Abstract
Obesity-related euglycaemic insulin resistance clusters with cardiometabolic risk factors, contributing to the development of both type 2 diabetes and cardiovascular disease. An increased thrombotic tendency in diabetes stems from platelet hyperactivity, enhanced activity of prothrombotic coagulation factors and impaired fibrinolysis. Furthermore, a low-grade inflammatory response and increased oxidative stress accelerate the atherosclerotic process and, together with an enhanced thrombotic environment, result in premature and more severe cardiovascular disease. The disruption of circadian cycles in man secondary to chronic obesity and loss of circadian cues is implicated in the increased risk of developing diabetes and cardiovascular disease. Levels of melatonin, the endogenous synchronizer of circadian rhythm, are reduced in individuals with vascular disease and those with deranged glucose metabolism. The anti-inflammatory, antihypertensive, antioxidative and antithrombotic activities of melatonin make it a potential therapeutic agent to reduce the risk of vascular occlusive disease in diabetes. The mechanisms behind melatonin-associated reduction in procoagulant response are not fully known. Current evidence suggests that melatonin inhibits platelet aggregation and might affect the coagulation cascade, altering fibrin clot structure and/or resistance to fibrinolysis. Large-scale clinical trials are warranted to investigate the effects of modulating the circadian clock on insulin resistance, glycaemia and cardiovascular outcome.
Keywords: Diabetes, freestyle libre, mean average relative difference, interstitial fluid glucose, hypoglycaemia
Introduction
Diabetes mellitus is a major health problem with almost 400 million adults with type 2 diabetes mellitus (T2DM) worldwide.1–3 A physiological phenomenon of insulin resistance results from circannual weight gain, which confers resistance to hypothermia and infections and is thus protective in overwintering and migrating animals.4–8 However, in man, the loss of regulation of body weight by the seasonal needs and artificial lighting leads to a loss of seasonal adjustment in adiposity, transforming what is a circannual phenomenon of insulin resistance in overwintering animals to a circadian pattern in humans.9–12 Fat-loaded adipose tissue provokes insulin resistance and contributes to metabolic syndrome through the dysregulated production of free fatty acids and pro-inflammatory adipokines affecting metabolic tissues (skeletal muscle and liver) and modifying inflammatory responses.12–14 The net result is a hyperglycaemic and pro-inflammatory environment that in turn accelerates vascular pathology, explaining the close association between diabetes and vascular ischaemic events.6,8
Cardiovascular disease (CVD) remains the main cause of morbidity and mortality in patients with T2DM, with around three-quarter of patients dying of CVD complications.15 In addition to increased risk of first vascular events, T2DM subjects have worse outcomes following vascular ischaemia compared to subjects with normal glucose metabolism.16 One of the key links between T2DM and CVD is insulin resistance, which is associated with clustering of vascular risk factors including deranged glucose metabolism, increased very-low-density lipoprotein triglyceride, decreased high-density lipoprotein cholesterol and hypertension.17–26
While insulin resistance has been attributed to lifestyle changes and the increase in obesity worldwide, understanding the mechanistic pathways underpinning insulin resistance will help to develop more effective management strategies. In particular, the production of the endogenous synchronizer of circadian rhythms, melatonin, is reduced in several cardiovascular diseases and in insulin-resistant subjects.27–35 The anti-inflammatory, antihypertensive, antioxidative activity of melatonin is suggested to favourably influence the development of atherothrombotic disease in diabetes.36–38 This review will use a foundation of circadian cardiometabolic physiology to summarize mechanisms for increased atherothrombotic events and evaluate the potential therapeutic opportunities for melatonin as an anti-atherothrombotic agent, particularly in the context of obesity-related insulin resistance.
A search of PubMed (MEDLINE) was performed starting from the year 1980 until the present, using the following terms either singly or in combination: ‘insulin resistance’, ‘type 2 diabetes’, ‘prediabetic state’, ‘cardiovascular disease’, ‘atherosclerosis’, ‘atherosclerotic plaque’, ‘inflammation’, ‘platelet aggregation’, ‘endothelial dysfunction’, ‘circadian rhythm’, ‘circadian misalignment’, ‘melatonin’, ‘thrombosis’, ‘coagulation’, ‘atherothrombosis’, ‘fibrinogen’, ‘plasminogen activator inhibitor - 1’. Research papers were considered on the basis of their relevance to diabetic atherothrombosis and potential atheroprotective effect of melatonin. Articles that covered circadian rhythm and diabetes, dysglycaemia or cardiovascular disease were selected for inclusion in this review.
Mechanisms for increased atherothrombotic events in T2DM
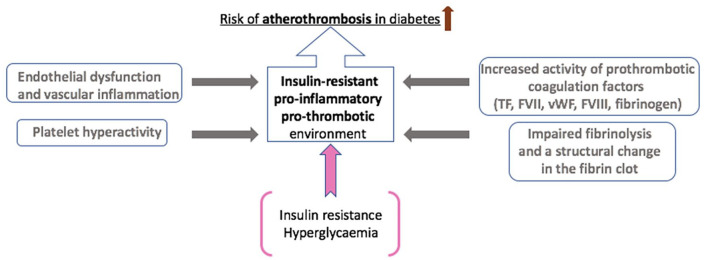
Insulin resistance is associated with endothelial dysfunction, a pro-inflammatory and a prothrombotic environment, which are all key to the development of atherothrombotic disease in T2DM (Figure 1).39–44
Figure 1.
Mechanisms for increased atherothrombotic events in diabetes. The underlying mechanisms for increased atherothrombotic risk in diabetes are complex and multifactorial. In addition to platelet hyperactivity following endothelial damage as a result of a low-grade chronic inflammatory response, increased thrombotic tendency primarily stems from enhanced activity or raised plasma levels of prothrombotic coagulation factors, including TF (tissue factor), FVII (Factor VII), vWF (von Willebrand factor), FVIII (Factor VIII) and fibrinogen. This results in fibrin networks, which form the backbone of the blood clot, that are compact with increased resistance to fibrinolysis. Insulin resistance and hyperglycaemia enable these underlying mechanisms and directly contribute to an insulin-resistant, pro-inflammatory and prothrombotic environment in diabetes, increasing the risk of atherothrombosis.
