Abstract
Background:
From September 2014, a tertiary care hospital in Karachi, Pakistan, started diagnosing 3–5 cases/month of a yeast locally identified as Saccharomyces spp. resistant to fluconazole. US Centers for Disease Control and Prevention identified the isolates as Candida auris. The Pakistan Field Epidemiology and Laboratory Training Program (FELTP) and the hospital investigated the outbreak from April 2015 to January 2016.
Objective:
The aim of the outbreak investigation was to determine the risk factors and to inform measures to limit the spread of the organism in the hospital.
Methods:
Medical records, nursing schedules and infection control practices were reviewed. Sixty-two age- and sex-matched hospital controls from the same wards were identified.
Results:
Thirty cases (17 males) were identified (mean age = 51.6 years, age range = 2–91 years), case fatality was 53%. Multivariate logistic regression showed that a history of surgery within 90 days of diagnosis, admission to the emergency department and history of chronic kidney disease were significantly associated with C. auris infection.
Discussion:
This is the report of the outbreak investigation that triggered a global exploration of C. auris as a newly identified multidrug-resistant nosocomial organism, spreading within the hospital, especially among patients with invasive procedures. Unfortunately, we could not identify any specific source of the outbreak nor stop the transmission of the organism.
Keywords: Candida auris, outbreak investigation, fluconazole resistant, infection control
Background
In 2009, a new strain of Candida, Candida auris, was identified from a patient’s ear canal (Satoh et al., 2009). However, its clinical significance was later realised when three cases of fungemia with a fatal outcome were reported from Korea in 2011 (Oh et al., 2011). These isolates showed varied resistance to common antifungals (Chowdhary et al., 2013). Until 2015, only a handful of countries had reported C. auris (Japan, Korea, India, South Africa and Kuwait) (Emara et al., 2015; Kathuria et al., 2015; Magobo et al., 2014). In 2015, a tertiary care hospital microbiology laboratory in Karachi, Pakistan started observing an increase in the isolation rate of invasive Saccharomyces infections. Previous isolation of invasive Saccharomyces species was limited to 1–2 isolates in a year. Review of culture data revealed an increasing trend in the frequency of blood cultures positive for Saccharomyces species. Another concerning finding was the high rate of resistance to fluconazole of these organisms, an unusual finding for Saccharomyces spp.
The Infection Control Committee (ICC) of the hospital, composed of four full-time Infection Control Nurses and led by an Infectious Diseases and a Clinical Microbiology Consultant, was notified and a local team tried to identify common exposures. However, no source of these infections was identified. In March 2015, the hospital authorities formally invited the U.S. Centers for Disease Control and Prevention (CDC), Atlanta, USA to assist with the species confirmation and provide guidance for the investigation. The U.S. CDC and the tertiary care hospital then requested the Field Epidemiology & Lab Training Program (FELTP) of Pakistan to conduct the investigation in March 2015.
The FELTP team visited the 570-bed hospital on 22 March 2015. After initial discussions, a formal plan and strategies for a detailed investigation was agreed upon. Objectives of the outbreak investigation were to identify the cause and risk factors for the outbreak and to make recommendations for infection control. A collaborative team consisting of FELTP fellows working in the Sindh Provincial Disease Surveillance and Response Unit (DSRU), Clinical Microbiology Laboratory, Infection Prevention and Control committee and federal office FELTP started a detailed investigation in April 2015. While performing the outbreak investigation, the U.S. CDC identified the isolates as C. auris instead of Saccharomyces spp. and the outbreak investigation team modified the case definition and risk factor information accordingly. We report here the outbreak investigation in a Pakistani hospital which triggered an inquiry into C. auris as a multidrug-resistant nosocomial pathogen which is now known to have spread among hospitalised patients in over 35 countries (Centers for Disease Control and Prevention, 2019a, 2019b).
