Summary
Homo sapiens is the only species alive able to take advantage of its cognitive abilities to inhabit almost all environments on Earth. Humans are able to culturally construct, rather than biologically inherit, their occupied climatic niche to a degree unparalleled within the animal kingdom. Precisely, when hominins acquired such an ability remains unknown, and scholars disagree on the extent to which our ancestors shared this same ability. Here, we settle this issue using fine-grained paleoclimatic data, extensive archaeological data, and phylogenetic comparative methods. Our results indicate that whereas early hominins were forced to live under physiologically suitable climatic conditions, with the emergence of H. heidelbergensis, the Homo climatic niche expanded beyond its natural limits, despite progressive harshening in global climates. This indicates that technological innovations providing effective exploitation of cold and seasonal habitats predated the emergence of Homo sapiens.
Subject Areas: Ecology, Evolutionary History, Paleobiology
Graphical Abstract
Highlights
-
•
Homo sapiens oversteps our ecological niche limits by means of culture
-
•
The origin of Homo niche-construction ability is unknown
-
•
We found Homo species other than H. sapiens were able to construct their own niche
Ecology; Evolutionary History; Paleobiology
Introduction
The genus Homo has existed for some three million years (Harmand et al., 2015; Villmoare et al., 2015). For one third of this stretch of time, human species were confined to tropical and sub-tropical Africa, which is the homeland of the genus (Carotenuto et al., 2016; Lordkipanidze et al., 2007) and is rich in the warm, savanna-like environments to which most early hominins were best adapted (Lee-Thorp et al., 2010; White et al., 2009). With the emergence of Homo erectus some 2 Ma ago, Homo began to disperse outside of Africa but remained confined to low latitudes, possibly because of physiological limits to cold tolerance (Dunbar et al., 2014) combined with the inevitable constraints of biogeographical barriers and habitat variability. However, later Homo species were able to expand their distribution to Northern Europe and Western Siberia, even as the contemporaneous establishment of full glacial cycles was making global temperatures colder than ever before during the history of the genus. Findings in Happisburgh and Pakefield (UK) date the earliest occurrence of Homo at the southern edge of the boreal zone at some 0.7–0.9 Ma (Parfitt et al., 2010). The occupation of such northern temperate and boreal zones presents a number of notable challenges. Not only was the cold itself challenging for hominins physiologically adapted to African climates but also seasonality imposes extreme annual resource fluctuations, which imply a reliance on hunted meat for survival (Pearce et al., 2014). Adaptations facilitating survival in cold environments may have included the use of fire, shelters or clothing, weapons useful to bring down large game species (Thieme, 1997), as well as extended social networks, with vulnerable infants being particularly susceptible to mortality (Spikins et al., 2019; Martin et al., 2020).
Unfortunately, clothing manufacturing leaves very little in the way of fossil remains (Hosfield, 2016). The first microwear evidence of hide scraping (for manufacturing clothes) at Hoxne (UK), Biâche-Saint-Vaast, Pech de l’Azé and Abri Peyrony (France), and Shöningen (Germany) (d'Errico and Henshilwood, 2007; Gilligan, 2010; Henshilwood et al., 2002) is just some 50 ka old at the most (Kittler et al., 2003; Gilligan, 2007). Only the two most recent human species, H. neanderthalensis and H. sapiens, left incontrovertible evidence that they were able to produce complex, cold-proof clothing at that time. To make things more complex, in the particular case of H. neanderthalensis, biological adaptation, besides material culture, was possibly involved in their ability to withstand the cold. H. neanderthalensis possessed relatively short limbs, and a large midface and nasal cavity proposed to be specific cold adaptations, to heat and humidify inspired air, although the issue is far from resolved and there is evidence for the contrary (Rae et al., 2011; Benito et al., 2017; Wroe et al., 2018). In contrast to any other Homo, H. sapiens is considered the only species in the genus able to occupy cold regions through a genuinely cultural process, driven by our technology, including the mastering of fire, ever improving clothing craftsmanship, and construction of shelters (Boivin et al., 2016; Gilligan, 2010; Hiscock, 2013; Laland et al., 2001). The archaeological record of Homo sapiens shows our own species was able to construct its own niche, using technologies transmitted over large regions and across generations via cultural interactions. Homo sapiens could thus exploit climatic variability over time and space, rather than being physiologically limited by it (Banks et al., 2006, 2008, 2011, 2013; Dunbar et al., 2014; Spikins et al., 2019; Nicholson, 2019; Xu et al., 2020).
This view sets H. sapiens apart from any other human species in terms of cognitive skills and implicitly rejects the idea that older Homo may have had sufficiently modern material culture to overcome climatic harshness (Roberts and Stewart, 2018). With such a poor fossil record of clothes and tools to produce them and because of great uncertainty about deep past local paleoclimates and human dispersal timing and direction, the issue of when humans first became cognitively and culturally able to extend their climatic tolerance beyond their physiological limits remains very difficult to decipher.
