Abstract
The development of the most successful cancer immunotherapies in solid tumors, immune-checkpoint blockade, has focused on factors regulating T cell activation. Until recently, the field has maintained a predominately T-cell centric view of immunotherapy, leaving aside the impact of innate immunity and especially myeloid cells. Dendritic cells (DC) are dominant partners of T cells, necessary for initiation of adaptive immune responses. Emerging evidence supports a broader role for DCs in tumors including the maintenance and support of effector functions during T cell responses. This relationship is evidenced by the association of activated DCs with immune-checkpoint blockade responses and transcriptional analysis of responding tumors demonstrating the presence of type I interferon transcripts and DC relevant chemokines. T cell-inflamed tumors preferentially respond to immunotherapies compared to non-T cell inflamed tumors and this model suggests a potentially modifiable spectrum of tumor microenvironmental immunity. While host and commensal factors may limit the T cell-inflamed phenotype, tumor cell intrinsic factors are gaining prominence as therapeutic targets. For example, tumor WNT/β-catenin signaling inhibits production of chemokine gradients and blocking DC recruitment to tumors. Conversely, mechanisms of innate immune nucleic acid sensing, normally operative during pathogen response, may enhance DC accumulation and make tumors more susceptible to cancer immunotherapy. Elucidating mechanisms whereby DCs infiltrate and become activated within tumors may provide new opportunities for therapeutic intervention. Conceptually, this would facilitate conversion of non-T cell-inflamed to T cell-inflamed states or overcome secondary resistance mechanisms in T cell-inflamed tumors, expanding the proportion of patients who benefit from cancer immunotherapy.
Introduction
Antibodies directed against T cell surface co-inhibitory receptors such as Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) and the Programmed cell death protein 1 (PD-1) pathway can reinvigorate anti-tumor T cell responses. These treatments, collectively termed Immune Checkpoint Blockade (ICB), have become critical pillars in cancer treatment and demonstrate activity in a broad range of cancer types. However, the majority of patients receiving ICB do not have durable therapeutic responses. As clinical use of these antibodies has grown immensely, a key area of research aims to define patients likely to respond, or not, to checkpoint blockade. Key to this understanding are the principles of T cell activation in cancer, and in particular how immunotherapies shift the balance of tolerance towards anti-cancer immunity.
Although hypotheses have been advanced as to why some patient’s tumors respond, or fail to respond, to checkpoint immunotherapy, most in the field have been in agreement that T cells, particularly CD8+ cytotoxic T cells are the drivers of therapeutic response (1). Abundance of tumor infiltrating CD8+ T cells, tumor mutational burden, and interferon- γ signatures are correlated with response to anti-PD-1 therapy (2–4). While biomarkers have become instrumental in understanding ICB, they do not explain the totality of treatment response and resistance. T cell presence alone in tumors may not be enough to induce anti-tumor immunity as numerous non-tumor specific T cells also infiltrate tumors (5). Therefore, this suggests an important need in the field to further understand and distinguish T cell-infiltrated from T cell-inflamed tumor microenvironments and further elucidate factors in the tumor environment driving antigen-specific T cell recruitment and activation.
Gene expression analysis of metastatic melanoma lesions identified a strong separation of samples based on T cell associated gene transcripts (6). Particularly, T cell inflammation in tumors was associated with tumor chemokine expression, particularly CXCL9, CXCL10, CCL5, CCL4, CCL3, and CCL2. T cell attracting chemokines can be produced by a variety of cell types, including tumor cells, dendritic cells (DCs), and macrophages (6–9). The importance and relative contribution of each cell type may depend upon the context and cancer type. However, some tumors grow progressively even when infiltrated with antigen specific T cells, which may be due to immune suppressive feedback and exhaustion of T cell responses (10,11). T cell activation is tightly controlled and requires initiation signals provided by antigen presenting cells, predominately DCs, such as TCR stimulation, co-stimulatory receptor ligation, and cytokine support. Sustained CD8+ T cell responses against tumors are associated with DC supportive niches within the tumor bed, and patients experiencing progressive disease exhibit breakdown of these niches (12). Moreover, DC activation phenotypes, as measured by DC gene signatures, positively correlate with the T cell-inflamed state as well as response to inhibition of the PD-1/PD-L1 pathway (13–15) suggesting a priority need in the field to further understanding of how DCs populate tumors and how to activate DCs to facilitate anti-cancer immunity.
