectroscopy assay and a spectral change was noted, where HC106A caused a shift of the dithionite reduced Fe2+ Soret peak from 430 to approximately 420 nm. This shift is neither associated with oxidized or reduced iron; however, NO and CO binding of heme causes a shift of the Soret peak to a similar position of approximately 420 nm [23]. An amino acid substitution in the heme exposing channel, DosS (G117L), resulted in resistance to HC106 in the UV–visible spectroscopy assay, supporting that HC106A modulates DosS heme by a mechanism that is distinct from artemisinin, but is accessing the heme via the same G117L dependent channel. Notably, NO/CO induces DosS, however, phenotypically we observe HC106A represses DosS/T signaling. This suggests that the HC106A–heme interactions are causing a new modification of the DosS kinase that potentially locks the kinase in an ‘off’ state. Understanding the molecular basis of this physiology will provide novel insights in the activation of the DosS/T kinases.
HC104A was examined for interactions with DosS in the UV-visible spectroscopy and kinase autophosphorylation assays and had no impact. Notably, HC104A bears similarity to the compound virstatin [84], which interferes with the dimerization of the ToxT transcription factor in Vibrio cholera [84,85]. Therefore, it was hypothesized that HC104A may be functioning by interfering with the dimerization of the response regulator transcription factor DosR and its binding to DNA. EMSA showed that HC104A inhibits the ability of DosR to bind DNA and functions with an IC50 of approximately 10 μM [23]. Notably, virstatin did not impact DosR binding of promoter DNA, nor did virstatin inhibit the DosRST pathway in the whole cell Mtb assay. Interestingly, the transcriptional profiling shows that HC104A only strongly inhibits the hspX operon, suggesting that it may specifically interfere with DosR binding to the hspX promoter, and not broadly impact DNA binding. This specificity explains why HC104 did not impact Mtb survival during hypoxic shock.
Overall, multiple distinct mechanisms have been discovered for inhibitors of DosRST signaling including oxidation/alkylation of heme, heme-binding, inhibition of autophosphorylation, inhibition of phosphotransfer to DosR and inhibition of DosR DNA binding. It is possible that combinations of these approaches may result in enhanced additive or synergistic functions of these compounds. Indeed, pairwise comparisons of all combinations of HC101–HC016, identified additive interactions and in the case of HC106 and artemisinin, strong synergistic interactions. Given that both target DosS/T heme, this observation is surprising, and suggests the mechanisms of these compounds against DosS/T in whole cells, may be more complicated than defined in the biochemical assays. A key development from these studies was the demonstration that the heme-based sensor in DosS/T is vulnerable to chemical inhibition. Given that structures are available for the DosS and DosT GAF domains, this opens the path for in silico screening or structure guided development of novel inhibitors of DosS or DosT that may function by targeting the heme, or possibly independent of heme, but binding in this vulnerable sensory domain.
Future perspective
Questions still remain about the utility of targeting DosRST as a new approach to TB therapy. Several lines of evidence support the importance of the pathway to pathogenesis. The DosR regulon is strongly induced in clinical human samples and there is evidence for its selection in more virulence strains, where HN878 ‘Beijing’ strain constitutively overexpresses the DosR regulon [86]. The pathway is required to establish NRP in response to hypoxia. And, as discussed above, mutants in the pathway are strongly attenuated in nonhuman primates and other animals that generate hypoxic granulomas. Thus, there is strong evidence supporting the hypothesis the pathway is a required for virulence and persistence. However, the virulence studies relied on knockout mutants, which will have altered physiology at the beginning of the stress and do not adequately reflect the consequences of conditional inhibition. When the genes are knocked out at the beginning of the assay (in the ΔdosR mutant, or compound treated), a significant decrease of survival is observed during NRP [63–65]. However, a critical question still needs to be addressed: what is the impact of inhibiting DosRST after Mtb has established NRP or is encased in a granuloma? Generating conditionally expressed mutants or using CRISPR interference approaches [87,88], to genetically deplete DosR following the establishment of NRP in vitro or granuloma formation in vivo, will enable comparison of the impact of controlled genetic depletion or chemical inhibition of dosR, dosS and dosT. Such studies will provide important data regarding the validity of targeting DosRST as a therapeutic approach.