Here, we address the more restricted issue of when during the history of Homo the limits of climatic tolerance expanded and which species were involved. We do not specifically address the cultural and social adaptations that might underlie such tolerance but rather consider the implications of our findings for the timing of such adaptations. We model the evolution of climatic tolerance (i.e. niche) limits in the Homo genus by associating paleoclimatic values with fossil occurrences in the archaeological record. Specifically, we test the hypothesis that H. sapiens developed greater climatic tolerance relative to H. heidelbergensis and H. neanderthalensis against the alternative that the exploration of climates outside natural physiological limits had already begun with the earliest of these species.
To test this hypothesis, we estimated the rate of change of climatic tolerance limits across the human phylogenetic tree and searched for possible shifts in the rate. We apply a method which allows us to compute the rate of evolution of climatic niche limits at each branch in the tree. In the present context, shifts in the rate of evolution of climatic tolerance that accrue to the clade including the Happisburgh/Pakefield hominins, H. heidelbergensis, plus H. neanderthalensis, and H. sapiens (modern Homo species, MHS, hereafter) would indicate these hominins were the first to acquire the capacity to develop cold climate-related technological skills and cultural adaptations. Conversely, if either no rate shift occurs or the rate shift coincides with different clades (e.g. early Homo species, EHS, hereafter), the colonization of Northern habitats would not be indicative of any sudden increase in the ability to face environmental harshness.
The human fossil data set we used includes 2,597 occurrences of hominid remains and artifacts associated with 727 archaeological sites. The time range of our record spans from the first occurrence of Australopiths in East Africa dated to some 4.2 Ma to the definitive advent of H. sapiens in Eurasia almost coincident with the demise of H. neanderthalensis dated at 0.040 Ma (see Data S1, Raia et al., 2020). Such a wide range of hominin taxa provides a thorough phylogenetic context for the analyses.
Deriving spatiotemporally detailed climate data for the past requires dynamic climate modeling, but the timescales for human evolution exceed the possibilities of direct model simulation by several orders of magnitude. To circumvent this limitation, we combine direct simulation using a computationally efficient, intermediate complexity Earth system model, the Planet Simulator–Grid-Enabled Integrated Earth system model (PLASIM-GENIE), with statistical modeling, to create PALEO-PGEM, a paleoclimate emulator, capable of performing multi-million year simulations forced by observationally derived proxy time series for ice sheet state, CO2 concentration, and orbital forcing (Holden et al., 2016, 2019). To model the realized climatic niche evolution, we applied phylogenetic ridge regression (“RRphylo”, Castiglione et al., 2018). “RRphylo” allows us to compute evolutionary rates for each branch of the phylogeny and to estimate the ancestral phenotypes (Raia et al., 2018; Melchionna et al., 2020b; Baab, 2018). Here, the “phenotype” comprises climatic tolerance limits.
By using past annual maxima and minima for temperature, precipitation, and net primary productivity from PALEO-PGEM, we reconstructed and projected onto the geographical space the climatic niche limits corresponding to the ancestral species distributions (the nodes in the tree) in our fossil database. Using “RRphylo”, we were then able to infer climatic niche tolerance limits (Quintero and Wiens, 2013) for each node in the tree and to assess whether the rate of climatic niche evolution shows any shift (i.e. acceleration or deceleration) consistent with our starting hypothesis, while accounting for the effect of shared inheritance. We accounted for phylogenetic uncertainty by perturbing the tree node ages and the tree topology randomly one hundred times. By incorporating phylogenetic uncertainty in this way, we were able to define an overall “habitat quality” (HQ) metric, representing the number of times (out of 100 repetitions) a geographic cell was found habitable (i.e. fell within climatic tolerance limits) for a given ancestor in the tree.
Results
The Association between the Distribution of Fossil Species and Habitat Quality
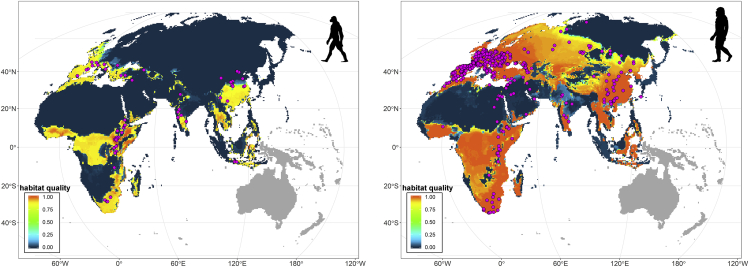
We used the area under the curve (AUC) metric to measure the association between HQ and the location of fossil occurrences. At AUC = 1, the association would be perfect. AUC = 0 would indicate perfect inverse relation, whereas AUC ∼ 0.5 indicates random association. We found that despite the enormous geographic variation in both the preservation potential and the intensity of paleontological sampling (Carotenuto et al., 2010), there is a strong association between the geographic position of archaeological remains and the inferred suitability of the environmental conditions, for both EHS (AUC = 0.80, Figure 1 left, AUC after subsampling the most abundant species = 0.71) and MHS (AUC = 0.81, Figure 1 right, AUC after subsampling the most abundant species = 0.82). This strong association remains valid for all nodes in the hominin tree (Figures S1 and S2, Tables 1 and S2) and suggests that climatic variation in time and space strongly controlled the geographic ranges of our ancestors. Excluding extreme climatic values (i.e. climatic records beyond the 90th percentile of the individual variable distributions) in order to mitigate the effect of potential errors in the paleoclimate emulator, the AUC value for EHS decreased to 0.68, whereas it increased to as much as 0.82 for MHS (Table S3, Figure S3). We repeated this test by randomly placing species fossil occurrences throughout their biogeographical domain (Table S4, Figure S4) to simulate a scenario of no association between the archaeological record and HQ. Under this simulation, the AUC values drop toward 0.5, which indicate non-significant association between the two variables (EHS AUC = 0.56; 95%, confidence interval: 0.52–0.61; MHS AUC = 0.58, confidence interval: 0.56–0.60). This finding reinforces the notion that the geographic position of archaeological sites is a non-random process guided by climatic variability.