Dendritic cell sub-sets and maturation
Dendritic cells arise from a bone marrow derived DC specific pre-cursor cell (pre-cDC) and depend upon factors such as FMS-like tyrosine kinase 3 ligand (Flt3L) and granulocyte-macrophage colony-stimulating factor (GM-CSF) for their development. DC ontogeny and classification has been thoroughly reviewed by other authors (16–18) and here we will focus only on key DC functions and subtypes. Pre-cDC seed tissues and proliferate to form peripheral DC networks. DCs can be resident within lymphoid organs, or they can surveil peripheral tissues and blood. Inflammatory conditions, including cancer, enhance DC accumulation within tissues from bone marrow sourced pre-cDCs (19). DCs endocytose material from their environment, and if appropriate stimulatory triggers are present, such as pattern recognition receptor (PRR) ligation, DCs mature and transition from antigen sampling to antigen presentation functions. Once mature, DCs upregulate surface expression of the chemokine receptor CCR7 and are attracted towards the CCR7 ligand CCL21 (produced by lymphatic endothelium) to migrate from peripheral tissues to T cell zones of local draining lymph nodes (20). DCs traffic from tissues to draining lymph nodes in the steady state, though when tissues are inflamed DCs traffic in greater numbers. Homeostatic DC migration promotes immune tolerance, in contrast DCs migrating in response to inflammation engender T cell immunity. This is likely due to the upregulation of inflammatory cytokines and co-stimulatory molecules in inflammation matured DCs, signals which are not present on steady state migrating DCs (18).
There are multiple sub-classifications of DCs – which expand further with enhanced profiling approaches (21,22), however these can broadly be grouped into conventional DC type 1 (cDC1), conventional DC type 2 (cDC2), and plasmacytoid DCs (pDCs; Table 1). An additional subset of DCs, monocyte-derived DC, have been described to arise during inflammation and promote context-dependent differentiation of CD4+ T cells. The role for these cells relative to cancer immunotherapy is unclear however and will not be discussed further here. cDC1 are the DC sub-set most well known for their ability to cross present antigens to stimulate CD8+ cytotoxic T cells. These DCs express specialized antigen presentation pathways which allow exogenous cross-presented antigens to be processed and presented on major compatibility complex class I (MHC-I) molecules on the DC’s surface (23). These cells express CD11c, MHC II, CD8α, XCR1, CLEC9a, CD24, and CD103. In humans, cDC1 also express CD141. cDC1 development depends upon the transcription factors IRF8, ID2, and BATF3, and loss of these transcription factors eliminates this DC subtype (24,25). Mechanistically, BATF3 maintains activation of IRF8 to specify cDC1 lineage commitment, as transgenic overexpression of IRF8 can rescue cDC1 development (26). In contrast to cDC1, cDC2 potently stimulate CD4+ T cell responses. These cells express CD11c and MHC II, and have surface CD11b, Sirpα, and CD301b (27). CD1c is a marker of cDC2 in humans, however mice lack CD1c genes. cDC2 function and development requires the transcription factors IRF4, RBPJ, KLF4, and RELB (18,27). pDCs are major producers of type I interferon when activated through TLR7 or 9, although identification of the Axl+ Siglec6+ DC (asDC) subpopulation has called the T cell stimulatory ability of pure pDCs into question (22). pDCs require the transcription factors TCF4 (E2–2), IRF8, and RUNX1 for development. These cells express CD11c and MHC II, and they can be distinguished by B220, Siglec-H, CD317, CD123, and CLEC4C in humans. pDC are present in blood and lymphoid organs, and can sometimes be found in tissues. In the setting of viral infection, pDC are known to augment CD8+ T cell responses through type 1 interferon activation of cDC1(28), though this mechanism is unexplored in cancer.
TABLE 1.
Dendritic Cell Subtypes
| DC Type | Cell Surface Markers | Key Transcription Factors | Primary Functions |
|---|---|---|---|
| cDC1 | CD11c, MHC II, CD8α (lymphoid resident), XCR1, CLEC9a, CD103, CD141 (human), CD24 | BATF3, IRF8, ID2 | Cross presentation of antigens to activate CD8+ T cell mediated immunity, T-helper 1 type immune response, Secretion of IL-12, CXCL9 and CXCL10 mediated T cell recruitment |
| cDC2 | CD11c, MHC II, CD11b, Sirpα, CD301b, CD1c (human) | IRF4, RBPJ, KLF4, RELB | CD4+ T cell activation, Humoral immune responses, Allergic immunity and T-helper 2 type immune response |
| pDC | CD11clow, MHC IIlow, B220, SIGLEC-H, CD317, CLEC4C (human), CD123 | TCF4 (E2-2), IRF8, RUNX1 | Type 1 interferon production, Limited antigen presentation to T cells, Augmenting DC responses through interferon signals |
DC = dendritic cell
Impact of Tumor Intrinsic Signaling on DC Exclusion and Infiltration
Analysis of T cell-inflamed melanomas demonstrated positive correlation with cDC1 gene expression signatures, indicating that the degree of tumor T cell infiltration is strongly tied to cDC1 presence within tumors (13). In contrast to the hypothesis that only overall antigenic load dictates ICB response, it seems that the recruitment and activation status of tumor DCs, in particular cDC1, factor strongly into tumor T cell-inflammation. In support of this, melanoma cell intrinsic secretion of CCL4 can attract cDC1, although this can be blocked by activated β-catenin signaling (29). Studies of hepatocellular carcinoma have also connected the β-catenin pathway with cDC1 tumor infiltration and anti-PD-1 response (30). Indeed, activated β-catenin is associated with non-T cell-inflamed tumors across a range of cancers (31,32). Inhibition of tumor DC recruitment appears to be a dominant mechanism of tumor intrinsic β-catenin activation, though it is unclear whether β-catenin activation has diverse signaling effects across cell types. Tumor intrinsic mutations that strongly alter host immune parameters argue for a cancer cell dependent mechanism of DC recruitment to tumors.