Methods
Outbreak investigation and data collection were carried out in two phases: 6–17 April 2015 and 16 November 2015 to 6 January 2016. The first phase was when the isolate was suspected to be Saccharomyces spp., resistant to fluconazole by disc diffusion. A case was defined as any patient admitted to the hospital with a chronic illness or for a surgical procedure with a positive sample for Saccharomyces spp., resistant to fluconazole, between 1 September 2014 and 31 March 2015. However, later, sequencing of the D1–D2 region of the 28S subunit of rDNA performed at the Mycotic Diseases Branch, CDC, Atlanta, USA identified the yeast as C. auris. Cases continued to be identified so the second phase of the investigation was undertaken from November 2015 to January 2016. The case definition was revised to include any patient admitted with a chronic illness or for a surgical procedure yielding a positive sample for confirmed or suspected C. auris between 1 September 2014 and 30 November 2015. A ‘confirmed isolate’ was identified as an isolate identified as C. auris by molecular identification, while a ‘suspected isolate’ was defined as a white yeast resistant to fluconazole by disc diffusion and biochemically identified either as either Saccharomyces spp. (profile nos. 2000130, 2000173) or Rhodotorula glutinis (profile nos. 2102173, 6102173) on API 20C AUX (bioMe’rieux, France), as described in the literature (Kathuria et al., 2015). The rest of the isolates were all later confirmed to be C. auris on Bruker MALDI Biotyper at the Mycotic Diseases Branch, CDC, Atlanta, USA.
Medical records of all the case patients were comprehensively reviewed. The records were obtained from the Health Information Management System (HIMS) and were reviewed for variables including demographics, major underlying medical/surgical conditions, date and site of C. auris infection, fungal infections within 90 days before culture positive for C. auris, history of previous hospitalisations, presence of central venous catheter, presence of urinary catheter and any antifungal treatment within 90 days of a positive C. auris culture. Movement of the patients during their hospitalisation within the various wards, Emergency Department (ED), special care units and intensive care unit (ICU) was also tracked. Since the ED was the one risk factor common to all cases, nursing records from the ED were reviewed to identify common staff members assigned to cases, to evaluate them as the source of spread of the infection. The nursing staff were interviewed to evaluate hand washing and other infection control practices such as the use of gloves and aseptic techniques. Moreover, maps of bed layouts in the ED were reviewed to identify clustering among certain areas of the department. It was also observed that staff assigned to particular areas of the ED moved to other areas within the ED to provide support. Records of rotation within the units of ED, ICU and ward staff, especially residents, nursing assistants and technicians, were also reviewed to find personnel common to case occurrences.
A case-control study was carried out for the evaluation of risk factors. Using the case definition, cases who had been admitted at the hospital, from 1 September 2014 to 30 November 2015, with culture positive for confirmed or suspected C. auris were included. To avoid over-matching as well as overestimating the odds, two controls for every case were randomly selected by an individual blinded to patient identification, from two different lists of patients admitted at the hospital, matched for age and sex. One control was also matched on admission via the ED or clinic, and the other had a hospital stay of > 8 days. Bivariate and multivariate logistic regression analyses were performed and odds ratios were calculated for various exposure related variables. Confidence intervals (CI) at 95% were calculated and P values < 0.05 were considered statistically significant. Statistical analysis was conducted using EpiInfo® version 7.1.5.0.
The Ethical Review Committee of the Ethical Review Board of our University exempted the study from full approval (ERC exemption no. 2019-2192-5790).
Results
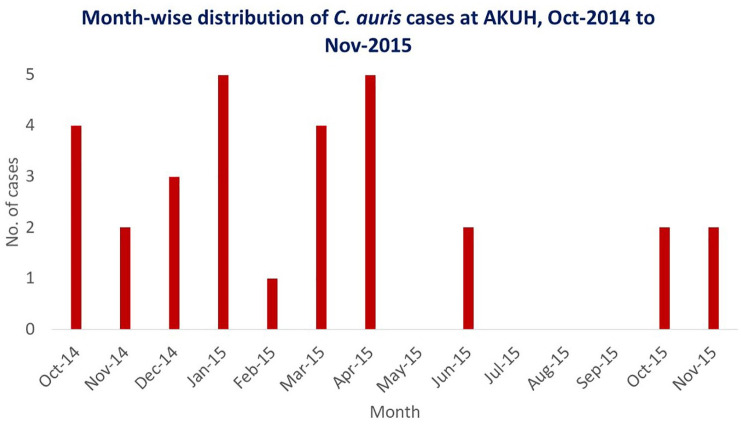
A total of 30 cases (17 males; 13 females) were included, 15 of which were confirmed and 15 suspected C. auris infections. The mean age was 51.6 years (age range = 2–91 years). Out of the 30 cases, 14 (47%) had candidemia; the rest were isolated from urine (n = 11), tissue (n = 2), central line tips (n = 2), and conjunctival (n = 1) and ear swabs (n = 1). Fourteen cases were most likely colonised while 16 (14 candidemias and two urinary tract infections) were considered to be infections. Sixteen patients had died and 14 were alive on discharge from the hospital: overall case fatality rate was 53%, but slightly higher (8/14 [57%]) in candidemic patients. Twelve cases (40%) were aged 41–60 years while 9 (30%) were aged 61–80 years. When plotted against the month of sample collected, the highest number of cases recorded per month (n = 5 each) were in January and April 2015 (Figure 1).