Endothelial dysfunction and vascular inflammation
Insulin resistance is associated with a cluster of risk factors that result in endothelial dysfunction, a key abnormality that contributes to the atherothrombotic process (reviewed in Sena et al.71). Briefly, endothelial dysfunction causes endothelial cells to express adhesion molecules, thus attracting inflammatory cells, and also increases inter-cellular permeability resulting in accumulation of lipoproteins and inflammatory cells within the vessel wall. Lipid particles migrating to vessel wall are oxidized into highly atherogenic molecules that are taken up by inflammatory cells to form foam cells. An accumulation of foam cells forms the fatty streak, the earliest abnormality in the atherosclerotic process. This is followed by deposition of collagen, gradually resulting in the development of the mature atherosclerotic plaque. Enhanced production of cytokines and growth factors by these inflammatory cells leads to smooth muscle migration and proliferation in the intima, which further contributes to the inflammatory process.42,49,54,55
Increased inflammatory cytokines, including tumour necrosis factor α (TNFα) and interleukin (IL)-6, impairs production of a vasodilatory molecule NO (nitric oxide) and also modulates activation of PI3K/Akt pathways further accelerating atherothrombosis in diabetes.72–79 In addition, the activation of the pro-inflammatory nuclear transcription factor nuclear factor-kappa B is permitted by the loss of NO, increasing the expression of adhesion molecules, cytokines and chemokines, creating a vicious cycle.50,52 Finally, increased production of advanced glycation end product (AGE) and formation of reactive oxygen species (ROS) diminishes endothelial-derived NO and leads to enhanced synthesis of vasoconstrictor prostanoids and endothelin.49,80–82
Role of circadian rhythm in T2DM and increased thrombotic environment
The underlying mechanisms for increased thrombosis risk in diabetes subjects are complex and multifactorial. Both the cellular and protein arms of coagulation in diabetes are affected leading to a prothrombotic environment. Platelet activation is increased in diabetes and a key mechanism appears to be reduced NO production, an important inhibitor of platelet activation, secondary to endothelial dysfunction.46,83–96 Furthermore, under conditions of hyperglycaemia and insulin resistance, chronic low-grade systemic inflammation drives the haemostatic system towards a prothrombotic state.39,40–44 Plasma levels of a number of procoagulant factors are elevated in diabetes patients, including tissue factor, factor VII, von Willebrand Factor, factor VIII and fibrinogen, increasing thrombotic tendency.45,49,59–65,80 In addition, diabetes is associated with reduced concentration of antithrombotic factors, such as antithrombin III and protein C,83 further adding the prothrombotic milieu. Moreover, the fibrinolytic system is directly affected in diabetes secondary to elevated levels of plasminogen activator inhibitor (PAI)-1 and impaired plasminogen to plasmin conversion, together with reduced enzyme activity.16 Interestingly, the EuroClot study involving healthy subjects reported an association between clustering of cardiometabolic risk factors and suppression of fibrinolytic activity, making modulation of hypofibrinolysis in diabetes a possible target to reduce atherothrombotic disease.97 Hypofibrinolysis has been recently reported to be an independent predictor of cardiovascular mortality in individuals with acute coronary syndrome treated with modern antithrombotic therapies.98 In summary, inflammation-mediated endothelial dysfunction, platelet hyperactivity, increased activation of pro-coagulation factors and impaired fibrinolysis represent a wide spectrum of biological targets with most of them exhibiting circadian variation, thus implicating loss of circadian rhythmicity in the increased thrombotic tendency in diabetes.99–103
The constant exposure to food and the loss of the dark/night stimulus translate into loss of environmental control of circadian rhythmicity, which is implicated in the above-mentioned processes and thus increased risk of developing both diabetes and cardiovascular disease.104–106 The master circadian pacemaker in the suprachiasmatic nucleus generates diurnal circadian rhythms secondary to cyclical expression of clock-controlled genes in response to light and food intake.107 Clock genes, the Clock and Bmal1, encode transcriptionally activating proteins of the clock system in response to light, whereas Per 1-3 and Cry 1 and 2 genes encode transcriptionally inhibiting proteins of the clock system.35 Rudic et al.108 reported that an adequate insulin response depends on normal function of the circadian clock. Indeed, Clock mutant Bmal1−/− mice develop a diabetes phenotype with metabolic abnormalities, including impaired gluconeogenesis, hyperglycaemia and hypercholesterolaemia.108 Mutations in the Clock gene have also been associated with the metabolic syndrome and vascular dysfunction in man, implicating circadian disruption in the development of both diabetes and associated cardiovascular disease.109–111
In human subjects, data linking circadian misalignment with development of T2DM and adverse cardiovascular events comes either from epidemiological studies in shift workers or from short-term sleep deprivation studies (Table 1). Factors opposing aligned circadian rhythmicity are proposed to underlie higher rates of cardiovascular disease events in shift workers when compared to day workers.112–117 Women of the Nurses’ Health Study cohort with 6 years or more of rotating shift work experience were 1.51 [95% confidence interval (CI): 1.12–2.03] more likely to develop coronary heart disease compared to women who had never been shift workers.118 A systematic review conducted in 2012 reported that shift work was associated with 41% increased relative risk of coronary events.119 Furthermore, circadian misalignment has been shown to have a negative impact on the predictors of cardiovascular disease risk, including pro-inflammatory markers, such as serum IL-6 and C-reactive protein (CRP), and was also associated with hypercoagulability.120,121 There is, however, evidence of metabolic and cardiovascular disease markers being improved when shift work schedules are adapted to biological rhythmicity.122
Table 1.
Summary of studies showing evidence on association between circadian misalignment and development of adverse cardiovascular events and T2DM in human subjects.112–115,118–126,127
| Study name (reference) | Number of individuals recruited | Follow-up period | Main conclusions |
|---|---|---|---|
| Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines (Yamasaki et al.112) | 99 nurses | 24-h period (either day, evening or night shift) | Two independent predictors of higher systolic blood pressure are being African American and working evening or night shifts, which could contribute to adverse effects of shift work |
| Adverse metabolic and cardiovascular consequences of circadian misalignment (Scheer et al.113) | 10 adults | 10-day laboratory protocol | Circadian misalignment decreases leptin, increases glucose, insulin and blood pressure, and reverses cortisol rhythm |
| Melatonin rhythms in night shift workers (Sack et al.114) | 9 night-shift workers | 24-h period | The sleep times of night-shift workers is not synchronized with melatonin rhythm |
| The circadian melatonin and cortisol secretion pattern in permanent night shift workers (Roden et al.115) | 9 night-shift workers | 28 h | Shift work is associated with a rise in melatonin secretion |
| Prospective study of shift work and risk of coronary heart disease in women (Kawachi et al.118) | 79,109 nurses | 4 years | Shift work increases the risk of coronary heart diseases in women |
| Shift work and vascular events: systematic review and meta-analysis (Vyas et al.119) | 34 studies in 2,011,935 people | N/A | Shift work is associated with an increased risk of atherothrombotic events |
| C-reactive protein and fibrin clot strength measured by thrombelastography after coronary stenting (Kreutz et al.120) | 54 adults | 16–24 h | Thrombotic risk is associated with raised CRP in patients undergoing coronary stenting and may be linked to procoagulant changes and higher maximal fibrin clot strength independently of fibrinogen concentration |
| Circadian misalignment increases cardiovascular disease risk factors in humans (Morris et al.121) | 14 adults | 8 days | Short-term circadian misalignment (12-h inverted behavioural and environmental cycles for 3 days) increases inflammatory markers and blood pressure in healthy adults |
| Intervention on coronary risk factors by adapting a shift work schedule to biologic rhythmicity (Orth-Gomer122) | 45 adults | 4 weeks of clockwise rotation and 4 weeks of counter-clockwise rotation | Adapting shift rotation to biological rhythmicity could favourably influence risk factors for ischaemic heart disease, including triglycerides, glucose and blood pressure |
| Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women (Pan et al.123) | 69,269 women in Nurses Health Study I and 107,915 women in Nurses Health Study II | 18–20 years | Duration of rotated night-shift work is associated with an increased risk of T2DM |
| Incidence of metabolic syndrome among night-shift healthcare workers (Pietroiusti et al.124) | 738 adults | 4 years | Night-shift work is associated with the risk of developing metabolic syndrome |
| Rotating shift work and the metabolic syndrome: a prospective study (De Bacquer et al.125) | 1529 adults | 6.6 years | Rotating shift work increases the risk of developing the metabolic syndrome |
| Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women (Eriksson et al.126) | 5227 adults | 8-10 years | Psychological distress, including fatigue and insomnia, increases the risk of developing prediabetes and T2DM in men |
| Sleep disturbance and onset of type 2 diabetes (Kawakami et al.127) | 2649 adults | 8 years | Sleep disturbance is associated with an increased risk of developing T2DM independently of known risk factors for T2DM |
CRP: C-reactive protein; T2DM: type 2 diabetes mellitus.