Many tumor cells are genomically unstable which results in the activation of cell intrinsic DNA sensing pathways such as the STING pathway (33). Tumor cGAS/STING is induced by DNA damage, notably that seen in the context of irradiation (34) (35). This pathway is activated upon double stranded DNA (dsDNA) binding to cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS). cGAMP then functions as a second messenger and binds STING to activate downstream signaling (36). This triggers interferon response genes and production of CCL5, which can attract cDC1s (37). Extracellular tumor DNA, or cGAMP can activate tumor DCs and initiate T cell responses in the draining lymph node (34,38,39). Tumor cell loss of LKB1 is associated with poor T cell infiltration and non-response to ICB therapy (40,41), and correspondingly STING signaling pathways can be inhibited by LKB1 loss. This blunted T cell recruitment to tumors may explain why LKB1 mutant tumors respond poorly to ICB (42). STING activity is regulated by several proteins notably including the DNA exonuclease Trex1(35), which degrades cytosolic DNA substrates that activate cGAS/STING, or via viral proteins that bind directly to STING (43). Further mechanisms of cGAS/STING pathway inhibition will likely emerge and blockade of these may represent therapeutic strategies to induce type I interferon.
Downmodulation of tumor suppressor genes and oncogene expression are now frequently associated with immune exclusion phenotypes, though at this point only β-catenin activation has been mechanistically tied to cDC1 biology (44). Absence of PTEN leads to decreased T cell infiltration in melanoma with levels of CXCL10 transcripts decreased in PTEN mutant tumors (45). Emerging evidence demonstrates that IDH1 mutations are strongly correlated with non-T cell inflamed tumors and reductions in factors such as CXCL10 (46,47). The oncometabolite R-2 hydroxyglutarate (R-2-HG), produced by mutant IDH1, has also been linked to direct metabolic suppression T cell function (48). DC presence and activity has not yet been assessed in mutant LKB1, PTEN, or IDH1 tumors. It will be of substantial interest to learn whether lack of DCs mediates the non-T cell-inflamed tumor microenvironment in these tumors.
Other mechanisms also likely influence cDC1 recruitment noting that NK cells are primary producers of the cDC1 attractive chemokines CCL5 and XCL1 (49), and the DC differentiation factor Flt3L (14). NK cells precede cDC1 recruitment in some models suggesting that molecular mediators controlling NK accumulation within tumors could be important determinants of cDC1 infiltration. Tumor cell derived prostaglandin E2 (PGE2) can inhibit both NK and cDC1 recruitment (50), leading to a non-T cell-inflamed tumor microenvironment. Oncogene influence on NK cell responses is not well described though DNA damage responses within tumors are also known to upregulate NK activating ligands, suggesting that tumor intrinsic features could drive NK activation and recruitment (51).
Alternatively, tumor alterations have been identified that promote T cell-inflamed tumors. Exacerbating tumor intrinsic nucleic acid sensing, via deletion of the double stranded RNA (dsRNA) binding protein Adar1, leads to activation of the PKR and MDA5 cytosolic dsRNA sensing pathways, type I interferon induction, and enhanced response to anti-PD-1 (52). In this setting, tumor intrinsic type I interferon production is independent of RIG-I, another cytosolic dsRNA sensor, however tumor cell intrinsic RIG-I has been implicated in anti-CTLA-4 response, and combining anti-CTLA-4 with RIG-I agonist could enhance anti-tumor responses through cDC1 cross-presentation of tumor antigens (53). TREX1 knockdown in irradiated tumor cells led to enhanced levels of cytosolic DNA that rendered tumor cells more immunogenic through activating the cGAS/STING pathway (35). Loss of function of PBRM1 in murine melanoma sensitizes tumor cells to interferon γ and T cell killing (54), likewise PBRM1 loss of function in clear cell renal cell carcinoma patients was associated with response to ICB therapy (55).