Numerous challenges exist for developing a DosRST-targeting therapy. The pathway is not required for growth or survival under aerobic conditions, therefore, it is not a traditional antibiotic. Rather, it is a targeted antivirulence approach that is intended to selectively deplete the reservoir of drug-tolerant persisters, that are thought to drive the long course of therapy. Thus, DosRST inhibitors are proposed to be employed as adjunct therapies to current or next generation combination TB treatments. The compounds are intended to shorten the course of therapy, by killing persisters and reducing the population of drug-tolerant Mtb, therefore, the compounds will need to be shown to shorten time to cure or reduce relapse in animal models. Efficacy in clinical trials will also be challenging, as there is not expected to by any effect when used as monotherapy in a two week early bactericidal activity study. Thus, should a DosRST inhibitor be found to shorten treatment duration in an animal model, and be safe in humans, it would need to be studied for efficacy in combination with current therapies with the positive outcomes being shortened time to cure, reduction of relapse or radiological evidence of enhancing standard of care therapy. Thus, new positron emission tomography imaging approaches could play an important role in establishing the efficacy of using a DosRST inhibitors as an adjunct therapy in animal models or humans [89–91]. Developing new methods to shorten TB treatment is essential for the long-term control of TB, and new approaches, such as antivirulence therapies targeting DosRST or other factors required for persistence, may play an important role in achieving the goal of shortening therapy.
Executive summary.
New treatments are required to control the tuberculosis epidemic
Tuberculosis (TB) treatment requires 6 months of therapy and approaches to shorten TB therapy are required to improve treatment outcomes.
New drug targets are important to treat drug-resistant TB. Targeting pathways required for Mycobacterium tuberculosis (Mtb) virulence represents a promising new approach for therapy.
Mtb establishes nonreplicating persistence during infection, a physiological adaptation that is associated with phenotypic drug tolerance.
Role of DosRST signaling in Mtb pathogenesis
DosRST is a two-component regulatory pathway that is induced by host immune cues such as hypoxia, nitric oxide and carbon monoxide.
The DosR regulon is composed of approximately 50 genes and is required for Mtb to establish nonreplicating persistence and survival during hypoxia.
Mtb mutants in DosRST have reduced virulence in animal models that form hypoxic granulomas, supporting its potential as a drug target.
Inhibiting DosRST during infection may reduce the reservoir of persistent, drug-tolerant bacteria.
Inhibitors of DosRST signaling
Small molecules targeting a heme sensor embedded in DosS/T, including Artemisinin and HC106A, inhibit DosRST signaling and persistence. This finding shows that DosS/T heme is a vulnerable target for inhibiting the DosRST pathway.
Small molecules (HC102A and HC103A) and peptides have been discovered that inhibit DosS/T autophosphorylation activity and phosphotransfer to DosR.
Small molecules (HC104A and compound 10) have been identified that inhibit DosR–DNA binding.
Inhibitors of DosRST selectively inhibit Mtb growth during hypoxia.
Footnotes
Financial & competing interests disclosure
This project was supported by the Bill and Melinda Gates Foundation (grant no. OPP1059227 and OPP1119065) and the National Institute of Allergy and Infectious Diseases (grant no. R01AI116605 and R21AI105867). RB Abramovitch is the founder of Tarn Biosciences, a company developing new TB drugs. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Russell DG. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 5(1), 39–47 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol. Rev. 240(1), 252–268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 219(1) 37–54 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Bretl DJ, Demetriadou C, Zahrt TC. Adaptation to environmental stimuli within the host: two-component signal transduction systems of Mycobacterium tuberculosis . Microbiol. Mol. Biol. Rev. 75(4), 566–582 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63, 133–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahrt TC, Deretic V. An essential two-component signal transduction system in Mycobacterium tuberculosis. J. Bacteriol. 182(13), 3832–3838 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haydel SE, Malhotra V, Cornelison GL, Clark-Curtiss JE. The prrAB two-component system is essential for Mycobacterium tuberculosis viability and is induced under nitrogen-limiting conditions. J. Bacteriol. 194(2), 354–361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejesus MA, Gerrick ER, Xu W. et al. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8(1), e02133 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 9(2), 117–128 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Johnson BK, Abramovitch RB. Small molecules that sabotage bacterial virulence. Trends Pharmacol. Scis. 38(4), 339–362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parish T. Two-component regulatory systems of mycobacteria. Microbiol. Spectr. 1, MGM2-0010-2013 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Perez E, Samper S, Bordas Y, Guilhot C, Gicquel B, Martin C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 41(1), 179–187 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Mehra S, Foreman TW, Didier PJ. et al. The DosR regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am. J. Respir. Crit. Care Med. 191(10), 1185–1196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows that Mycobacterium tuberculosis mutants in dosR, dosS or dosT are attenuated during infection of nonhuman primates.