Figure 1.
Habitat Quality Map for Early Homo Species (EHS, Left) and Modern Homo Species (MHS, Right)
The maps show the quality of the habitats potentially suitable for occupation by the common ancestors of EHS and MHS, respectively. Quality varies from little (blue) to highly suitable (red) areas. The fossil occurrences of EHS (H. habilis, H. ergaster, and H. erectus) and MHS (H. heidelbergensis, H. neanderthalensis, and H. sapiens) are superimposed on each map (pink dots). See also Figure S1 and Table S1.
Table 1.
Percentage of Significant Rate Shifts in Niche Width Calculated through Phylogenetic Reshuffling
| Species | Shift | Node with Two Species | Node with Three Species | H. heidelbergensis | H. neanderthalensis | H. sapiens |
|---|---|---|---|---|---|---|
| H. heidelbergensis | 86 | 23 | 63 | / | 75 | 74 |
| H. neanderthalensis | 85 | 22 | 63 | 74 | / | 74 |
| H. sapiens | 86 | 23 | 63 | 75 | 74 | / |
The table lists the percentage of significant shifts that occurred at nodes with two or three species, as well as the occurrence of each of the three Homo species in each significant shift.
Rates of Hominin Climatic Niche Limit Evolution
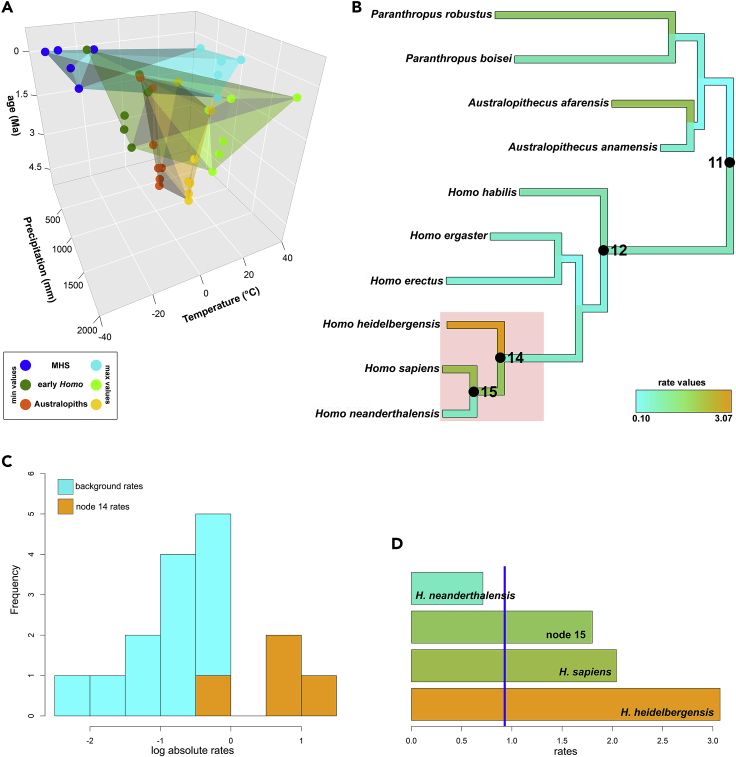
We found that the clade identified by H. heidelbergensis, H. neanderthalensis, and H. sapiens and their common ancestor experienced a significant evolutionary rate shift toward wider climatic tolerance (Figure 2). The rate shift does not depend on the specific phylogenetic hypothesis (tree topology) assumed, neither does it depend on the selection of species we used. Randomly changing the tree node ages (to account for dating uncertainty) and species positions in the hominin tree (to account for phylogenetic uncertainty) 100 times, the shift appears for this clade 95 times (Table 1). Subsampling the most abundant species (randomly selecting no more than 100 fossil occurrences per species) to account for sampling differences between species, the shift appears 91 times out of a hundred. We also repeated the phylogenetic reshuffling randomly removing one species at once. Under this latter design, the MHS shift occurs 63 times out of 100, and 23 additional times the shift involves two, rather than three, MHS species. Individually, H. sapiens and H. heidelbergensis appear in 86 rate shifts, H. neanderthalensis in 85, and no shift appears outside the MHS clade, demonstrating that the rate shift pertains to these species only and is not guided preferentially by any of the three (Table 1).
Figure 2.