Epidemiological evidence also indicates associations between shift work and development of T2DM.27,123 An important prospective clinical study, including 402 night-shift workers and 336 daytime workers followed up for a median of 4 years, identified a five-fold increased risk of developing T2DM/obesity in night-shift workers.124 In addition, a prospective study on a cohort of 1529 workers reported a 77% higher incidence of the metabolic syndrome in shift workers.125 Misalignment of circadian rhythm and sleep disorders have been associated with the development of metabolic syndrome and prediabetes, correlated with an elevated risk of T2DM.27,126 There is also mounting evidence to demonstrate a close association between sleep deprivation, insomnia, restless leg syndrome and T2DM.128 Poor sleep quality is associated with increased sympathetic activity, decreased leptin levels and elevated ghrelin levels among other disturbances, leading to a number of metabolic abnormalities, including glucose intolerance.127,129,130 Moreover, loss of circadian clock synchronization has been suggested as one mechanism for increased thrombotic tendency in diabetes.131 That view is supported by in vitro studies demonstrating that the Clock/Bmal heterodimers regulate PAI-1 gene expression.132 The involvement of Clock in the regulation of PAI-1 was also reported in the setting of obesity, supporting the link between obesity, diabetes and CVD through circadian regulation and PAI-1 gene expression.133
The above evidence raises the possibility of using circadian rhythm modulators to ‘reset’ normal circadian physiology, in turn reducing metabolic abnormality and vascular risk.134
Melatonin and the circadian rhythm
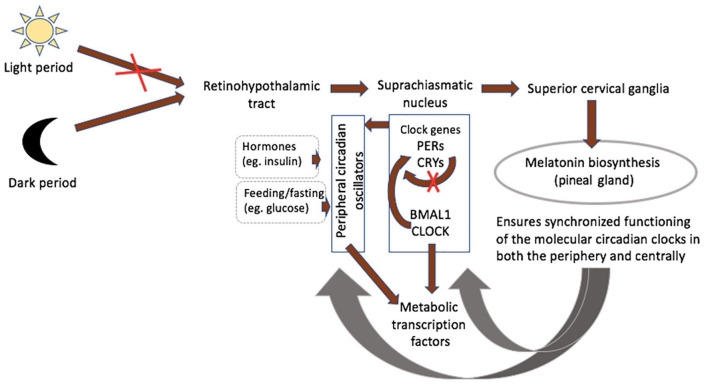
The pineal gland is essential as a rhythmic synchronizer, connecting the nervous and the endocrine systems.135,136 The retinohypothalamic-pineal system becomes activated by norepinephrine with the onset of darkness, leading to melatonin release (Figure 2).36,135,136 Activity of the central pacemaker, being regulated by the humoral system, triggers peripheral circadian oscillators, controlling their rhythmicity to coordinate metabolic activity throughout the body.136–138 These peripheral pacemakers share similar organization at the molecular level and regulate circadian variation in local transcription clusters.138,139 Metabolically active organs including skeletal muscles, liver and adipose tissue display circadian variability in the expression of clock genes and genes that are involved in hormone synthesis and encode proteins participating in key metabolic events.139,140 Although animal studies demonstrate that environmental stimuli, such as light exposure, regulate the phase of the master clock, experiments of restricted feeding show that the phase of circadian expression in mouse liver could be inverted without affecting central clock gene expression.141–143 Such desynchrony, whereby the peripheral pacemakers are uncoupled from the master clock in the suprachiasmatic nucleus, may worsen circadian-related metabolic disorders.144
Figure 2.
Melatonin an internal synchronizer of circadian biological rhythmicity. The retinohypothalamic-pineal system becomes activated with the onset of darkness, leading to the transmission of neural signals from the suprachiasmatic nucleus (central pacemaker) to the superior cervical ganglia. Noradrenalin release from postganglionic fibres stimulates synthesis of melatonin by pinealocytes. Clock genes encoding transcriptionally activating or inhibiting proteins of the clock system in response to variation in light exposure are the Clock and Bmal1, PERs and CRYs, respectively. Activity of the central pacemaker triggers peripheral circadian oscillators, which are also influenced by local factors, including hormones and glucose. Metabolically active organs including skeletal muscles, liver and adipose tissue display circadian variability in the expression of metabolic transcription factors. Melatonin prevents desynchrony, whereby the peripheral pacemakers are uncoupled from the master clock in the suprachiasmatic nucleus, which may worsen circadian-related metabolic disorders.
Melatonin plays an essential role in the synchronized functioning of the molecular circadian clocks in both the periphery and centrally in the suprachiasmatic nucleus.36,137,146,147 Its circadian rhythm-entraining effects are mediated via melatonin receptors MT1 and MT2, which have been implicated in jet lag phenomenon and in the context of sleeping disorders.148–150 Animal studies report reductions in visceral adiposity, glucose, insulin and triglycerides following melatonin administration.36,137,151–154 Although the evidence on the effect of melatonin is limited in human individuals and in the context of insulin resistance, decreased melatonin production found in patients with T2DM, prediabetes and cardiovascular disease suggests a protective role for this hormone in insulin resistance and atherosclerosis.27–35
Role of melatonin in vascular complications in T2DM
Evidence gathered in the last 20 years indicates that melatonin influences multiple aspects of cardiometabolic function.36,137,151–154 Strong evidence comes from studies demonstrating that genetic variants in the melatonin receptor as a result of single nucleotide polymorphisms are related to obesity and are associated with atherosclerosis and the risk of myocardial infarction (MI). Genome-wide association studies indicate a causal relationship between one of the single nucleotide polymorphisms of melatonin receptor MTNR1B and reduced glucose-regulating ability. Furthermore, studies on hypercholesterolemic rats indicated that melatonin leads to lowering of total cholesterol, low-density lipoprotein (LDL) and very-low-density lipoprotein cholesterol levels, suggesting it may protect from atherosclerosis.155 In addition, animal studies provide evidence on the antioxidant and anti-inflammatory actions of melatonin, which attenuates thermal-induced activation of blood clot formation.156,157 These observations together with studies demonstrating low melatonin levels in individuals with coronary artery disease, insulin resistance and T2DM led to the speculation that melatonin has a role in vascular pathology.27–35 Therefore, melatonin treatment may prove to be an effective strategy in the management of atherothrombotic disease, particularly in high-risk subjects with deranged circadian rhythm such shift workers. Experimental work provided evidence that melatonin supplementation improves insulin resistance induced by disruption of internal circadian rhythms.158 In addition to preclinical evidence suggesting exogenous melatonin could also reduce leptin resistance, hyperinsulinaemia and hyperglycaemia in animal models of obesity and metabolic syndrome, melatonin supplementation has also been shown to be associated with significant reduction in HbA1c in a small group of patients with diabetes and insomnia.159–161 More recently, studies show that the low levels of melatonin secretion predicts the onset of T2DM in women and that adequate melatonin secretion is thought to reduce the incidence of T2DM.34,162
Antihypertensive and antioxidant properties of melatonin: potential impact on the atherosclerotic process
Studies have shown associations between melatonin secretion and reduction in night-time systolic blood pressure.36,163 Obayashi et al.,163 however, did not find a significant relationship between melatonin excretion and diastolic blood pressure, suggesting the relationship is mainly with systolic blood pressure by mechanisms that are not entirely clear.