- 14.Gautam US, Mcgillivray A, Mehra S. et al. DosS Is required for the complete virulence of Mycobacterium tuberculosis in mice with classical granulomatous lesions. Am. J. Respir. Cell Mol. Biol. 52(6), 708–716 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahrt TC, Deretic V. Mycobacterium tuberculosis signal transduction system required for persistent infections. Proc. Natl Acad. Sci. USA 98(22), 12706–12711 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewann F, Jackson M, Pethe K. et al. Transient requirement of the PrrA-PrrB two-component system for early intracellular multiplication of Mycobacterium tuberculosis. Infect Immun. 70(5), 2256–2263 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parish T, Smith DA, Roberts G, Betts J, Stoker NG. The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149(Pt 6), 1423–1435 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Boon C, Dick T. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184(24), 6760–6767 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta N, Kapur V, Singh KK. et al. Characterization of a two-component system, devR-devS, of Mycobacterium tuberculosis. Tuber Lung Dis. 80(3), 141–159 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Bagchi G, Mayuri, Tyagi JS. Hypoxia-responsive expression of Mycobacterium tuberculosis Rv3134c and devR promoters in Mycobacterium smegmatis . Microbiology 149(Pt 9), 2303–2305 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 279(22), 23082–23087 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini DK, Malhotra V, Dey D, Pant N, Das TK, Tyagi JS. DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology 150(Pt 4), 865–875 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Toledo JC, Patel RP, Lancaster JR, Jr, Steyn AJ. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl Acad. Sci. USA 104(28), 11568–11573 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A, Mai D, Kumar A, Steyn AJ. Dissecting virulence pathways of Mycobacterium tuberculosis through protein–protein association. Proc. Natl Acad. Sci. USA 103(30), 11346–11351 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saini DK, Malhotra V, Tyagi JS. Cross talk between DevS sensor kinase homologue, Rv2027c, and DevR response regulator of Mycobacterium tuberculosis. FEBS Lett. 565(1–3), 75–80 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Agrawal R, Pandey A, Rajankar MP, Dixit NM, Saini DK. The two-component signalling networks of Mycobacterium tuberculosis display extensive cross-talk in vitro. Biochem. J. 469(1), 121–134 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Taneja NK, Dhingra S, Mittal A, Naresh M, Tyagi JS. Mycobacterium tuberculosis transcriptional adaptation, growth arrest and dormancy phenotype development is triggered by vitamin C. PLoS ONE 5(5), e10860 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 3(5), 323–330 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Deshane JS, Crossman DK. et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 283(26), 18032–18039 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa EHS, Gonzalez G, Gilles-Gonzalez MA. Target DNA stabilizes Mycobacterium tuberculosis DevR/DosR phosphorylation by the full-length oxygen sensors DevS/DosS and DosT. FEBS J. 284(22), 3954–3967 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Park HD, Guinn KM, Harrell MI. et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 48(3), 833–843 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galagan JE, Minch K, Peterson M. et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499(7457), 178–183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minch KJ, Rustad TR, Peterson EJ. et al. The DNA-binding network of Mycobacterium tuberculosis. Nat. Commun. 6, 5829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur K, Kumari P, Sharma S, Sehgal S, Tyagi JS. DevS/DosS sensor is bifunctional and its phosphatase activity precludes aerobic DevR/DosR regulon expression in Mycobacterium tuberculosis. FEBS J. 283(15), 2949–2962 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Sardiwal S, Kendall SL, Movahedzadeh F, Rison SC, Stoker NG, Djordjevic S. A GAF domain in the hypoxia/NO-inducible Mycobacterium tuberculosis DosS protein binds haem. J. Mol. Biol. 353(5), 929–936 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Sivaramakrishnan S, De Montellano PR. The DosS-DosT/DosR mycobacterial sensor system. Biosensors 3(3), 259–282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho HY, Cho HJ, Kim MH, Kang BS. Blockage of the channel to heme by the E87 side chain in the GAF domain of Mycobacterium tuberculosis DosS confers the unique sensitivity of DosS to oxygen. FEBS Lett. 585(12), 1873–1878 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Cho HY, Cho HJ, Kim YM, Oh JI, Kang BS. Structural insight into the heme-based redox sensing by DosS from Mycobacterium tuberculosis. J. Biol. Chem. 284(19), 13057–13067 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madrona Y, Waddling CA, Ortiz De Montellano PR. Crystal structures of the CO- and NO-bound DosS GAF-A domain and implications for DosS signaling in Mycobacterium tuberculosis. Arch. Biochem. Biophys. 612, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podust LM, Ioanoviciu A, Ortiz De Montellano PR. 2.3 A x-ray structure of the heme-bound GAF domain of sensory histidine kinase DosT of Mycobacterium tuberculosis. Biochemistry 47(47), 12523–12531 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lobao J, Gondim ACS, Guimaraes WG, Gilles-Gonzalez MA, Lopes LGF, Sousa EHS. Oxygen triggers signal transduction in the DevS (DosS) sensor of Mycobacterium tuberculosis by modulating the quaternary structure. FEBS J. 286(3), 479–494 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Barreto GA, Carepo MSP, Gondim ACS. et al. A spectroelectrochemical investigation of the heme-based sensor DevS from Mycobacterium tuberculosis: a redox versus oxygen sensor. FEBS J. 286(21), 4278–4293 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Ioanoviciu A, Yukl ET, Moenne-Loccoz P, De Montellano PR. DevS, a heme-containing two-component oxygen sensor of Mycobacterium tuberculosis. Biochemistry 46(14), 4250–4260 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sousa EH, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA. DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis. Protein Sci. 16(8), 1708–1719 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson BK, Colvin CJ, Needle DB, Mba Medie F, Champion PA, Abramovitch RB. The carbonic anhydrase inhibitor ethoxzolamide inhibits the Mycobacterium tuberculosis PhoPR regulon and Esx-1 secretion and attenuates virulence. Antimicrob. Agents Chemother. 59(8), 4436–4445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vashist A, Malhotra V, Sharma G, Tyagi JS, Clark-Curtiss JE. Interplay of PhoP and DevR response regulators defines expression of the dormancy regulon in virulent Mycobacterium tuberculosis. J. Biol. Chem. 293(42), 16413–16425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abramovitch RB, Rohde KH, Hsu FF, Russell DG. aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol. Microbiol. 80(3), 678–694 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker JJ, Johnson BK, Abramovitch RB. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol. Microbiol. 94(1), 56–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker JJ, Dechow SJ, Abramovitch RB. Acid fasting: modulation of Mycobacterium tuberculosis metabolism at acidic pH. Trends Microbiol. 27(11), 942–953 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichlen MJ, Leistikow RL, Scobey MS, Born SEM, Voskuil MI. Anaerobic Mycobacterium tuberculosis cell death stems from intracellular acidification mitigated by the DosR regulon. J. Bacteriol. 199(23), e00320–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malhotra V, Agrawal R, Duncan TR, Saini DK, Clark-Curtiss JE. Mycobacterium tuberculosis response regulators, DevR and NarL, interact in vivo and co-regulate gene expression during aerobic nitrate metabolism. J. Biol. Chem. 290(13), 8294–8309 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chao JD, Papavinasasundaram KG, Zheng X. et al. Convergence of Ser/Thr and two-component signaling to coordinate expression of the dormancy regulon in Mycobacterium tuberculosis. J. Biol. Chem. 285(38), 29239–29246 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S, Kumari P, Vashist A, Kumar C, Nandi M, Tyagi JS. Cognate sensor kinase-independent activation of Mycobacterium tuberculosis response regulator DevR (DosR) by acetyl phosphate: implications in anti-mycobacterial drug design. Mol Microbiol. 111(5), 1182–1194 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Sha W, Liu Z. et al. Lysine acetylation of DosR regulates the hypoxia response of Mycobacterium tuberculosis. Emerg Microbes Infect. 7(1), 34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan S, Sukumar N, Abramovitch RB, Parish T, Russell DG. Mycobacterium tuberculosis responds to chloride and pH as synergistic cues to the immune status of its host cell. PLoS Pathog. 9(4), e1003282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 215(4), 1135–1152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harper J, Skerry C, Davis SL. et al. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J. Infect. Dis. 205(4), 595–602 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gautam US, Mehra S, Kaushal D. In-vivo gene signatures of Mycobacterium tuberculosis in C3HeB/FeJ Mice. PLoS ONE 10(8), e0135208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hudock TA, Foreman TW, Bandyopadhyay N. et al. Hypoxia sensing and persistence genes are expressed during the intragranulomatous survival of Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 56(5), 637–647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study demonstrates that DosR regulon genes are strongly induced in intragranulomatous tissues isolated from nonhuman primates.
- 60.Garton NJ, Waddell SJ, Sherratt AL. et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 5(4), 634–645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gengenbacher M, Kaufmann SH. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol. Rev. 36(3), 514–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gengenbacher M, Rao SP, Pethe K, Dick T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology 156(Pt 1), 81–87 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol. 192(6), 1662–1670 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng H, Williams JT, Alewei B, Ellsworth E, Abramovitch RB. Inhibiting Mycobacterium tuberculosis DosRST signaling by targeting response regulator DNA binding and sensor kinase heme. ACS Chem. Biol. 15(1), 52–62 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that HC106A interacts with DosS and DosT heme to inhibit DosRST signaling and survival during hypoxia.