Figure 1.
Epi Curve showing month-wise case distribution of C. auris from October 2014 to November 2015 at our University Hospital in Karachi.
Tracking and plotting the cases on a spot map showed clustering of cases in the resuscitation bays and the 10-bed bays in the front and back of the ED. Only one case was admitted via the paediatric wing of the ED. No significant clustering could be seen in any other areas of the hospital including ICU, coronary care unit, special care units, wards and private wing. Out of 30 cases, 28 (93.33%) were admitted through the ED; however, since the C. auris infection was acquired after > 72 h, it was unlikely that the acquisition was from the ED; rather, these patients were acutely ill to begin with. This theory was supported by the fact that all 30 (100%) were found to be seriously ill before acquiring C. auris, requiring a prolonged hospital stay of > 8 days, special care or individual nursing at some point, or repeated hospital admissions. Moreover, all the cases were given broad spectrum antibiotics and 15 (50%) had received an antifungal agent within 90 days preceding the diagnosis of C. auris infection.
Review of the duty rosters of nursing and paramedical staff did not reveal or identify any common staff attending to cases. Central line maintenance audit compliance reports for medical and surgical ICUs revealed a compliance rate of < 95% for the third and fourth quarters of 2014 and first quarter of 2015. The investigation team’s observation of the medical staff revealed unsatisfactory compliance with certain infection control practices, such as adherence to hand hygiene, but these were not specifically audited by the OI team. Hand hygiene compliance data were obtained from the infection control committee of the hospital and it revealed an average of 87% (95% CI = 83.8–89.0) compliance over the investigation period. Six months earlier, the hand hygiene rate average was 81% (95% CI = 74–92).
Risk factor analysis was conducted on a sample size of 92: 30 cases and 62 controls. Among other variables, admission through the ED, surgery within 90 days of diagnosis and insertion of a central venous line showed stronger statistical association. Results of the bivariate analysis are given in Table 1.
Table 1.
Bivariate analysis: association of risk factors.
| Variables | Cases exposed | Controls exposed | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Admit through ED | 28 (93.33) | 37 (59.68) | 9.27 | 2.30–62.39 | < 0.001 |
| Surgery < 90 days | 15 (50) | 8 (12.90) | 6.57 | 2.36–19.38 | < 0.001 |
| Central venous line | 23 (77) | 23 (37.10) | 5.46 | 2.0–15.6 | < 0.001 |
| Chronic kidney disease | 10 (33.33) | 6 (9.68) | 4.57 | 1.46–15.22 | 0.004 |
| Previous fungal Infection | 11 (37) | 7 (11.29) | 4.46 | 1.50–13.89 | 0.003 |
| Urinary catheter | 26 (87) | 38 (61.29) | 4.04 | 1.31–15.06 | 0.006 |
| ICU stay | 16 (53.33) | 14 (22.58) | 3.85 | 1.51–10.05 | 0.002 |
| Endotracheal tube | 24 (80) | 35 (57.38) | 2.93 | 1.07–8.89 | 0.017 |
Values are given as n (%).
CI, confidence interval; ED, Emergency Department; ICU, intensive care unit; OR, odds ratio.
However, on multivariable analysis (logistic regression modelling) only history of surgery 90 days before diagnosis, admission through the ED and having chronic kidney disease (CKD) were found to be significantly associated with C. auris infection. Table 2 gives the details of the multivariable analysis. No single point of transmission was identified which makes a breach in standard precautions more likely.
Table 2.