Climatic Niche Evolution in Hominins
(A) Three-dimensional plot of the climatic niche space occupied by the hominin clades through time.
(B) The hominin tree used in this study. The branch colors are proportional to the multivariate rate of climatic niche evolution for each branch in the tree. At the MHS common ancestor (14), an acceleration in the rate of evolution in climatic tolerance limits occurs (shaded area). The common ancestor to all species within Homo is indicated by node 12.
(C) The distribution of the rates of niche evolution for the MHS clade (orange) compared to the rest of the branches in the tree (light blue).
(D) The individual rates of niche evolution for the tree branches forming the MHS clade. The average rate for the entire tree is indicated by the vertical blue line. MHS = modern Homo species, EHS = Homo species exclusive of MHS, Australopiths = species in the genus Paranthropus and Australopithecus.
Discussion
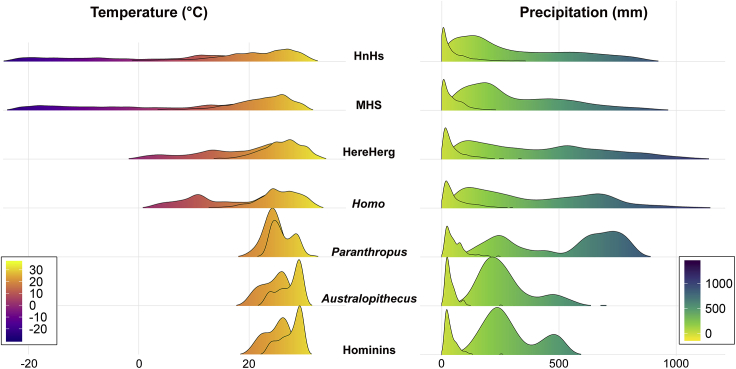
The estimated values of realized climatic niche limits at nodes in the hominin phylogeny suggest that the rate shift in the climatic niche limits for the MHS clade was not an exclusively biological process. At the root of the hominin tree (node 11, Table S1), the predicted range in annual temperatures spans from 20°C (coldest quarter of the year) to 29.9°C (warmest quarter) and in mean rainfall from 12 mm (driest quarter) to 512 mm (wettest quarter). This is entirely consistent with today's African savannah environment (Hijmans et al., 2005). At the node subtending the pair H. ergaster plus H. erectus (which is the first hominin to disperse over Southern Eurasia), the corresponding figures are 0.7°C–31.9°C for temperature range and from 4.8 mm to 1080 mm for precipitation range. These estimates are reasonable considering both the range expansion into temperate regions and the colonization of warm and humid environments (Indonesia) by H. erectus (Carotenuto et al., 2016; Joordens et al., 2015; Rizal et al., 2019). Yet, at the common ancestor to the three MHS, the estimates for annual temperature extremes span from minus 21.1°C to plus 31.4°C and for annual precipitation from 0.7 mm to 905 mm. Although the common ancestor to MHS was an African species which probably never experienced these extreme climates (Profico et al., 2016), the values agree qualitatively with the notion that a sudden widening of climatic niche limits occured with the advent of this ancestor, whose offspring lived after the onset of fully glacial Pleistocene conditions (Churchill, 1998). The massive increase in the estimated range of thermal conditions suitable for the MHS clade taxa (marked by a 20°C decrease in minimum temperature of the coldest season of the year as compared to the hominin tree root, Figures 3 and S5) does not depend on the phylogenetic hypothesis we applied and surpasses what is expected by a random process of increased phenotypic variance over time (namely the Brownian motion model of evolution, see Supplemental Information for full explanation). Using 100 different tree topologies and branch lengths to account for phylogenetic uncertainty, we found a significant trend in the temperature of the coldest season realized by hominins 97 times (Figure 3), whereas no trend was found in the maximum temperatures of the warmest season. We found that in African species and ancestors, the average temperature of the coldest quarter of the year was no less than 9.4°C, meaning that the winter chill is unlikely to have been a problem for them (Table S5). In contrast, within the range of temperatures experienced by H. heidelbergensis, the coldest quarter of the year was as cold as −12.3°C, suggesting specific technological and cultural adaptations were needed to fend off the risk of hypothermia and to live in the highly seasonal, cold northern environments (Ulijaszek and Strickland, 1993; Ellison et al., 2005; Gilligan, 2007; Rivals et al., 2009; El Zaatari et al., 2016). These adaptations may have included fitted clothing (Amanzougaghene et al., 2019), thrown spears (Lenoir and Villa, 2006) or adhesives (Cârciumaru et al., 2012), and enhanced healthcare practices (Spikins et al., 2019).
Figure 3.
Estimated Temperature and Precipitation Ranges at Several Nodes in the Human Phylogenetic Tree
The individual rows represent the density distribution of minimum and maximum temperature and precipitation, respectively, collapsed together. HnHs = common ancestor to H. neanderthalensis and H. sapiens, MHS = common ancestor to H. heidelbergensis, H. neanderthalensis, and H. sapiens HereHerg = common ancestor to H. erectus and H. ergaster, Homo = common ancestor to Homo species, Paranthropus = common ancestor to all Paranthropus species, Australopithecus = common ancestor to all Australopithecus species, Hominins = common ancestor to hominins.