- 65.Zheng H, Colvin CJ, Johnson BK. et al. Inhibitors of Mycobacterium tuberculosis DosRST signaling and persistence. Nat. Chem. Biol. 13(2), 218–225 (2017). [DOI] [PubMed] [Google Scholar]; •• Describes a phenotypic screen for inhibitors of DosRST signaling, including the structures of six new inhibitors of the DosRST pathway.
- 66.Honaker RW, Leistikow RL, Bartek IL, Voskuil MI. Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy. Infect. Immun. 77(8), 3258–3263 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartek IL, Rutherford R, Gruppo V. et al. The DosR regulon of M. tuberculosis and antibacterial tolerance. Tuberculosis (Edinb.). 89(4), 310–316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 9(5), e1001065 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 7(6), e1002093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE. 3(1), e1502 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Converse PJ, Karakousis PC, Klinkenberg LG. et al. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect. Immun. 77(3), 1230–1237 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6(1), 17–27 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rasko DA, Moreira CG, Li De R. et al. Targeting QseC signaling and virulence for antibiotic development. Science 321(5892), 1078–1080 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goswami M, Espinasse A, Carlson EE. Disarming the virulence arsenal of Pseudomonas aeruginosa by blocking two-component system signaling. Chem. Sci. 9(37), 7332–7337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chase OM, Espinasse A, Wilke KE, Carlson EE. Exploration of the effects of gamma-phosphate-modified ATP analogues on histidine kinase autophosphorylation. Biochemistry 57(29), 4368–4373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilke KE, Fihn CA, Carlson EE. Screening serine/threonine and tyrosine kinase inhibitors for histidine kinase inhibition. Bioorg. Med. Chem. 26(19), 5322–5326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bem AE, Velikova N, Pellicer MT, Baarlen P, Marina A, Wells JM. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 10(1), 213–224 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Kaur K, Taneja NK, Dhingra S, Tyagi JS. DevR (DosR) mimetic peptides impair transcriptional regulation and survival of Mycobacterium tuberculosis under hypoxia by inhibiting the autokinase activity of DevS sensor kinase. BMC Microbiology 14, 195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the use of phage-display screen to discover DosS-binding peptides that inhibit DosRST signaling and Mycobacterium tuberculosis survival during hypoxia.
- 79.Dhingra S, Kaur K, Taneja NK, Tyagi JS. DevR (DosR) binding peptide inhibits adaptation of Mycobacterium tuberculosis under hypoxia. FEMS Microbiol. Lett. 330(1), 66–71 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Banerjee SK, Kumar M, Alokam R. et al. Targeting multiple response regulators of Mycobacterium tuberculosis augments the host immune response to infection. Sci. Rep. 6, 25851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta RK, Thakur TS, Desiraju GR, Tyagi JS. Structure-based design of DevR inhibitor active against nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 52(20), 6324–6334 (2009). [DOI] [PubMed] [Google Scholar]; •• Describes the an in silico screen that identified several new inhibitors of DosR dimerization, promoter DNA binding and DosRST signaling.
- 82.Mak PA, Rao SP, Ping Tan M. et al. A high-throughput screen to identify inhibitors of ATP homeostasis in non-replicating Mycobacterium tuberculosis. ACS Chem. Biol. 7(7), 1190–1197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deb C, Lee CM, Dubey VS. et al. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS ONE 4(6), e6077 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310(5748), 670–674 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc. Natl Acad. Sci. USA 104(7), 2372–2377 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Domenech P, Zou J, Averback A. et al. Unique regulation of the DosR regulon in the beijing lineage of Mycobacterium tuberculosis. J. Bacteriol. 199(2), e00696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rock JM, Hopkins FF, Chavez A. et al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat. Microbiol. 2, 16274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schnappinger D, Ehrt S. Regulated expression systems for mycobacteria and their applications. Microbiol. Spectrum 2(1), MGM2-0018-2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Via LE, England K, Weiner DM. et al. A sterilizing tuberculosis treatment regimen is associated with faster clearance of bacteria in cavitary lesions in marmosets. Antimicrob. Agents Chemother. 59(7), 4181–4189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin PL, Maiello P, Gideon HP. et al. PET CT identifies reactivation risk in cynomolgus macaques with latent M. tuberculosis. PLoS Pathog. 12(7), e1005739 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malherbe ST, Shenai S, Ronacher K. et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat. Med. 22(10), 1094–1100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