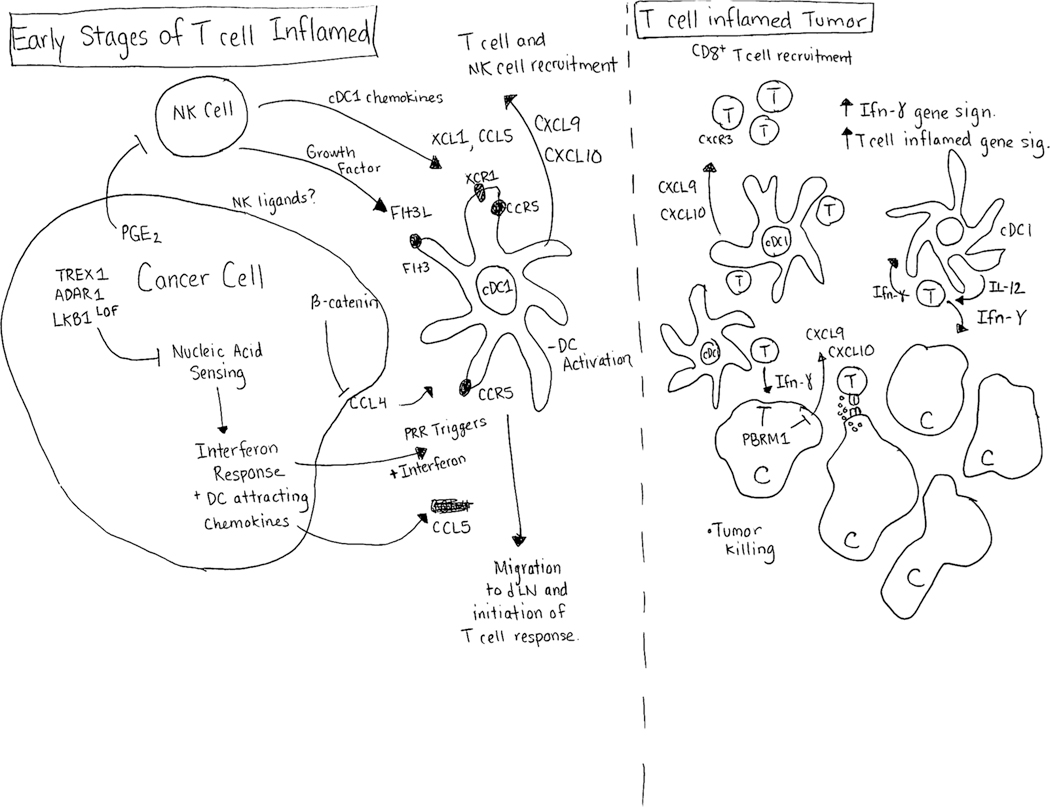
Collectively, these studies suggest a model where tumor intrinsic handling of innate immune pathways, in particular nucleic acid sensing pathways, plays a critical role controlling tumor immunogenicity through downstream recruitment and activation of DCs. These responses are then critical for initialization of the T cell response and the T-cell inflamed tumor state (Figure 1).
Figure 1 Legend:
Determinants of Tumor Dendritic Cell Infiltration. (Left Panel) Tumor cell intrinsic innate sensing of nucleic acids (both DNA and RNA substrates) induces interferon responses that attract dendritic cells (DC). Blocking innate nucleic acid sensing by limiting activating substrates (ADAR11 – RNA, TREX1 – DNA) or inhibiting STING signaling tempers tumor cell immunogenicity. cDC1 attractive chemokines produced by tumor cells can include CCL4 and CCL5, though production of CCL4 can be blocked by activated β -catenin. Natural killer cells (NK) can also produce the cDC1 tropic chemokines XCL1 and CCL5, and can support DC functions by producing the DC growth factor Flt3L. Tumor cell derived prostaglandin E2 (PGE2) can inhibit NK functions and cDC1 recruitment into tumors. Activation of DCs leads to initiation of anti-tumor T cell responses. (Right Panel) T cell inflamed tumor replete with DCs and cytotoxic T cells. T cell derived interferon γ inhibits tumor cell growth and activates local tumor cDC1 to produce IL-12, and the chemokines CXCL9 and CXCL10. DC produced IL-12 further activates tumor CD8+ T cells, and CXCL9/CXCL10 recruit T cells into the tumor microenvironment.
DCs Induce and Maintain Anti-Tumor T Cell Responses
Beyond the association of DCs and the T cell-inflamed tumor microenvironment, the presence of the Batf3 transcription factor within DCs is becoming apparent as a necessary condition for anti-cancer immunity. This has been emphasized by Batf3 murine knockout systems which manifest with cDC1 deficiency (25). Batf3 deficiency prevents spontaneous immunogenic tumor regression and impacts on therapeutic efficacy of many types of cancer immunotherapy including but likely not limited to anti-PD-1/L1 response, adoptive transfer T cell therapy, and tumor specific CD8+ T cell responses (25) (9) (56) (57) (58). Specific dissection of cDC1 function has identified that cross presentation defective mouse strains, lacking Wdfy4 or Sec22b, still retain cDC1 but these cells are incapable of inducing CD8+ T cell responses to reject tumors (59,60). cDC1 deficiency in Batf3 knockout results in similar findings as CD8+ T cells are not primed effectively and produce far less IFN-γ compared to T cells primed from wild-type DCs (58). cDC1 are also important T cell chemoattractors to the tumor microenvironment through their production of CXCL9 and CXCL10, though it is unclear if cross-presentation is required in this setting (9,61). CXCR3, the receptor for CXCL9 and CXCL10, is expressed by CD8+ T cells and is required for anti-PD-1 therapy in murine models (8). Anti-PD-1 responsive mouse tumors such as MC38 and MCA1956 had substantially higher CXCL9 and CXCL10 producing DCs compared to anti-PD-1 resistant models such as B16F10 and AT-3, and patients responding to anti-PD-1 had higher induction of these chemokines (8).