Oxidative stress plays a central role in the atherosclerotic process, particularly in diabetes.44 The antioxidant properties of melatonin stem from its detoxifying characteristics, effective scavenging of reactive oxygen and nitrogen species and its ability to enhance antioxidative enzymes.36 Studies have demonstrated that the rhythm of antioxidant defence is obliterated in pinealectomized rats and by light in humans.36 In prothrombotic stroke models of ischaemia-induced oxidative damage, in vivo melatonin administered post-ischaemia exhibited a concentration-dependent protection mediated through a reduction in ROS production, associated with decreased infarct size.164 Similarly, melatonin was shown to have a cardioprotective effect against oxidative injury in the ischaemic/reperfused heart.155,165,166 Decreased serum melatonin and an increase in oxidative stress in patients with MI may indirectly suggest a protective effect of melatonin against cardiovascular disease in man.167–169 Finally, subjects with elevated levels of LDL, oxidation of which is implicated in the progression of atherosclerosis, have been reported to have low levels of melatonin.153 Some studies, but not all, support prevention of LDL oxidation by melatonin, providing another potential mechanism of protection from vascular pathology.153,170
Melatonin deficiency augments sympathetic activity and influences atherosclerosis and thrombus formation
Nocturnal secretion of melatonin is reduced in subjects with coronary artery disease, and especially those with acute coronary syndrome (ACS), compared to healthy individuals, and in patients with unstable compared to stable angina.29,30,168,171–173 Impaired circadian biological rhythmicity and the lack of the blunting effect of melatonin on sympathetic activity lead to sympathetic activation, contributing to endothelial injury, platelet activation and predisposing vulnerable plaques to rupture.29,113 Increased sympathetic activity and activation of the coagulation cascade in the early morning might contribute to the well-described morning peaks in cardiovascular events in patients with coronary artery disease (CAD).28,173,174 In addition, increased sympathetic activity might affect production of a key inhibitor of fibrinolysis, PAI-1, thus contributing to hypofibrinolysis and increasing the risk of vascular events.175
Urinary 6-sulfatoxymelatonin excretion, a urinary metabolite of melatonin serving as an index of its secretion, is inversely associated with arterial stiffness after adjusting for diabetes and hypertension.176 While this does not prove causality, the association warrants further investigation. Previous work has also shown that melatonin has antithrombotic properties when administered at low doses before or during coronary occlusion, although the exact mechanisms remain to be elucidated.177
Melatonin affects activated coagulation and platelet aggregation
A few animal studies have investigated the effect of melatonin on heat-induced inflammatory and coagulation responses. Platelet morphology and elevated levels of fibrinogen, fibrin degradation products, prothrombin activity and CRP following thermal injury were normalized after melatonin administration.156,157 Antithrombotic effects of melatonin were also investigated in randomized-controlled trials in healthy volunteers under acute psychosocial stress conditions. Emotionally triggered catecholamine-induced hypercoagulable state promotes thrombus formation following plaque rupture.178–181 Stress-induced increases in FVII:c and fibrinogen contribute to a hypercoagulable state; however, no statistically significant reductions in these factors were observed after melatonin administration, arguing against a beneficial role for melatonin during thrombotic vascular occlusion.181–183 In healthy young men, the administration of melatonin was associated with a significant reduction in FVIII:c and fibrinogen levels, whereas levels of FVII:c and D-dimer showed no change.173 On the contrary, melatonin could attenuate elevations in d-dimer, suggesting limited thrombus formation and providing support for melatonin potential in reducing atherothrombotic risk.172,183
In addition to the protein arm of coagulation, melatonin is thought to influence circadian variation in platelet activity and several studies have suggested melatonin having direct effects on platelet function. Melatonin has been implicated in the inhibition of platelet aggregation, both spontaneous and induced, mainly through the cyclooxygenase-dependent pathway.184–189 In addition, some studies, but not all, suggest that melatonin elevates the apoptotic events in platelets.190,191 Although both of these effects of melatonin on platelets could potentially limit the process of thrombogenesis, the evidence behind them is not consistent and therefore warrants further exploration.
Conclusion and future directions
There is convincing evidence stemming from genetic and epidemiological studies to implicate deranged circadian rhythm in the development of both diabetes and cardiovascular disease. Although the evidence for melatonin as an agent that restores the deranged rhythm is limited in man, indirect evidence suggests a central role for melatonin in abnormal glucose metabolism and/or vascular pathology.
An increased thrombotic tendency in the setting of low-grade chronic inflammation primarily stems from platelet hyperactivity, enhanced activity of prothrombotic coagulation factors and impaired fibrinolysis. Anti-inflammatory and antioxidative properties of melatonin may favourably modulate the prothrombotic and inflammatory environment typically found in atherothrombotic disease.36–38 In particular, the antioxidant activity of melatonin may be atheroprotective in diabetes since hyperglycaemia-induced free radical formation increases oxidative stress, which predisposes to vascular pathology.49,192,193 A cardioprotective and antithrombotic effect of melatonin might also stem from a selective effect on the plasma levels of coagulation measures, including fibrinogen and FVIII:c, both of which are found at increased concentrations in diabetes and are associated with an elevated risk of coronary thrombotic events.84,173 In addition, the atheroprotective potential of melatonin comes from evidence of melatonin deficiency increasing the risk of cardiovascular events by augmenting sympathetic activity, which contributes to platelet activation and hypertension-induced injury to the endothelial cell layer.29
The potential mechanisms of melatonin-mediated atheroprotection are summarized in Table 2. Studies to date suggest that melatonin should be explored as an agent to reduce insulin resistance and prevent diabetes and/or vascular disease, particularly in individuals with deranged circadian rhythm. An advantage of melatonin supplementation is the favourable side effect profile, potentially giving it an advantage over established therapies.145 However, the effects of melatonin to prevent desynchrony are not one-sided and melatonin supplementation could affect endogenous serotonin concentrations and functions of pineal and hypothalamic systems, thus influencing appetitive, emotional and cognitive processes.194 In addition, persistent exposure to melatonin could lead to continuous activation of both melatonin receptors, generating off-target as well as on-target effects. For example, activation of MT1 receptor could generate continuous effects on downstream pathways, such as PLC/DAG/PKC pathway. This in turn could impact neuronal firing and immune system response via a continuous inhibition of leukocyte rolling in the microvasculature.195 In addition, the effects of melatonin metabolites have to be explored and evaluated, in particular, 6-hydroxymelatonin-induced oxidative DNA damage.196 Therefore, more mechanistic studies are needed in different population of patients to not only further understand the role of melatonin in modifying the inflammatory and thrombotic environment that predisposes to vascular disease, but also consider the effect of melatonin and its metabolites on the variety of biological systems.
Table 2.
Mechanisms responsible for the cardioprotective effect of melatonin in atherothrombotic disease.
| Potential mechanisms of melatonin-mediated atheroprotection |
|---|
| • Significant reduction in HbA1c in patients with diabetes and insomnia |
| • Restores insulin resistance induced by disruption of internal circadian rhythms, preventing the onset of diabetes, a strong independent risk factor for atherothrombotic disease |
| • Reduction in leptin resistance, hyperinsulinaemia and hyperglycaemia in the models of obesity and metabolic syndrome |
| • Anti-inflammatory effect |
| • Antihypertensive effect: reduction in the night-time systolic blood pressure |
| • Antioxidant effect: detoxification ability, effective scavenging of reactive oxygen and nitrogen species and ability to enhance antioxidative enzymes |
| ◦ Prevention of low-density lipoproteins from oxidation |
| • Blunting effect on sympathetic activity |
| • Altered levels/activity of proteins involved in the coagulation cascade |
| • Inhibition of platelet aggregation |
Key messages
An increased thrombotic tendency in diabetes stems from platelet hyperactivity, enhanced activity of prothrombotic coagulation factors and impaired fibrinolysis.
Levels of melatonin, the endogenous synchronizer of circadian rhythm, are reduced in individuals with vascular disease and those with deranged glucose metabolism.
Anti-inflammatory, antioxidative, antihypertensive and antithrombotic activities of melatonin may favourably modulate the prothrombotic and inflammatory environment typically found in atherothrombotic disease, which make it a potential therapeutic agent to reduce the risk of vascular occlusive disease in diabetes.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Anastasia Otamas  https://orcid.org/0000-0002-2796-434X
https://orcid.org/0000-0002-2796-434X
References
- 1. Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J 2012; 27: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37: S81–S90. [DOI] [PubMed] [Google Scholar]
- 3. WHO. Global report on diabetes 2016, https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=F0E50588BDA1091F000D377575E55AAE?sequence=1 (accessed October 2019).