For some, the process of cultural niche construction (Laland et al., 2001; Laland and O'Brien, 2012) through which human cultural traits have changed the human adaptive niche and in turn selective pressures and ecological inheritance (Odling-Smee and Laland, 2011) traces back to the very emergence of the genus Homo at some 2.5 million years ago (Antón and Snodgrass, 2012; Antón et al., 2014). At that time, increasing dependence on stone artifact production and social learning (Hiscock, 2014) and on collaboration (Fuentes et al., 2010; Fuentes, 2015) may have been particularly influential in allowing hominins to not only escape their biological constraints but also actively change the environmental and ecological niches of other species (Low et al., 2019). The occasional use of fire has similarly deep roots in human history (Gowlett, 2016; Organ et al., 2011; Pruetz and Herzog, 2017). Yet, the habitual use of fire (Shimelmitz et al., 2014) and the ability to work hide, wood and ivory (d'Errico and Henshilwood, 2007; Thieme, 1997) is attested at a much later date, during the Middle Stone Age (d'Errico, 2003) and attached to MHS only. Brain asymmetry and right handiness, usually linked with advanced cognitive skills (Crow, 1993; Xiang et al., 2019; Melchionna et al., 2020a), similarly characterize MHS (Frayer et al., 2012; Lozano et al., 2009; Poza-Rey et al., 2017). In contrast to MHS, EHS either did not venture outside Africa or went across Eurasia longitudinally. Homo erectus spread across Africa and Eurasia up to Java at some 1.7 Ma but never settled north of the Mediterranean area or southeast China (Carotenuto et al., 2016). From the appearance of H. heidelbergensis onward, northern, presumably colder habitats were no longer completely uninhabitable.
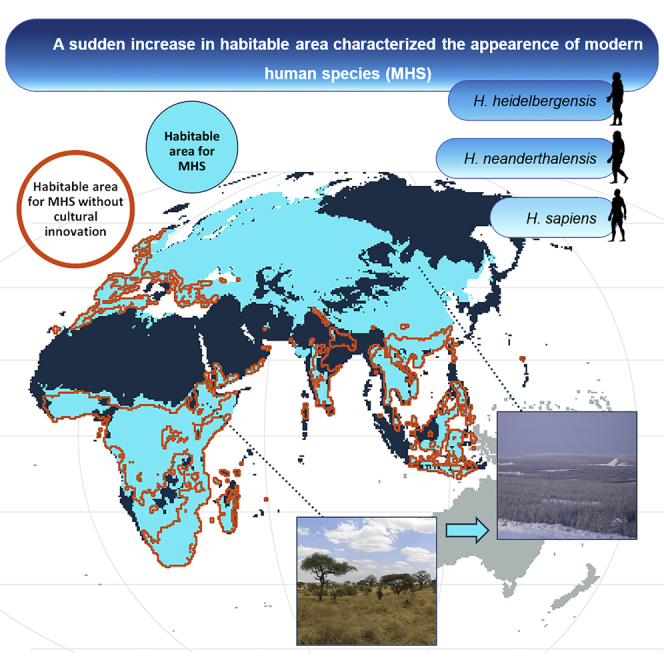
The jump in the rates of evolution in climatic niche width (driven by a sudden increase in tolerance to the cold, Figure 3) had enormous consequence in terms of geographic range. By modeling climatic niche limits according to a random walk with constant variance process (i.e. the Brownian motion model of evolution, BM) and assuming as habitable all geographic cells with HQ >0.25, the rate shift accounts for a twofold increase in viable geographic range at the ancestor of MHS (node 14 in the tree) for a net gain of some 30 × 106 km2 (roughly the land surface of the African continent). At node 15, the ancestor of H. sapiens and H. neanderthalensis, the habitable area becomes nearly three times larger than expected under BM, corresponding to a geographic extension of some 50 × 106 km2. This massive increase in habitable area mostly represents expansion into northern latitudes, testifying to the importance of the rate shift in the colonization of Eurasia (Figure S5).
Although there is consistent evidence that Homo species may have exchanged genes with positive fitness consequences in cold environments by means of genetic introgression, this evidence is limited to the last 40 kya and invariably pertains to local Homo sapiens populations (Huerta-Sánchez et al., 2014; Sánchez-Quinto and Lalueza-Fox, 2015), meaning it occurs much later than the rate shift, and after the actual colonization of northern territories.
Although the real consequences of any individual cultural or technological adaptation introduced by MHS will almost certainly be a matter for debate for some time, our results indicate that these hominins were able to overcome the challenges imposed by life in northern habitats by a non-biological process, suggesting that behavioral modernity, interpreted as the capacity to use technology and culture to overcome the constraints imposed by natural climate variability on the geographic distribution, is not limited to H. sapiens.