Multivariable analysis showing factors significantly associated with C. auris infection.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Surgery < 90 days | 9.0 | 2.15–36.35 | 0.00 |
| Admit through ED | 7.6 | 1.16–49.60 | 0.03 |
| Chronic kidney disease | 5.2 | 1.17–23.50 | 0.02 |
| ICU stay | 2.7 | 0.72–10.64 | 0.13 |
| Urinary catheter | 1.6 | 0.37–7.72 | 0.48 |
| Central venous line | 1.5 | 0.36–6.87 | 0.53 |
| Previous fungal Infection | 1.4 | 0.30–6.44 | 0.66 |
| Endotracheal tube | 0.79 | 0.17–3.57 | 0.77 |
CI, confidence interval; ED, Emergency Department; ICU, intensive care unit; OR, odds ratio.
Microbiological
Results of the 17 clinical samples from patients admitted at the hospital, sent to US-CDC labs, were received on 6 May 2015. They showed 15 of the samples to be positive for C. auris, one positive for C. parapsilosis and one positive for Saccharomyces spp. Table 3 gives the details of the laboratory results from CDC. Whole genome sequencing was also performed on these 15 isolates and all were found to belong to the same clade with nearly identical strains differing by < 2 single-nucleotide polymorphisms, confirming the outbreak (Lockhart et al., 2017). These were found to have MIC90 of 256 µg/mL against fluconazole, 4 µg/mL against voriconazole, 1 µg/mL against anidulafungin, 0.5 µg/mL for caspofungin and 1.8 against amphotericin, respectively. Five isolates had MIC > 1 µg/mL against Amphotericin, thus rendering one-third of them resistant to multiple antifungals. The remaining 15 isolates were later sent to US-CDC in 2019 and were also confirmed as C. auris by Bruker MALDI Biotyper. The absence of an appropriate diagnostic technology which could reliably confirm C. auris isolates in Pakistan considerably delayed identification of suspected cases.
Table 3.
Specimen wise distribution of fungal species identified among the isolates sent to the US-CDC in Atlanta by D1/D2 sequencing, during the outbreak.
| Source of sample | Fungal species | n |
|---|---|---|
| Clinical (n = 17) | ||
| Blood (n = 7) | Candida auris | 7 |
| Urine (n = 7) | Candida auris | 7 |
| CVC tip (n = 2) |
Candida auris
Candida parapsilosis |
1 1 |
| Peritoneal fluid (n = 1) | Saccharomyces spp. | 1 |
Discussion
C. auris is a newly emerging yeast first identified in 2009 (only two earlier isolates have been identified, in Korea in 1996 and in Pakistan in 2008) (Lockhart et al., 2017; Satoh et al., 2008). The report is one of very few outbreak investigation reports of C. auris which has a full epidemiological investigation along with microbiological results. However, we failed to identify a single source, similar to other outbreak reports published recently from London, UK and Tel Aviv, Israel (Ben-Ami et al., 2017; Schelenz et al., 2016).
Around the same time, one multicentre study, involving 19 ICUs across India, compared risk factors for candidemia with C. auris to that due to other Candida species (Rudramurthy et al., 2017). They identified public sector hospitals and those in northern India to be associated with C. auris candidemia. These factors could obviously not be determined from a single centre outbreak investigation; however, our facility was a large private sector hospital in southern Pakistan. The only other facility, from which we had documented C. auris isolation, was a burns unit in another private sector hospital from northern Pakistan. An interesting similarity between our findings and the Indian study was regarding ICU stay. C. auris candidemia was associated with longer stay compared to other Candida species, while cases of C. auris in our hospital also had higher odds for ICU stay compared to other admitted patients. Similarly, vascular surgery in India, and any type of surgery in our study, was also a significant risk factor in cases of C. auris. Prior antifungal exposure was an important risk factor for C. auris candidemia versus other Candida in the Indian study, while prior fungal infection, suggesting antifungal exposure, did not achieve statistical significance in our single-centre data. Amongst co-morbidities, respiratory illness was more common in Indian cases of C. auris, while CKD was associated with C. auris infection in our study. Another study from our centre described candidemia with C. auris and infection with other multidrug-resistant organisms (MDRO) to be associated with crude mortality among patients with C. auris, while source control rather than appropriate antifungal therapy as the only protective factor (Sayeed et al., 2019).
The most probable risk factor of this outbreak appeared to be the history of a surgical procedure within 90 days preceding diagnosis of C. auris infection, admission through the ED and history of the CKD. All these risk factors seemed to predispose patients with lengthy hospitalisation to C. auris infection, possibly via hospital supplies and materials, soiled hands of the healthcare staff and/or contaminated environment as also suggested by other reports from UK and US (Kerins et al., 2017; Schelenz et al., 2016; Tsay et al., 2017). The investigation was limited by our inability to explore the above stated sources through microbiological sampling. We recommended review of the infection control protocols and their compliance especially in critical care facilities such as operating room, ICU and ED.