- 4. Meier AH, Cincotta AH. Circadian rhythms regulate the expression of the thrifty genotype/phenotype. Diabetes Rev 1996; 4: 464–487. [Google Scholar]
- 5. Martin SL. Mammalian hibernation: a naturally reversible model for insulin resistance in man. Diab Vasc Dis Res 2008; 5: 76–81. [DOI] [PubMed] [Google Scholar]
- 6. Reaven GM, Scott EM, Grant PJ. Hemostatic abnormalities associated with obesity and the metabolic syndrome. J Thromb Haemost 2005; 3: 1074–1085. [DOI] [PubMed] [Google Scholar]
- 7. Bloomgarden ZT. Insulin resistance concepts. Diabetes Care 2007; 30: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 8. Scott EM, Grant PJ. Neel revisited: the adipocyte, seasonality and type 2 diabetes. Diabetologia 2006; 49: 1462–1466. [DOI] [PubMed] [Google Scholar]
- 9. Sowers JR. Obesity as a cardiovascular risk factor. Am J Med 2003; 115: 37–41. [DOI] [PubMed] [Google Scholar]
- 10. Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001; 286: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 11. Seidell JC. Obesity, insulin resistance and diabetes: a worldwide epidemic. Br J Nutr 2000; 83: S5–S8. [DOI] [PubMed] [Google Scholar]
- 12. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014; 15: 6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JI, Huh JY, Sohn JH, et al. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol 2015; 35: 1686–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013; 2013: 139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care 2010; 33: 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearney K, Tomlinson D, Smith K, et al. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol 2017; 16: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stern MP. Diabetes and cardiovascular disease: the ‘common soil’ hypothesis. Diabetes 1995; 44: 369–374. [DOI] [PubMed] [Google Scholar]
- 18. Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for developing diabetes. Lancet 2012; 379: 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol 2011; 108: 3B–24B. [DOI] [PubMed] [Google Scholar]
- 20. Smith U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance: is insulin resistance initiated in the adipose tissue? Int J Obes Relat Met Disord 2002; 26: 897–904. [DOI] [PubMed] [Google Scholar]
- 21. Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bloomgarden ZT. World congress on insulin resistance, diabetes, and cardiovascular disease: part 1. Diabetes Care 2011; 34: e115–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fletcher B, Lamendola C. Insulin resistance syndrome. J Cardiovasc Nurs 2004; 19: 339–345. [DOI] [PubMed] [Google Scholar]
- 24. Haffner SM, Stern MP, Hazuda HP, et al. Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 1990; 263: 2893–2898. [DOI] [PubMed] [Google Scholar]
- 25. Reaven GM. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 26. Alshehri AM. Metabolic syndrome and cardiovascular risk. J Fam Community Med 2010; 17: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27 485 people. Occup Environ Med 2001; 58: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lercher P, Stoschitzky K, Sakotnik A, et al. Melatonin in patients with coronary artery disease, hypertensive heart disease and dilated cardiomyopathy. Atherosclerosis 2000; 151: P203. [Google Scholar]
- 29. Yaprak M, Altun A, Vardar A, et al. Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int J Cardiol 2003; 89: 103–107. [DOI] [PubMed] [Google Scholar]
- 30. Girotti L, Lago M, Ianovsky O, et al. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. J Pineal Res 2000; 29: 138–142. [DOI] [PubMed] [Google Scholar]
- 31. Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez M, et al. Elevated levels of oxidized low-density lipoprotein and impaired nocturnal synthesis of melatonin in patients with myocardial infarction. Atherosclerosis 2005; 180: 101–105. [DOI] [PubMed] [Google Scholar]
- 32. Tutuncu NB, Batur MK, Yildirir A, et al. Melatonin levels decrease in type 2 diabetic patients with cardiac autonomic neuropathy. J Pineal Res 2005; 39: 43–49. [DOI] [PubMed] [Google Scholar]
- 33. Girotti L, Lago M, Ianovsky O, et al. Low urinary 6-sulfatoxymelatonin levels in patients with severe congestive heart failure. Endocrine 2003; 22: 245–248. [DOI] [PubMed] [Google Scholar]
- 34. McMullan CJ, Schernhammer ES, Rimm EB, et al. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013; 309: 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peschke E, Bahr I, Muhlbauer E. Experimental and clinical aspects of melatonin and clock genes in diabetes. J Pineal Res 2015; 59: 1–23. [DOI] [PubMed] [Google Scholar]
- 36. Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005; 9: 11–24. [DOI] [PubMed] [Google Scholar]
- 37. Viles-Gonzalez JF, Fuster V, Badimon JJ. Links between inflammation and thrombogenicity in atherosclerosis. Curr Mol Med 2006; 6: 489–499. [DOI] [PubMed] [Google Scholar]
- 38. Muller G, Goettsch C, Morawietz H. Oxidative stress and endothelial dysfunction. Hamostaseologie 2007; 27: 5–12. [PubMed] [Google Scholar]
- 39. Esmon CT. Inflammation and thrombosis. J Thromb Haemost 2003; 1: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 40. Hess K, Grant PJ. Inflammation and thrombosis in diabetes. J Thromb Haemost 2011; 105: S43–S54. [DOI] [PubMed] [Google Scholar]
- 41. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014; 10: 293–302. [DOI] [PubMed] [Google Scholar]
- 42. Myers DD, Wakefield TW. Inflammation-dependent thrombosis. Front Biosci 2005; 10: 2750–2757. [DOI] [PubMed] [Google Scholar]
- 43. Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas 2004; 47: 305–314. [DOI] [PubMed] [Google Scholar]
- 44. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004; 24: 816–823. [DOI] [PubMed] [Google Scholar]
- 45. Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res 2010; 7: 260–273. [DOI] [PubMed] [Google Scholar]
- 46. Vinik AI, Erbas T, Park TS, et al. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001; 24: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 47. Baldi S, Natali A, Buzzigoli G, et al. In vitro effect of insulin on intracellular calcium concentrations: relation to insulin resistance. Metabolism 1996; 45: 1402–1407. [DOI] [PubMed] [Google Scholar]
- 48. Ishibashi KI, Kageyama S, Sakurai T, et al. Inhibitory effects of insulin on intracellular calcium and aggregatory response of platelets are impaired in hypertensive subjects with insulin resistance. Hypertens Res 1997; 20: 225–231. [DOI] [PubMed] [Google Scholar]
- 49. Eckel RH, Wassef M, Chait A, et al. Prevention conference VI: diabetes and cardiovascular disease: writing group II: pathogenesis of atherosclerosis in diabetes. Circulation 2002; 105: e138–e143. [DOI] [PubMed] [Google Scholar]
- 50. Paneni F, Beckman JA, Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 2013; 34: 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh RB, Mengi SA, Xu YJ, et al. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol 2002; 7: 40–53. [PMC free article] [PubMed] [Google Scholar]
- 52. Hasegawa Y, Saito T, Ogihara T, et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circ 2012; 125: 1122–1133. [DOI] [PubMed] [Google Scholar]
- 53. Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest 1991; 87: 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis update and therapeutic implications. Circulation 2007; 116: 1832–1844. [DOI] [PubMed] [Google Scholar]
- 55. Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 2012; 1: 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Margetic S. Inflammation and hemostasis. Biochemia Medica 2012; 22: 49–62. [PMC free article] [PubMed] [Google Scholar]