Limitations of the Study
The very concept of niche construction in Homo implies cultural advancements (fitted clothing manufacture, intentional fire, the production of tools made of perishable material such as bone, hide and wood) and improved social connections and skills that leave little to no archaeological evidence (Riede, 2019). Rather than focusing on such scarce evidence, we therefore focused on one of the major consequences of these cultural advances, that is, the occupation of areas and climates outside the physiological niche limits of humans. A limitation of our findings is that the precise connection between the expansion of the climatic niche limits and advancements in material culture cannot easily be determined. Still, it relies on paleoclimate modeling that necessarily comes with uncertainty around the estimates. Nevertheless, our study confidently demonstrates the importance of cultural niche construction in the evolution of Homo and how the sudden evolution of such niche construction abilities shaped the geography of our own lineage in the deep past.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to Pasquale Raia (pasquale.raia@unina.it).
Materials Availability
This study did not generate any new material.
Data and Code Availability
The human fossil record and phylogenetic tree of hominins are available as supplemental data files. The functions used in this study are freely available as parts of the package RRphylo. Environmental niche limits (climatic variables) for each hominin species to generate estimates at the tree nodes (ancestors) are available in Table S1.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to Fabio di Vincenzo and Giorgio Manzi for critical discussion about the main findings presented here.
Author Contributions
P.R., A.M., M.M., and M.D.F. conceived the study. A.M., M.M., M.Mod., T.R., A.P., N.E., and P.H. produced and collected the data. A.M., M.M., M.D.F., S.C., and C.S. performed the analyses. P.O.H., F.C., L.M., L.R., J.A.D.F., T.R., A.P., N.E., and P.H. contributed in critique of analyses and interpretation. P.S. contributed in discussion of cultural and social contexts. All the authors contributed to writing.
Declaration of Interests
The authors declare no conflict of interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101693.
Supplemental Information
References
- Antón S.C., Snodgrass J.J. Origins and evolution of genus homo: new perspectives. Curr. Anthropol. 2012;53:S479–S496. [Google Scholar]
- Antón S.C., Potts R., Aiello L.C. Human evolution. Evolution of early homo: an integrated biological perspective. Science. 2014;345:1236828. doi: 10.1126/science.1236828. [DOI] [PubMed] [Google Scholar]
- Amanzougaghene N., Fenollar F., Raoult D., Mediannikov O. Where are we with human lice? A review of the current state of knowledge. Front. Cell. Infect. Microbiol. 2019;9:213. doi: 10.3389/fcimb.2019.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baab K.L. Evolvability and craniofacial diversification in genus Homo. Evolution. 2018;72:2781–2791. doi: 10.1111/evo.13637. [DOI] [PubMed] [Google Scholar]
- Banks W.E., d'Errico F., Dibble H.L., Krishtalka L., West D., Olszewski D., Townsend Peterson A., Anderson D.G., Gillam G.C., Montet-White A. Eco-cultural niche modeling: new tools for reconstructing the geography and ecology of past human populations. Palaeoanthropology. 2006;4:68–83. [Google Scholar]
- Banks W.E., d'Errico F., Peterson A.T., Vanhaeren M., Kageyama M., Sepulchre P., Ramstein G., Jost A., Lunt D. Human ecological niches and ranges during the lgm in Europe derived from an application of eco-cultural niche modeling. J. Archaeol. Sci. 2008;35:481–491. [Google Scholar]
- Banks W.E., Aubry T., d'Errico F., Zilhão J., Lira-Noriega A., Townsend Peterson A. Eco-cultural niches of the badegoulian: unraveling links between cultural adaptation and ecology during the last glacial maximum in France. J. Anthr. Archaeol. 2011;30:359–374. [Google Scholar]
- Banks W.E., d'Errico F., Zilhão J. Human–climate interaction during the early upper paleolithic: testing the hypothesis of an adaptive shift between the proto-aurignacian and the early aurignacian. J. Hum. Evol. 2013;64:39–55. doi: 10.1016/j.jhevol.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Benito B.M., Svenning J.-C., Kellberg-Nielsen T., Riede F., Gil-Romera G., Mailund T., Kjaergaard P.C., Sandel B.S. The ecological niche and distribution of neanderthals during the last interglacial. J. Biogeogr. 2017;44:51–61. [Google Scholar]
- Boivin N.L., Zeder M.A., Fuller D.Q., Crowther A., Larson G., Erlandson J.M., Denham T., Petraglia M.D. Ecological consequences of human niche construction: examining long-term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. U S A. 2016;113:6388–6396. doi: 10.1073/pnas.1525200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cârciumaru M., Ion R.-M., Niţu E.-C., Ştefănescu R. New evidence of adhesive as hafting material on middle and upper palaeolithic artefacts from Gura Cheii-Râşnov Cave (Romania) J. Archaeol. Sci. 2012;39:1942–1950. [Google Scholar]