We advised refresher trainings of the healthcare workers on infection control practices and strict implementation of standard precautions for all patients and monitoring rates of C. auris using passive surveillance. In light of the outbreak, a number of infection prevention measures were taken. First, C. auris was added to the list of MDROs targeted in the MDRO programme. This programme includes enhanced communication between the laboratory and the Infection Prevention and Control committee to ensure early identification of patients with suspected C. auris, before final identification, followed by placement of patients in contact precautions. Once identified, patients are marked in the system and flagged at any visit to the hospital, whether outpatient, emergency or inpatient, so that prompt initiation of precautions are carried out. Similarly, on discharge from the hospital, terminal cleaning of the bed and equipment using 10% hypochlorite (HOCl) was carried out. Surveillance of C. auris was also initiated by modifying the LabID methodology of the CDC (Centers of Disease Control and Prevention, 2019a) and rates were disseminated to each unit on a monthly basis. Thus, we realised that beyond ensuring hand hygiene and contact precautions, active surveillance and isolation precautions cannot be maintained in resource-limited settings. With a facility managing almost 150 admissions per day, active surveillance must be supported by adequate number of trained laboratory staff or rapid and easy diagnostics, and sufficient isolation rooms which are not currently available.
The crude mortality reduced from 53% to 36.5%, possibly due to early appropriate empiric antifungal coverage with amphotericin rather than fluconazole. Since November 2015, the number of cases of C. auris still remain quite high (n = 336 till December 2019); over the last four years, 119/305 (39%) were invasive, causing candidemia. There was a short-term reduction in candidemia in the last quarter of 2015 and first quarter of 2016, most likely due to better line care. While there was no documented change in the recorded line bundle adherence rates, during this period intensive trainings on line handling were performed and the line bundle compliance tool was under revision. However, the alarming increase in the following years is very likely due to attrition of experienced healthcare personnel and a high turnover of nursing staff, a problem listed by the World Health Organization among the top health challenges for the next decade (World Health Organization, 2020).
Limitations of the investigation
Our outbreak investigation was limited mainly by lack of resources. The laboratory was not equipped with technologies that can reliably identify C. auris, thus delaying case identification. We could not conduct any exhaustive environmental microbiological investigation as the identification method was too time-consuming and there was a lot of demand for patient beds. It was considered easier to apply terminal cleaning protocols in units with high rates of C. auris. There were not enough rooms in the hospital to allow single room isolation for C. auris when the hospital was already trying to accommodate patients with other MDROs, measles and pulmonary tuberculosis. Thus, active surveillance was not thought to be feasible. However, refresher trainings of strict contact precautions with regular audits of infection control protocols were instituted. This outbreak of C. auris has added to the workload of the microbiology laboratory staff and the department of infection control and prevention: ensuring early contact precautions on suspected patients and preventing transmission requires a hefty investment in hospital resources.
Acknowledgments
From our University Hospital: Mycology bench technologists Samia Tariq, Sidra Laiq and Kanwal Iqbal for their vigilance in identifying suspected cases and keeping the investigation team informed. Microbiology post-graduate residents and Infectious Diseases fellows Salima Qamar, Fizza Farooqui, Pushpa Bhawan Mal, Yusra Shafquat and Aneela Hussain were extremely helpful in reviewing patient files for both cases and controls with the investigation team and rechecking the data entered in the case report forms and datasheets. We would also like to thank the Health Information Management Department, Nutrition and Food Services Department, Pharmacy Department and all hospital nursing and administrative staff for their utmost cooperation during the investigation.
From CDC: The strain confirmation would not have been possible without the support and guidance of our collaborators, specifically those involved in the C. auris working group: Shawn Lockhart, Snigdha Vallabhaneni at the Mycotic Diseases Branch, CDC, both the current and the prior Branch Chiefs, Dr Tom Chiller and Dr Mary E Brandt, in laboratory identification and confirmation, and epidemiological investigation
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Some of the bench work, related to antifungal susceptibility testing, and the shipment of isolates to CDC, Atlanta, were supported by HEC, US Bio-Engagement Program, USAID and Ministry of Science and Technology, grant no. 4-338/PAK-US/HEC/2010/932.