- 57. Carter AM. Inflammation, thrombosis and acute coronary syndromes. Diab Vasc Dis Res 2005; 2: 113–121. [DOI] [PubMed] [Google Scholar]
- 58. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009; 78: 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zumbach M, Hofmann M, Borcea V, et al. Tissue factor antigen is elevated in patients with microvascular complications of diabetes mellitus. Exp Clin Endocrinol Diabetes 1997; 105: 206–212. [DOI] [PubMed] [Google Scholar]
- 60. Heywood DM, Mansfield MW, Grant PJ. Factor VII gene polymorphisms, factor VII: C levels and features of insulin resistance in non-insulin-dependent diabetes mellitus. Thromb Haemost 1996; 75: 401–406. [PubMed] [Google Scholar]
- 61. Frankel DS, Meigs JB, Massaro JM, et al. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: the Framingham Offspring Study. Circulation 2008; 118: 2533–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Babic N, Dervisevic A, Huskic J, et al. Coagulation factor VIII activity in diabetic patients. Med Glas 2011; 8: 134–139. [PubMed] [Google Scholar]
- 63. Soares AL, Sousa MD, Fernandes AP, et al. Hemostatic changes in patients with type 2 diabetes mellitus. Revista Brasileira de Hematologia e Hemoterapia 2010; 32: 482–488. [Google Scholar]
- 64. Kloczko J, Wojtukiewicz M, Bielawiec M, et al. Plasma factor XIII and some other haemostasis parameters in patients with diabetic angiopathy. Acta Haematol 1986; 76: 81–85. [DOI] [PubMed] [Google Scholar]
- 65. Bembde AS. A study of plasma fibrinogen level in type-2 diabetes mellitus and its relation to glycemic control. Indian J Hematol Blood Transfus 2012; 28: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yarmolinsky J, Bordin Barbieri N, Weinmann T, et al. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep 2016; 6: 17714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dunn EJ, Ariens RA, Grant PJ. The influence of type 2 diabetes on fibrin structure and function. Diabetologia 2005; 48: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 68. Dunn EJ, Philippou H, Ariens RA, et al. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia 2006; 49: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 69. Howes JM, Richardson VR, Smith KA, et al. Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diab Vasc Dis Res 2012; 9: 216–225. [DOI] [PubMed] [Google Scholar]
- 70. Hess K, Alzahrani SH, Mathai M, et al. A novel mechanism for hypofibrinolysis in diabetes: the role of complement C3. Diabetologia 2012; 55: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 71. Sena CM, Pereira AM, Seica R. Endothelial dysfunction: a major mediator of diabetic vascular disease. Biochim Biophys Acta 2013; 1832: 2216–2231. [DOI] [PubMed] [Google Scholar]
- 72. Laine H, Yki-Jarvinen H, Kirvela O, et al. Insulin resistance of glucose uptake in skeletal muscle cannot be ameliorated by enhancing endothelium-dependent blood flow in obesity. J Clin Invest 1998; 101: 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Steinberg H, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996; 97: 2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Perticone F, Ceravolo R, Candigliota M, et al. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress. Diabetes 2001; 50: 159–165. [DOI] [PubMed] [Google Scholar]
- 75. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev 2005; 26: 19–39. [PMC free article] [PubMed] [Google Scholar]
- 76. Engin AB, Engin A. Endothelium: molecular aspects of metabolic disorders. Cleveland, OH: CRC Press, 2013. [Google Scholar]
- 77. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 2014; 6: a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 2013; 14: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am 2008; 37: 685–711, ix–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol 2004; 44: 2293–2300. [DOI] [PubMed] [Google Scholar]
- 81. Yamagishi SI, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev 2010; 3: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Singh VP, Bali A, Singh N, et al. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 2014; 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schneider DJ, Sobel BE. Diabetes and thrombosis. In: Johnstone MT, Veves A. (eds) Diabetes and cardiovascular disease. Totowa, NJ: Humana Press, 2001, pp. 107–128. [Google Scholar]
- 84. Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med 2007; 262: 157–172. [DOI] [PubMed] [Google Scholar]
- 85. Watson KE, Peters Harmel AL, Matson G. Atherosclerosis in type 2 diabetes mellitus: the role of insulin resistance. J Cardiovasc Pharmacol Ther 2003; 8: 253–260. [DOI] [PubMed] [Google Scholar]
- 86. Lowe GD, Drummond MM, Third JL, et al. Increased plasma fibrinogen and platelet-aggregates in type II hyperlipoproteinaemia. Thromb Haemost 1980; 42: 1503–1507. [PubMed] [Google Scholar]
- 87. Zahavi J, Betteridge J, Jones N, et al. Enhanced in vivo platelet release reaction and malondialdehyde formation in patients with hyperlipidaemia. Am J Med 1981; 70: 59–64. [DOI] [PubMed] [Google Scholar]
- 88. Avogaro A, Albiero M, Menegazzo L, et al. Endothelial dysfunction in diabetes the role of reparatory mechanisms. Diabetes Care 2011; 34: S285–S290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Randriamboavonjy V, Fleming I. Insulin, insulin resistance, and platelet signaling in diabetes. Diabetes Care 2009; 32: 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Westerbacka J, Yki-Järvinen H, Turpeinen A, et al. Inhibition of platelet-collagen interaction. An in vivo action of insulin is abolished by insulin resistance in obesity. Arterioscler Thromb Vasc Biol 2002; 22: 167–172. [DOI] [PubMed] [Google Scholar]
- 91. Trovati M, Anfossi G. Influence of insulin and insulin resistance on platelet and vascular smooth muscle cell function. J Diabetes Complications 2002; 16: 35–40. [DOI] [PubMed] [Google Scholar]
- 92. Betteridge D, El Tahir K, Reckless J, et al. Platelets from diabetic subjects show diminished sensitivity to prostacycline. Eur J Clin Invest 1982; 12: 395–398. [DOI] [PubMed] [Google Scholar]
- 93. Akai T, Naka K, Okuda K, et al. Decreased sensitivity of platelets to prostacyclin in patients with diabetes mellitus. Horm Metab Res 1983; 15: 523–526. [DOI] [PubMed] [Google Scholar]
- 94. Carrizzo A, Izzo C, Oliveti M, et al. The main determinants of diabetes mellitus vascular complications: endothelial dysfunction and platelet hyperaggregation. Int J Mol Sci 2018; 19: 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cola C, Brugaletta S, Martin Yuste V, et al. Diabetes mellitus: a prothrombotic state implications for outcomes after coronary revascularization. Vasc Health Risk Manag 2009; 5: 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Angiolillo DJ, Suryadevara S, Capranzano P, et al. Antiplatelet drug response variability and the role of platelet function testing: a practical guide for interventional cardiologists. Catheter Cardiovasc Interv 2009; 73: 1–14. [DOI] [PubMed] [Google Scholar]
- 97. Carter AM, Cymbalista CM, Spector TD, et al. Heritability of clot formation, morphology, and lysis: the EuroCLOT study. Arterioscler Thromb Vasc Biol 2007; 27: 2783–2789. [DOI] [PubMed] [Google Scholar]
- 98. Sumaya W, Wallentin L, James SK, et al. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: a PLATO substudy. Eur Heart J 2018; 39: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res 2010; 106: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pinotti M, Bertolucci C, Portaluppi F, et al. Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arterioscler Thromb Vasc Biol 2005; 25: 646–649. [DOI] [PubMed] [Google Scholar]
- 101. Kanabrocki EL, George M, Hermida RC, et al. Day-night variations in blood levels of nitric oxide, T-TFPI, and E-selectin. Clin Appl Thromb Hemost 2001; 7: 339–345. [DOI] [PubMed] [Google Scholar]