- Carotenuto F., Barbera C., Raia P. Occupancy, range size, and phylogeny in Eurasian Pliocene to Recent large mammals. Paleobiology. 2010;36:399–414. [Google Scholar]
- Carotenuto F., Tsikaridze N., Rook L., Lordkipanidze D., Longo L., Condemi S., Raia P. Venturing out safely: the biogeography of Homo erectus dispersal out of Africa. J. Hum. Evol. 2016;95:1–12. doi: 10.1016/j.jhevol.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Castiglione S., Tesone G., Piccolo M., Melchionna M., Mondanaro A., Serio C., Di Febbraro M., Raia P. A new method for testing evolutionary rate variation and shifts in phenotypic evolution. Methods Ecol. Evol. 2018;9:974–983. [Google Scholar]
- Churchill S.E. Cold adaptation, heterochrony, and Neandertals. Evol. Anthropol. 1998;7:46–60. [Google Scholar]
- Crow T.J. Sexual selection, Machiavellian intelligence, and the origins of psychosis. Lancet. 1993;342:594–598. doi: 10.1016/0140-6736(93)91415-i. [DOI] [PubMed] [Google Scholar]
- d'Errico F. The invisible frontier. A multiple species model for the origin of behavioral modernity. Evol. Anthropol. 2003;12:188–202. [Google Scholar]
- d'Errico F., Henshilwood C.S. Additional evidence for bone technology in the southern African middle stone age. J. Hum. Evol. 2007;52:142–163. doi: 10.1016/j.jhevol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M., Gamble C., Gowlett J.A.J. Oxford University Press; 2014. Lucy to Language. [Google Scholar]
- El Zaatari S., Grine F.E., Ungar P.S., Hublin J.-J. Neandertal versus modern human dietary responses to climatic fluctuations. PLoS One. 2016;11:e0153277. doi: 10.1371/journal.pone.0153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison P.T., Valeggia C.R., Sherry D.S. Human birth seasonality. In: Brockman D.K., van Schaik C.P., editors. Seasonality in Primates: Studies of Living and Extinct Human and Non-human Primates. Cambridge University Press; 2005. p. 379. [Google Scholar]
- Frayer D.W., Lozano M., Bermúdez de Castro J.M., Carbonell E., Arsuaga J.-L., Radovčić J., Fiore I., Bondioli L. More than 500,000 years of right-handedness in Europe. Laterality. 2012;17:51–69. doi: 10.1080/1357650X.2010.529451. [DOI] [PubMed] [Google Scholar]
- Fuentes A. Integrative anthropology and the human niche: toward a contemporary approach to human evolution. Am. Anthropol. 2015;117:302–315. [Google Scholar]
- Fuentes A., Wyczalkowski M.A., MacKinnon K.C. Niche construction through cooperation: a nonlinear dynamics contribution to modeling facets of the evolutionary history in the genus Homo. Curr. Anthropol. 2010;51:435–444. [Google Scholar]
- Gilligan I. Neanderthal extinction and modern human behaviour: the role of climate change and clothing. World Archaeol. 2007;39:499–514. [Google Scholar]
- Gilligan I. The prehistoric development of clothing: archaeological implications of a thermal model. J. Archaeol. Method Theor. 2010;17:15–80. [Google Scholar]
- Gowlett J.A.J. The discovery of fire by humans: a long and convoluted process. Philos. Trans. R. Soc. B. 2016;371:20150164. doi: 10.1098/rstb.2015.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmand S., Lewis J.E., Feibel C.S., Lepre C.J., Prat S., Lenoble A., Boës X., Quinn R.L., Brenet M., Arroyo A. 3.3-million-year-old stone tools from lomekwi 3, West Turkana, Kenya. Nature. 2015;521:310–315. doi: 10.1038/nature14464. [DOI] [PubMed] [Google Scholar]
- Henshilwood C.S., d'Errico F., Yates R., Jacobs Z., Tribolo C., Duller G.A.T., Mercier N., Sealy J.C., Valladas H., Watts I., Wintle A.G. Emergence of modern human behavior: middle stone age engravings from South Africa. Science. 2002;295:1278–1280. doi: 10.1126/science.1067575. [DOI] [PubMed] [Google Scholar]
- Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Hiscock P. Blackwell Publishing Ltd; 2013. 5 Early Old World Migrations of Homo sapiens: Archaeology. [Google Scholar]
- Hiscock P. Learning in lithic landscapes: a reconsideration of the hominid ‘toolmaking’ niche. Biol. Theory. 2014;9:27–41. [Google Scholar]
- Holden P.B., Edwards N.R., Fraedrich K., Kirk E., Lunkeit F., Zhu X. PLASIM–GENIE v1. 0: a new intermediate complexity AOGCM. Geosci. Model Dev. 2016;9:3347–3361. [Google Scholar]
- Holden P.B., Edwards N.R., Rangel T.F., Pereira E.B., Tran G.T., Wilkinson R.D. PALEO-PGEM v1. 0: a statistical emulator of Pliocene–Pleistocene climate. Geosci. Model Dev. 2019;12:5137–5155. [Google Scholar]
- Hosfield R. Walking in a winter wonderland? Strategies for early and middle Pleistocene survival in midlatitude Europe. Curr. Anthropol. 2016;57:653–682. [Google Scholar]