cDC1 also provide cytokine support to T cells assisting in their effector functions. IL-12 is a well-known cDC1 produced factor that can drive CD8+ T cell cytolytic activity and secretion of IFN-γ (62,63). Supplying exogenous IL-12 intratumorally in combination with a T cell agonizing CD137 antibody results in durable tumor rejection in MC38 murine models, however this effect is lost in Batf3 knockout mice (58). Direct administration of IL-12 alone is sufficient to regress tumors in wild-type mice (64), suggesting that T cell priming by Batf3 dependent cDCs is required for response to IL-12 and that T cells are stimulated by IL-12. This is further supported by the finding that tumor infiltrating T cells must receive both T cell stimuli and IL-12 to upregulate IFN-γ production. Specific T cells, and the degree to which they are exhausted, responding to IL-12 are unclear, though T cells isolated from mouse and human tumors can respond to IL-12 (63). IL-12 can drive CD8+ T cells to short lived effector cell (SLEC) fates in models of infectious disease, and in this setting IL-12 can be supplied by non-antigen presenting bystander DCs (65).This could possibly be relevant in cancer as compartmentalized models of DC function have been proposed, but these models remain to be tested.
T cell engagement can occur locally in the tumor microenvironment, or in the context of the lymph node after tumor antigen bearing DCs migrate. cDC1 migrating from the tumor are the major antigen carriers that initiate CD8+ T cell responses (66), however some important differences exist between studies. For example, migratory cDC1 have been shown to carry antigen to the B16 melanoma draining lymph node (57), however both cDC1 and cDC2 have also been shown to traffic antigen to the lymph nodes in other systems (67). Aggregation of evidence strongly supports that migratory DCs in cancer, as the mature DC, are the most efficient at stimulating T cell responses (57,66–68). This is in contrast to viral infection mechanisms that demonstrate antigen handoff from migratory DC to resident DC, with resident DC as the T cell stimulatory population (69,70). It is possible that the lack of strong inflammatory triggers, which are present in viral infection, in cancer prevents antigen handoff mechanisms that would otherwise amplify the immune response. Likewise, augmenting tumor nucleic acid sensing pathways, pathways which also serve anti-viral function can give stronger inflammatory triggers and render tumors immunogenic.
To date, evidence suggests a dominant role for cDC1 in anti-tumor immunity, however tumors are also frequently infiltrated by cDC2. These cDC2 are best able to initiate CD4+ T cell responses upon migrating to the lymph node. T regulatory cells (Treg) restrain cDC2 anti-tumor responses that activate CD4+ T cells (67) and Treg can inhibit DC maturation while hampering migration to draining lymph nodes. Removing this suppression enables IFN-γ producing CD4+ T cells to accumulate in the tumor microenvironment and is independent of CD8+ T cells, suggesting that IFN-γ responses may provide the dominant anti-tumor effect (71) (52). Tumors can also be shaped by MHC II neoantigens (72) as tumor cell expression of both MHC I and MHC II neoantigens elicited strong anti-tumor immunity. In this instance the CD4+ T cells assist in CD8+ T cell priming and maturation. Abscopal anti-tumor effects were not seen unless the tumor had expression of both neoantigens, suggesting that local CD4+ T cell activation in the tumor site, or draining lymph node, is required for a tumor immune response. These data imply that cDC2, which initiate CD4+ T cell responses, can provide anti-tumor functions in some settings.
Therapeutic Agonism of DCs
Modulation of DCs can be approached via a variety of methods, including but not limited to targeting of innate pattern recognition receptors, supplying DC growth factors, and agonizing cell surface receptors. Modulation strategies presented here are not meant to be an exhaustive list, but rather a general overview of recent attempts to activate anti-tumor DC responses. Diverse methods of therapeutic DC cancer vaccination have been attempted over the past 25 years. Longer summaries of such approaches have been previously reviewed (73) (74) (75).
Agonism of toll like receptors (TLRs) drives pro-inflammatory gene programs that can engender anti-tumor immunity. The endosomal TLRs (TLRs 3, 7, 8, and 9) make particularly attractive targets as these receptors naturally sense foreign nucleic acid patterns such as dsRNA, ssRNA, and unmethylated CpG and trigger type 1 interferon responses. Based on current understanding of interferon response and tumor immunogenicity, these pathways are attractive therapeutically toward recapitulating microenvironmental features of T cell-inflamed tumors. Agents such as Poly I:C (TLR3 agonist), NKTR-262 (TLR7/8 agonist), CMP-001 (TLR9 agonist) and Tilsotolimod (TLR9 agonist), among others, are in clinical development and testing. Clinical trial biomarker investigation of these agents has suggested increases in intratumoral interferon responses, T cell chemokines and tumor infiltrating CD8+ T cells (76–78).
Targeting the cGAS/STING pathway through STING agonism is another emerging therapeutic approach. Host DC uptake of tumor DNA induces a STING dependent interferon response that is needed for anti-tumor immunity (38). Agonizing this pathway pharmacologically could be an approach to elicit anti-tumor dendritic cell responses (36). Clinical data has been disclosed for ADU-S100 and MK-1454 (79) demonstrating the induction of systemic type I interferon response, however little intra-tumoral biomarker data has been released. In general, the intra-tumoral injection approaches pursued to date may require optimization to improve clinical efficacy, though randomized phase II and phase III trials have been launched for TLR9 and STING agonists in combination with ICB.