- 102. Soulban G, Labrecque G. Circadian rhythms of blood clotting time and coagulation factors II, VII, IX and X in rats. Life Sci 1989; 45: 2485–2489. [DOI] [PubMed] [Google Scholar]
- 103. Bremner WF, Sothern RB, Kanabrocki EL, et al. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J 2000; 139: 164–173. [DOI] [PubMed] [Google Scholar]
- 104. Gerhart-Hines Z, Lazar MA. Circadian metabolism in the light of evolution. Endocr Rev 2015; 36: 289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kramer A, Merrow M. (eds). Circadian clocks. Berlin; Heidelberg: Springer, 2013. [Google Scholar]
- 106. Olds W. Sleep, circadian rhythms, and metabolism: the rhythm of life. Toronto, ON, Canada: Apple Academic Press, 2014. [Google Scholar]
- 107. Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res 2012; 199: 337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. Plos Biol 2004; 2: e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Viswambharan H, Carvas JM, Antic V, et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007; 115: 2188–2195. [DOI] [PubMed] [Google Scholar]
- 110. Anea CB, Cheng B, Sharma S, et al. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res 2012; 111: 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes 2008; 32: 658–662. [DOI] [PubMed] [Google Scholar]
- 112. Yamasaki F, Schwartz JE, Gerber LM, et al. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension 1998; 32: 417–423. [DOI] [PubMed] [Google Scholar]
- 113. Scheer FA, Hilton MF, Mantzoros CS, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009; 106: 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep 1992; 15: 434–441. [DOI] [PubMed] [Google Scholar]
- 115. Roden M, Koller M, Pirich K, et al. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol 1993; 265: R261–R267. [DOI] [PubMed] [Google Scholar]
- 116. Buijs RM, Scheer FA, Kreier F, et al. Organization of circadian functions: interaction with the body. Prog Brain Res 2006; 153: 341–360. [DOI] [PubMed] [Google Scholar]
- 117. Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab 2007; 18: 4–11. [DOI] [PubMed] [Google Scholar]
- 118. Kawachi I, Colditz GA, Stampfer MJ, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation 1995; 92: 3178–3182. [DOI] [PubMed] [Google Scholar]
- 119. Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012; 345: e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kreutz RP, Owens J, Breall JA, et al. C-reactive protein and fibrin clot strength measured by thrombelastography after coronary stenting. Blood Coagul Fibrinolysis 2013; 24: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morris CJ, Purvis TE, Hu K, et al. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A 2016; 113: E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Orth-Gomer K. Intervention on coronary risk factors by adapting a shift work schedule to biologic rhythmicity. Psychosom Med 1983; 45: 407–415. [DOI] [PubMed] [Google Scholar]
- 123. Pan A, Schernhammer ES, Sun Q, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 2011; 8: e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pietroiusti A, Neri A, Somma G, et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med 2010; 67: 54–57. [DOI] [PubMed] [Google Scholar]
- 125. De Bacquer D, Van Risseghem M, Clays E, et al. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol 2009; 38: 848–854. [DOI] [PubMed] [Google Scholar]
- 126. Eriksson AK, Ekbom A, Granath F, et al. Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med 2008; 25: 834–842. [DOI] [PubMed] [Google Scholar]
- 127. Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004; 27: 282–283. [DOI] [PubMed] [Google Scholar]
- 128. Iyer SR. Sleep and type 2 diabetes mellitus: clinical implications. J Assoc Physicians India 2012; 60: 42–47. [PubMed] [Google Scholar]
- 129. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–850. [DOI] [PubMed] [Google Scholar]
- 130. Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev 2009; 10: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Aronson D. Impaired modulation of circadian rhythms in patients with diabetes mellitus: a risk factor for cardiac thrombotic events. Chronobiol Int 2001; 18: 109–121. [DOI] [PubMed] [Google Scholar]
- 132. Maemura K, de la Monte SM, Chin MT, et al. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem 2000; 275: 36847–36851. [DOI] [PubMed] [Google Scholar]
- 133. Oishi K, Ohkura N, Wakabayashi M, et al. CLOCK is involved in obesity-induced disordered fibrinolysis in ob/ob mice by regulating PAI-1 gene expression. J Thromb Haemost 2006; 4: 1774–1780. [DOI] [PubMed] [Google Scholar]
- 134. Wade AG, Ford I, Crawford G, et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med 2010; 8: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brzezinski A. Melatonin in humans. N Engl J Med 1997; 336: 186–195. [DOI] [PubMed] [Google Scholar]
- 136. Kostenko EV, Petrova LV, Eneeva MA, et al. Sleep impairments and circadian rhythms in diseases of the cardiovascular system. Zh Nevrol Psikhiatr Im S S Korsakova 2015; 115: 30–36. [DOI] [PubMed] [Google Scholar]
- 137. Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, et al. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res 2010; 49: 14–22. [DOI] [PubMed] [Google Scholar]
- 138. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012; 35: 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kwon I, Choe HK, Son GH, et al. Mammalian molecular clocks. Exp Neurobiol 2011; 20: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev 2013; 93: 107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ramkisoensing A, Meijer JH. Synchronization of biological clock neurons by light and peripheral feedback systems promotes circadian rhythms and health. Front Neurol 2015; 6: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Damiola F, Le Minh N, Preitner N, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000; 14: 2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 2003; 18: 250–260. [DOI] [PubMed] [Google Scholar]
- 144. West AC, Bechtold DA. The cost of circadian desynchrony: evidence, insights and open questions. Bioessays 2015; 37: 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P. The role of melatonin in acute myocardial infarction. Front Biosci 2012; 17: 2433–2441. [DOI] [PubMed] [Google Scholar]
- 146. Armstrong SM. Melatonin and circadian control in mammals. Experientia 1989; 45: 932–938. [DOI] [PubMed] [Google Scholar]
- 147. Cardinali DP, Pevet P. Basic aspects of melatonin action. Sleep Med Rev 1998; 2: 175–190. [DOI] [PubMed] [Google Scholar]
- 148. Peschke E, Muhlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab 2010; 24: 829–841. [DOI] [PubMed] [Google Scholar]
- 149. Srinivasan V, Singh J, Brzezinski A, et al. Jet lag: use of melatonin and melatonergic drugs. In: Srinivasan V, Brzezinski A, Oter S, et al. (eds) Melatonin and melatonergic drugs in clinical practice. New Delhi, India: Springer, 2014, pp. 367–378. [Google Scholar]
- 150. Williams WP, 3rd, McLin DE, 3rd, Dressman MA, et al. Comparative review of approved melatonin agonists for the treatment of circadian rhythm sleep-wake disorders. Pharmacotherapy 2016; 36: 1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci 2014; 15: 15924–15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sewerynek E. Melatonin and the cardiovascular system. Neuro Endocrinol Lett 2002; 23: 79–83. [PubMed] [Google Scholar]
- 153. Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Melatonin and cardiovascular disease: myth or reality? Rev Esp Cardiol 2012; 65: 215–218. [DOI] [PubMed] [Google Scholar]