Peer review statement: Not commissioned; blind peer-reviewed.
ORCID iD: Joveria Q Farooqi  https://orcid.org/0000-0002-9921-4660
https://orcid.org/0000-0002-9921-4660
References
- Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, Maor Y, Tarabia J, Schechner V, Adler A, Finn T. (2017) Multidrug-Resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerging Infectious Diseases 23(1): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2019. a) Tracking Candida auris. Fungal Diseases, Candida auris. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED). Atlanta, GA: CDC; Available at: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html#world (accessed 15 Sept 2019). [Google Scholar]
- Centers for Disease Control and Prevention. (2019) Protocols: Multidrug-Resistant Organism & Clostridioides difficile Infection (MDRO/CDI) Module. National Health Safety Network, Materials for enrolled facilities, Acute care Hospitals/Facilities, Surveillance for C. difficile, MRSA, and other Drug-resistant Infections. Atlanta, GA: CDC; Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf (accessed 7 Feb 2020). [Google Scholar]
- Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Jain S, Kathuria S, Randhawa HS, Hagen F, Meis JF. (2013) New Clonal Strain of Candida auris, Delhi, India. Emerging Infectious Diseases 19(10): 1670–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, Bafna R. (2015) Candida auris Candidemia in Kuwait, 2014. Emerging Infectious Diseases 21(6): 1091–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Meis JF, Chowdhary A. (2015) Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. Journal of Clinical Microbiology 53: 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerins JL, Kemble S, Pacilli M, Welsh R, Chow N, Gade L, Tsay S, Vallabhaneni S, Landon E, Ridgway J, Marrs R, Jackson BR, Lockhart S, Litvintseva A, Black SR. (2017) Evidence of Health Care Transmission of Candida auris: An Investigation of 2 Cases — Chicago, Illinois, 2016. 66th Annual EIS Conference, Atlanta, USA, 24–27 April, 2017, pp. 5 Atlanta: CDC. [Google Scholar]
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. (2017) Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clinical Infectious Diseases 64(2): 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magobo RE, Corcoran C, Seetharam S, Govender NP. (2014) Candida auris-associated Candidemia, South Africa. Emerging Infectious Diseases 20(7): 1250–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh BJ, Shin JH, Kim MN, Sung H, Lee K, Joo MY, Shin MG, Suh SP, Ryang DW. (2011) Biofilm formation and genotyping of Candida haemulonii, Candida pseudohaemulonii, and a proposed new species (Candida auris) isolates from Korea. Medical Mycology 49(1): 98–102. [DOI] [PubMed] [Google Scholar]
- Rudramurthy SM, Chakrabarti A, Paul RA, Sood P, Kaur H, Capoor MR, Kindo AJ, Marak RSK, Arora A, Sardana R, Das S, Chhina D, Patel A, Xess I, Tarai B, Singh P, Ghosh A. (2017) Candida auris candidaemia in Indian ICUs: analysis of risk factors; Journal of Antimicrobial Chemotherapy 72(6): 1794–1801. [DOI] [PubMed] [Google Scholar]
- Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. (2009) Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiology and Immunology 53(1): 41–44. [DOI] [PubMed] [Google Scholar]
- Sayeed MA, Farooqi J, Jabeen K, Awan S, Mahmood SF. (2019) Clinical spectrum and factors impacting outcome of Candida auris: a single center study from Pakistan. BMC Infectious Diseases 19(1): 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. (2016) First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrobial Resistance and Infection Control 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay S, Poirot E, Chow N, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Lutterloh E, Quinn M, Gade L, Welsh R, Berkow E, Jackson B, Lockhart S, Litvintseva A, Chiller T, Kallen A, Vallabhaneni S, Adams E. (2017) The First Eight Reported Cases of Candida auris, an Emerging, Multidrug-Resistant Yeast — New York, 2013–2016. 66th Annual EIS Conference, Atlanta, USA, 24–27 April, 2017, pp. 5 Atlanta: CDC. [Google Scholar]
- World Health Organization. (2020) Urgent health challenges for the next decade. Geneva: WHO; Available at: https://www.who.int/news-room/photo-story/photo-story-detail/urgent-health-challenges-for-the-next-decade (accessed 6 Feb 2020). [Google Scholar]