Several other intriguing approaches are also on-going in clinical trials. Combining poly I:C activation with CDX-301 (human Flt3L) and radiotherapy in an approach termed “in-situ vaccination” has been described as demonstrating abscopal anti-tumor responses in indolent non-Hodgkin’s Lymphoma with effect mediated by increased tumor T cell infiltration and tumor cross presenting DCs (80). Other dsRNA detection pathways like the cytosolic RIG-I sensor can be activated using the synthetic RNA oligonucleotide MK-4621. Phase 1/2 trials are underway with this agent and have demonstrated tolerable safety profiles and could enhance interferon gene expression in tumors (81). Targeting of CD40 via agonistic antibodies has demonstrated limited single agent activity to date, however emerging evidence suggests that effective CD40 agonism requires antibody Fc engagement of Fcγ receptor 2b. Newer CD40 agonist antibodies, such as APX-005M and 2141-V11, with enhanced Fcγ receptor 2b affinity show improved CD40 crosslinking and activation of DCs in pre-clinical models, and are currently undergoing early stage clinical trials (82).
Future for DC Agonism in Cancer Immunotherapy
Further elucidating the biology surrounding DC control of T cell immunity will be critical for selecting rational combination therapies moving into the future. The highest unmet need in cancer immunotherapy may be to overcome the non-T cell-inflamed tumor microenvironment and facilitate antigen-specific T cell recruitment. Early clinical trials of DC agonists, such as those above, have yet to consistently show such effects and novel approaches in delivery or drug development may be necessary to accomplish this. An unmet need also continues to exist in T cell-inflamed tumors that do not response to immunotherapy. For example, in non-small cell lung cancer the response rate to anti-PD-1 in patients with tumors that are PDL1+ > 50% remains only approximately 45%(83).
Recent studies have indicated that neoadjuvant checkpoint inhibitor treatment can produce enhanced anti-tumor T cell responses compared to adjuvant therapy, with more clonal and diverse tumor T cell infiltrates in the neoadjuvant setting (84,85). T cell responses to checkpoint inhibition can be rapid, and response can be seen in blood and normal adjacent tissue (86) (87). T cell clonotyping analysis also suggests that novel T cell clones not previously seen in the tumor can be observed following checkpoint inhibition (88) (85). It is likely these peripherally stimulated T cells are activated via DCs – possibly within tumor draining lymph nodes. Blockade of PD-1/PD-L1 can also enhance priming of naïve T cells (15), suggesting that these therapeutics do not solely act on pre-existing T cell clonotypes. Several of these studies also associated tumor T cell inflamed gene signatures with therapeutic benefit (84–86). DCs can have multiple roles in this setting, from initiating T cell expansion, to guiding T cell entry and effector function within tumors. It will be important to examine anti-tumor T cell clonalities in response to DC directed therapeutics, and the location of tumor antigens to understand where T cell re-invigoration occurs. Antigen location should also be taken into account when using DC agonist therapy. For example, peritumoral dosing of CD40 agonist antibodies drives stronger anti-tumor responses compared to systemically delivered agonist, and CD40 agonist delivered to an irrelevant tissue site failed to generate anti-tumor responses (89). Determining whether the application of DC agonists is optimally to the tumor or whether cross-presentation in the draining lymph nodes is operative may impact on combination partner selection. If this is indeed the case, a reasonable hypothesis may be entertained that optimal ICB combination therapy may include anti-CTLA-4. Further, combinations of multiple DC agonists may be necessary to optimally prime tumors for ICB response (53).
Effective cancer immunotherapy has been strongly associated with the presence of the T cell-inflamed tumor microenvironment and DC activation appears to be a primary driver of this phenotype. Conversely a major mechanism of resistance appears to be the lack of T cell-inflammation, raising agonism of DCs to generate type I interferon, in combination with ICB, as a priority approach. Multiple molecular targets are being actively explored however novel drug delivery approaches may be necessary. Many in the field have proposed to convert cold tumors to hot or make hot tumors hotter, we will observe with interest whether this simple paradigm can expand the numbers of patients benefiting from cancer immunotherapy over the next several years.
Acknowledgments
Funding Sources: CSG is supported by NIH F32 grant CA250147, JJL is supported by DOD W81XWH-17-1-0265
Footnotes
Disclosure: J. J. Luke holds stock in Actym, Alphamab Oncology, Kanaph, Mavu (now part of AbbVie), Onc.AI, Pyxis, and Tempest, is a paid consultant for TTC Oncology, 7 Hills, Alphamab Oncology, Kanaph, Mavu (now part of AbbVie), Onc.AI, Pyxis, Springbank, Tempest, Abbvie, Akrevia, Algios, Array, Astellas, Bayer, Bristol-Myers Squibb, Eisai, EMD Serono, Ideaya, Incyte, Janssen, Merck, Mersana, Novartis, PTx, RefleXion, Regeneron, Silicon, Tesaro, and Vividion, reports receiving commercial research support from (all to institution for clinical trials unless noted) AbbVie, Agios (IIT), Array (IIT), Astellas, Bristol-Myers Squibb, CheckMate (SRA), Compugen, Corvus, EMD Serono, Evelo (SRA), Five Prime, FLX Bio, Genentech, Immatics, Immunocore, Incyte, Leap, MedImmune, Macrogenics, Necktar, Novartis, Palleon (SRA), Merck, Springbank, Tesaro, Tizona, and Xencor, travel expenses from Akrevia, Bayer, Bristol-Myers Squibb, EMD Serono, Incyte, Janssen, Merck, Mersana, Novartis, Pyxis, and RefleXion, and is listed as an inventor on the following patents (both provisional): Serial #15/612,657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic, and Therapeutic Uses Thereof). C. S. Garris is listed as an inventor on the following provisional patents: serial #16/495,725 (Cancer Immunotherapy), PCT/US2018/023064 (Mitigating Fc-Fc Receptor Interactions in Cancer Immunotherapy). No other potential conflicts of interest were disclosed.