- 154. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities. Acta Physiol 2012; 205: 209–223. [DOI] [PubMed] [Google Scholar]
- 155. Sahna E, Parlakpinar H, Turkoz Y, et al. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res 2005; 54: 491–495. [PubMed] [Google Scholar]
- 156. Bekyarova G, Tancheva S, Hristova M. The effects of melatonin on burn-induced inflammatory responses and coagulation disorders in rats. Methods Find Exp Clin Pharmacol 2010; 32: 299–303. [DOI] [PubMed] [Google Scholar]
- 157. Tunali T, Sener G, Yarat A, et al. Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci 2005; 76: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 158. Sharma S, Singh H, Ahmad N, et al. The role of melatonin in diabetes: therapeutic implications. Arch Endocrinol Metab 2015; 59: 391–399. [DOI] [PubMed] [Google Scholar]
- 159. Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Diabetologia 2006; 49: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 160. Vinogradova I, Anisimov V. Melatonin prevents the development of the metabolic syndrome in male rats exposed to different light/dark regimens. Biogerontology 2013; 14: 401–409. [DOI] [PubMed] [Google Scholar]
- 161. Garfinkel D, Zorin M, Wainstein J, et al. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes 2011; 4: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res 2008; 44: 26–40. [DOI] [PubMed] [Google Scholar]
- 163. Obayashi K, Saeki K, Tone N, et al. Relationship between melatonin secretion and nighttime blood pressure in elderly individuals with and without antihypertensive treatment: a cross-sectional study of the HEIJO-KYO cohort. Hypertens Res 2014; 37: 908–913. [DOI] [PubMed] [Google Scholar]
- 164. Parada E, Buendia I, Leon R, et al. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J Pineal Res 2014; 56: 204–212. [DOI] [PubMed] [Google Scholar]
- 165. Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res 2003; 58: 10–19. [DOI] [PubMed] [Google Scholar]
- 166. Tan DX, Manchester LC, Reiter RJ, et al. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. J Pineal Res 1998; 25: 184–191. [DOI] [PubMed] [Google Scholar]
- 167. Reiter RJ, Tan DX, Sainz RM, et al. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol 2002; 54: 1299–1321. [DOI] [PubMed] [Google Scholar]
- 168. Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia MJ, et al. Decreased nocturnal melatonin levels during acute myocardial infarction. J Pineal Res 2002; 33: 248–252. [DOI] [PubMed] [Google Scholar]
- 169. Murata K, Yano E, Shinozaki T. Cardiovascular dysfunction due to shift work. J Occup Environ Med 1999; 41: 748–753. [DOI] [PubMed] [Google Scholar]
- 170. Abuja PM, Liebmann P, Hayn M, et al. Antioxidant role of melatonin in lipid peroxidation of human LDL. FEBS Lett 1997; 413: 289–293. [DOI] [PubMed] [Google Scholar]
- 171. Sakotnik A, Liebmann PM, Stoschitzky K, et al. Decreased melatonin synthesis in patients with coronary artery disease. Eur Heart J 1999; 20: 1314–1317. [DOI] [PubMed] [Google Scholar]
- 172. Wirtz PH, Ehlert U, Emini L, et al. Anticipatory cognitive stress appraisal and the acute procoagulant stress response in men. Psychosom Med 2006; 68: 851–858. [DOI] [PubMed] [Google Scholar]
- 173. Wirtz PH, Spillmann M, Bartschi C, et al. Oral melatonin reduces blood coagulation activity: a placebo-controlled study in healthy young men. J Pineal Res 2008; 44: 127–133. [DOI] [PubMed] [Google Scholar]
- 174. Muller JE. Circadian variation and triggering of acute coronary events. Am Heart J 1999; 137: S1–S8. [DOI] [PubMed] [Google Scholar]
- 175. Von Kanel R, Nelesen RA, Ziegler MG, et al. Relation of autonomic activity to plasminogen activator inhibitor-1 plasma concentration and the role of body mass index. Blood Coagul Fibrinolysis 2007; 18: 353–359. [DOI] [PubMed] [Google Scholar]
- 176. Obayashi K, Saeki K, Kurumatani N. Association between urinary 6-sulfatoxymelatonin excretion and arterial stiffness in the general elderly population: the HEIJO-KYO cohort. J Clin Endocrinol Metab 2014; 99: 3233–3239. [DOI] [PubMed] [Google Scholar]
- 177. Tengattini S, Reiter RJ, Tan DX, et al. Cardiovascular diseases: protective effects of melatonin. J Pineal Res 2008; 44: 16–25. [DOI] [PubMed] [Google Scholar]
- 178. Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog Cardiovasc Dis 2007; 49: 353–365. [DOI] [PubMed] [Google Scholar]
- 179. Servoss SJ, Januzzi JL, Muller JE. Triggers of acute coronary syndromes. Prog Cardiovasc Dis 2002; 44: 369–380. [DOI] [PubMed] [Google Scholar]
- 180. Krantz DS, Kop WJ, Santiago HT, et al. Mental stress as a trigger of myocardial ischemia and infarction. Cardiol Clin 1996; 14: 271–287. [PubMed] [Google Scholar]
- 181. Von Kanel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol 2000; 65: 357–369. [DOI] [PubMed] [Google Scholar]
- 182. Von Kanel R, Preckel D, Zgraggen L, et al. The effect of natural habituation on coagulation responses to acute mental stress and recovery in men. Thromb Haemost 2004; 92: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 183. Wirtz PH, Bartschi C, Spillmann M, et al. Effect of oral melatonin on the procoagulant response to acute psychosocial stress in healthy men: a randomized placebo-controlled study. J Pineal Res 2008; 44: 358–365. [DOI] [PubMed] [Google Scholar]
- 184. Del Zar MM, Martinuzzo M, Falcon C, et al. Inhibition of human platelet aggregation and thromboxane-B2 production by melatonin: evidence for a diurnal variation. J Clin Endocrinol Metab 1990; 70: 246–251. [DOI] [PubMed] [Google Scholar]
- 185. Scheer FA, Evoniuk H, Kelly EE, et al. Effect of circadian system and behavioral stressors on platelet activity and reactivity: implications for the morning peak in cardiovascular incidents. Sleep 2009; 32: A7. [Google Scholar]
- 186. Brezinski DA, Tofler GH, Muller JE, et al. Morning increase in platelet aggregability. Association with assumption of the upright posture. Circulation 1988; 78: 35–40. [DOI] [PubMed] [Google Scholar]
- 187. Kumari S, Dash D. Melatonin elevates intracellular free calcium in human platelets by inositol 1; 4, 5, trisphosphate independent mechanism. FEBS Lett 2011; 585: 2345–2351. [DOI] [PubMed] [Google Scholar]
- 188. Arushanian EB. Effect of melatonin on the thrombocyte hemostasis and its circadian organization. Eksp Klin Farmakol 2013; 76: 32–36 (in Russian). [PubMed] [Google Scholar]
- 189. Kornblihtt LI, Finocchiaro L, Molinas FC. Inhibitory effect of melatonin on platelet activation induced by collagen and arachidonic acid. J Pineal Res 1993; 14: 184–191. [DOI] [PubMed] [Google Scholar]
- 190. Girish KS, Paul M, Thushara RM, et al. Melatonin elevates apoptosis in human platelets via ROS mediated mitochondrial damage. Biochem Biophys Res Commun 2013; 438: 198–204. [DOI] [PubMed] [Google Scholar]
- 191. Sener A, Ozsavci D, Bingol-Ozakpinar O, et al. Oxidized-LDL and Fe3+/ascorbic acid-induced oxidative modifications and phosphatidylserine exposure in human platelets are reduced by melatonin. Folia Biol 2009; 55: 45–52. [PubMed] [Google Scholar]
- 192. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010; 107: 1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Matough FA, Budin SB, Hamid ZA, et al. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J 2012; 12: 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 2004; 28: 565–582. [DOI] [PubMed] [Google Scholar]
- 195. Dubocovich ML, Markowska M. Functional MT 1 and MT 2 melatonin receptors in mammals. Endocrine 2005; 27: 101–110. [DOI] [PubMed] [Google Scholar]
- 196. Sharman EH, Bondy SC. Melatonin: a safe nutraceutical and clinical agent. In: Gupta RC. (ed.) Innutraceuticals: efficacy, safety and toxicity. Cambridge, MA: Academic Press, 2016, pp. 501–509. [Google Scholar]