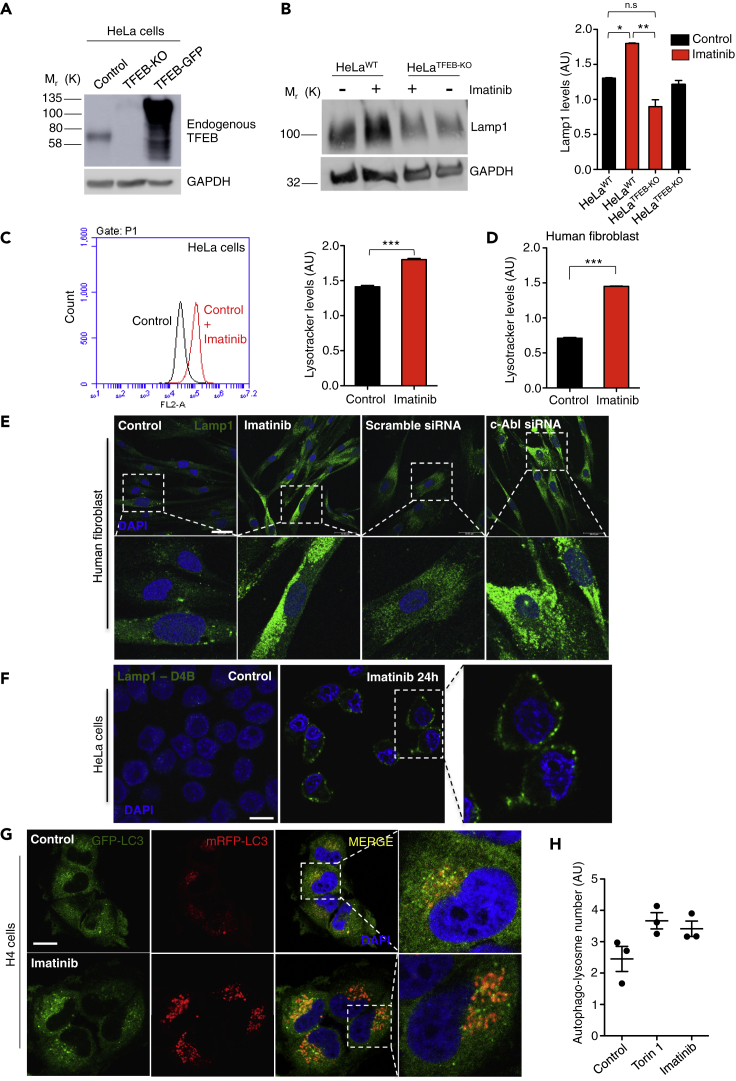
Figure 2.
c-Abl Inhibition Increases Lysotracker Positive Organelles and Lysosomal Protein Levels
(A) Representative Western blot of endogenous TFEB in HeLa cells (control), HeLa TFEB-KO cells (TFEB-KO), and HeLa TFEB-GFP cells (TFEB-GFP). GAPDH was used as loading control.
(B) Representative Western blot and quantification of HeLa cells treated with imatinib 10μM for 24 hr using a Lamp1 antibody. n = 3 independent experiments.
(C) Quantitative flow cytometry analysis of lysotracker in HeLa cells treated with imatinib 10μM for 24 hr n = 10,000 cells per conditions.
(D) Quantitative flow cytometry analysis of lysotracker in the human wild type fibroblasts treated with imatinib 10μM for 24 hr n = 10,000 cells per conditions.
(E) Representative immunofluorescence images of lysosomes using Lamp1 antibody in human fibroblast treated with imatinib 10μM for 24 hr, or transfected with a scramble siRNA or a siRNA against c-Abl for 48 hr n = 3 independent experiments. Scale bars, 50 μM.
(F) Representative immunofluorescence images of lysosomes attached to the plasma membrane using the antibody Lamp1-DB4 in HeLa cells treated with imatinib 10μM for 24 hr n = 3 independent experiments. Scale bars, 10 μM.
(G) H4 cells were treated with DMSO (control), Torin1 0.3 μM (positive control) and c-Abl inhibitor imatinib 10 μM for 3 h. The cells were then fixed and stained with DAPI. Representative confocal microscopy images. Scale bars, 10 μM.
(H) Graph of the autophago-lysosome number (spots negative for GFP and positive for RFP). For each condition 450–800 cells were analyzed (19 images per sample). n = 3 biological independent samples. Statistical analysis with one-way ANOVA followed by Tukey's post test and Student's t-tests when comparing two experimental groups. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001. Data represent mean ± SEM.