Literature Cited
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359(6382):1350–5 doi 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515(7528):568–71 doi 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348(6230):124–8 doi 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127(8):2930–40 doi 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557(7706):575–9 doi 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 6.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009;69(7):3077–85 doi 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.House IG, Savas P, Lai J, Chen AXY, Oliver AJ, Teo ZL, et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin Cancer Res 2019. doi 10.1158/1078-0432.CCR-19-1868. [DOI] [PubMed] [Google Scholar]
- 8.Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, et al. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019;50(6):1498–512 e5 doi 10.1016/j.immuni.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017;31(5):711–23 e4 doi 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5(200):200ra116 doi 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol 2019;37:457–95 doi 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 12.Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 2019;576(7787):465–70 doi 10.1038/s41586-019-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A 2016;113(48):E7759–E68 doi 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med 2018;24(8):1178–91 doi 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med 2020;12(534) doi 10.1126/scitranslmed.aav7431. [DOI] [PubMed] [Google Scholar]
- 16.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020;20(1):7–24 doi 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 17.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014;14(8):571–8 doi 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013;31:563–604 doi 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabeza-Cabrerizo M, van Blijswijk J, Wienert S, Heim D, Jenkins RP, Chakravarty P, et al. Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci Immunol 2019;4(33) doi 10.1126/sciimmunol.aaw1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol 2017;17(1):30–48 doi 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 21.See P, Dutertre CA, Chen J, Gunther P, McGovern N, Irac SE, et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 2017;356(6342) doi 10.1126/science.aag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017;356(6335) doi 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gros M, Amigorena S. Regulation of Antigen Export to the Cytosol During Cross-Presentation. Front Immunol 2019;10:41 doi 10.3389/fimmu.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tailor P, Tamura T, Morse HC 3rd, Ozato K The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood 2008;111(4):1942–5 doi 10.1182/blood-2007-07-100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008;322(5904):1097–100 doi 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grajales-Reyes GE, Iwata A, Albring J, Wu X, Tussiwand R, Kc W, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8alpha(+) conventional DC clonogenic progenitor. Nat Immunol 2015;16(7):708–17 doi 10.1038/ni.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durai V, Murphy KM. Functions of Murine Dendritic Cells. Immunity 2016;45(4):719–36 doi 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewitz A, Eickhoff S, Dahling S, Quast T, Bedoui S, Kroczek RA, et al. CD8(+) T Cells Orchestrate pDC-XCR1(+) Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity 2017;46(2):205–19 doi 10.1016/j.immuni.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523(7559):231–5 doi 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, Lindblad KE, Maier B, Sia D, et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov 2019;9(8):1124–41 doi 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin Cancer Res 2019;25(10):3074–83 doi 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweis RF, Spranger S, Bao R, Paner GP, Stadler WM, Steinberg G, et al. Molecular Drivers of the Non-T-cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol Res 2016;4(7):563–8 doi 10.1158/2326-6066.CIR-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 2018;215(5):1287–99 doi 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014;41(5):843–52 doi 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618 doi 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flood BA, Higgs EF, Li SY, Luke JJ, Gajewski TF. STING pathway agonism as a cancer therapeutic. Immunol Rev 2019;290(1):24–38 doi 10.1111/imr.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017;548(7668):461–5 doi 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014;41(5):830–42 doi 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carozza JA BV, Nguyen KC, Skariah G, Shaw KE, Brown JA, Rafat M, von Eyben R, Graves EE, Glenn JS, Smith M, Li L. Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nature Cancer 2020;1(2) doi 10.1038/s43018-020-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res 2016;76(5):999–1008 doi 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov 2018;8(7):822–35 doi 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitajima S, Ivanova E, Guo S, Yoshida R, Campisi M, Sundararaman SK, et al. Suppression of STING Associated with LKB1 Loss in KRAS-Driven Lung Cancer. Cancer Discov 2019;9(1):34–45 doi 10.1158/2159-8290.CD-18-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 2015;350(6260):568–71 doi 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 44.Trujillo JA, Sweis RF, Bao R, Luke JJ. T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol Res 2018;6(9):990–1000 doi 10.1158/2326-6066.CIR-18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov 2016;6(2):202–16 doi 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest 2017;127(4):1425–37 doi 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao R, Luke JJ. Molecular correlates and therapeutic targets in T cell-inflamed versus non-T cell-inflamed tumors across cancer types. bioRxiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 2018;24(8):1192–203 doi 10.1038/s41591-018-0095-6. [DOI] [PubMed] [Google Scholar]
- 49.Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018;172(5):1022–37 e14 doi 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015;162(6):1257–70 doi 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005;436(7054):1186–90 doi 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishizuka JJ, Manguso RT, Cheruiyot CK, Bi K, Panda A, Iracheta-Vellve A, et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature 2019;565(7737):43–8 doi 10.1038/s41586-018-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heidegger S, Wintges A, Stritzke F, Bek S, Steiger K, Koenig PA, et al. RIG-I activation is critical for responsiveness to checkpoint blockade. Sci Immunol 2019;4(39) doi 10.1126/sciimmunol.aau8943. [DOI] [PubMed] [Google Scholar]
- 54.Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 2018;359(6377):770–5 doi 10.1126/science.aao1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359(6377):801–6 doi 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 2016;22(12):1402–10 doi 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016;44(4):924–38 doi 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME, et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov 2016;6(1):71–9 doi 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theisen DJ, Davidson JTt, Briseno CG, Gargaro M, Lauron EJ, Wang Q, et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 2018;362(6415):694–9 doi 10.1126/science.aat5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alloatti A, Rookhuizen DC, Joannas L, Carpier JM, Iborra S, Magalhaes JG, et al. Critical role for Sec22b-dependent antigen cross-presentation in antitumor immunity. J Exp Med 2017;214(8):2231–41 doi 10.1084/jem.20170229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, et al. TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 2018;33(1):60–74 e6 doi 10.1016/j.ccell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014;26(5):623–37 doi 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity 2018;49(6):1148–61 e7 doi 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol 1994;153(4):1697–706. [PubMed] [Google Scholar]
- 65.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 2009;27(15):2177–87 doi 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016;30(2):324–36 doi 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 2019;177(3):556–71 e16 doi 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Theisen DJ, Ferris ST, Briseno CG, Kretzer N, Iwata A, Murphy KM, et al. Batf3-Dependent Genes Control Tumor Rejection Induced by Dendritic Cells Independently of Cross-Presentation. Cancer Immunol Res 2019;7(1):29–39 doi 10.1158/2326-6066.CIR-18-0138. [DOI] [PubMed] [Google Scholar]
- 69.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006;25(1):153–62 doi 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 70.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A 2004;101(23):8670–5 doi 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kammertoens T, Friese C, Arina A, Idel C, Briesemeister D, Rothe M, et al. Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature 2017;545(7652):98–102 doi 10.1038/nature22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019;574(7780):696–701 doi 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39(1):38–48 doi 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilboa E DC-based cancer vaccines. J Clin Invest 2007;117(5):1195–203 doi 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol 2000;18:245–73 doi 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 76.Diab A, Marcondes M, Kotzin B, Tagliaferri MA, Hoch U, Li YF, et al. Phase Ib: Preliminary clinical activity and immune activation for NKTR-262 [TLR 7/8 agonist] plus NKTR-214 [CD122-biased agonist] in patients (pts) with locally advanced or metastatic solid tumors (REVEAL Phase Ib/II Trial). Journal of Clinical Oncology 2019;37(8) doi DOI 10.1200/JCO.2019.37.8_suppl.26. [DOI] [Google Scholar]
- 77.Babiker H, Borazanci E, Subbiah V, Maguire O, Rahimian S, Minderman H, et al. Safety, efficacy, and immune effects of intratumoral tilsotolimod in patients with refractory solid tumours: Updated results from ILLUMINATE-101. Ann Oncol 2019;30:487-. [Google Scholar]
- 78.Milhem M, Gonzales R, Medina T, Kirkwood JM, Buchbinder E, Mehmi I, et al. Intratumoral toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase Ib trial in subjects with advanced melanoma. Cancer Research 2018;78(13) doi 10.1158/1538-7445.Am2018-Ct144. [DOI] [Google Scholar]
- 79.Harrington KJ, Brody J, Ingham M, Strauss J, Cemerski S, Wang M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol 2018;29:712-. [Google Scholar]
- 80.Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med 2019;25(5):814–24 doi 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 81.Middleton MR, Wermke M, Calvo E, Chartash E, Zhou H, Zhao X, et al. Phase I/II, multicenter, open-label study of intratumoral/intralesional administration of the retinoic acid-inducible gene I (RIG-I) activator MK-4621 in patients with advanced or recurrent tumors. Ann Oncol 2018;29 Suppl 8:viii712 doi 10.1093/annonc/mdy424.016. [DOI] [Google Scholar]
- 82.Vonderheide RH. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med 2020;71:47–58 doi 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- 83.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375(19):1823–33 doi 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 84.Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018;24(11):1649–54 doi 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018;24(11):1655–61 doi 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 86.Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019;25(3):454–61 doi 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen YJ, Chitre AS, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 2020. doi 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 88.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 2019;25(8):1251–9 doi 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Totterman TH, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res 2014;2(1):80–90 doi 10.1158/2326-6066.CIR-13-0067. [DOI] [PubMed] [Google Scholar]