- Huerta-Sánchez E., Jin X., Asan, Bianba Z., Peter B.M., Vinckenbosch N., Liang Y., Yi X., He M., Somel M. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature. 2014;512:194–197. doi: 10.1038/nature13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joordens J.C.A., d'Errico F., Wesselingh F.P., Munro S., de Vos J., Wallinga J., Ankjærgaard C., Reimann T., Wijbrans J.R., Kuiper K.F. Homo erectus at Trinil on Java used shells for tool production and engraving. Nature. 2015;518:228–231. doi: 10.1038/nature13962. [DOI] [PubMed] [Google Scholar]
- Kittler R., Kayser M., Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr. Biol. 2003;13:1414–1417. doi: 10.1016/s0960-9822(03)00507-4. [DOI] [PubMed] [Google Scholar]
- Laland K.N., Odling Smee J., Feldman M.W. Cultural niche construction and human evolution. J. Evol. Biol. 2001;14:22–33. doi: 10.1046/j.1420-9101.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Laland K.N., O’Brien M.J. Cultural niche construction: an introduction. Biol. Theory. 2012;6:191–202. [Google Scholar]
- Lee-Thorp J.A., Sponheimer M., Passey B.H., de Ruiter D.J., Cerling T.E. Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene. Philos. Trans. R. Soc. B. 2010;365:3389–3396. doi: 10.1098/rstb.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M., Villa P. Hunting weapons of the middle stone age and the middle palaeolithic: spear points from Sibudu, rose cottage and Bouheben. South. Afr. Humanit. 2006;18:89–122. [Google Scholar]
- Lordkipanidze D., Jashashvili T., Vekua A., de León M.S.P., Zollikofer C.P.E., Rightmire G.P., Pontzer H., Ferring R., Oms O., Tappen M. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature. 2007;449:305–310. doi: 10.1038/nature06134. [DOI] [PubMed] [Google Scholar]
- Low F.M., Gluckman P.D., Hanson M.A. Niche modification, human cultural evolution and the anthropocene. Trends Ecol. Evol. 2019;34:883–885. doi: 10.1016/j.tree.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Lozano M., Mosquera M., de Castro J.-M.B., Arsuaga J.-L., Carbonell E. Right handedness of Homo heidelbergensis from Sima de los Huesos (Atapuerca, Spain) 500,000 years ago. Evol. Hum. Behav. 2009;30:369–376. [Google Scholar]
- Martin J.S., Ringen E.J., Duda P., Jaeggi A.V. Harsh environments promote alloparental care across human societies. Proc. R. Soc. B. 2020;287:20200758. doi: 10.1098/rspb.2020.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionna M., Profico A., Castiglione S., Sansalone G., Serio C., Mondanaro A., Di Febbraro M., Rook L., Pandolfi L., Di Vincenzo F. From smart apes to human brain boxes. A uniquely derived brain shape in late hominins clade. Front. Earth Sci. 2020;8:273. [Google Scholar]
- Melchionna M., Mondanaro A., Serio C., Castiglione S., Di Febbraro M., Rook L., Diniz-Filho J.A.F., Manzi G., Profico A., Sansalone G., Raia P. Macroevolutionary trends of brain mass in primates. Biol. J. Linn. Soc. 2020;129:14–25. [Google Scholar]
- Nicholson C.M. Shifts along a spectrum: a longitudinal study of the western eurasian realized climate niche. Environ. Archaeol. 2019:1–16. [Google Scholar]
- Odling-Smee J., Laland K.N. Ecological inheritance and cultural inheritance: what are they and how do they differ? Biol. Theory. 2011;6:220–230. [Google Scholar]
- Organ C., Nunn C.L., Machanda Z., Wrangham R.W. Phylogenetic rate shifts in feeding time during the evolution of Homo. Proc. Natl. Acad. Sci. U S A. 2011;108:14555–14559. doi: 10.1073/pnas.1107806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt S.A., Ashton N.M., Lewis S.G., Abel R.L., Coope G.R., Field M.H., Gale R., Hoare P.G., Larkin N.R., Lewis M.D. Early Pleistocene human occupation at the edge of the boreal zone in northwest Europe. Nature. 2010;466:229–233. doi: 10.1038/nature09117. [DOI] [PubMed] [Google Scholar]
- Pearce E., Shuttleworth A., Grove M., Layton R. The costs of being a high latitude hominin. In: Dunbar R.I.M., Gamble C., Gowlett J.A.J., editors. Lucy to Language: The Benchmark Papers. Oxford University Press; 2014. pp. 356–379. [Google Scholar]
- Poza-Rey E.M., Lozano M., Arsuaga J.-L. Brain asymmetries and handedness in the specimens from the Sima de los Huesos site (Atapuerca, Spain) Quat. Int. 2017;433:32–44. [Google Scholar]
- Profico A., Di Vincenzo F., Gagliardi L., Piperno M., Manzi G. Filling the gap. Human cranial remains from Gombore II (Melka Kunture, Ethiopia; ca. 850 ka) and the origin of Homo heidelbergensis. J. Anthropol. Sci. 2016;94:1–24. doi: 10.4436/JASS.94019. [DOI] [PubMed] [Google Scholar]
- Pruetz J.D., Herzog N.M. Savanna chimpanzees at Fongoli, Senegal, navigate a fire landscape. Curr. Anthropol. 2017;58:S337–S350. [Google Scholar]
- Quintero I., Wiens J.J. What determines the climatic niche width of species? The role of spatial and temporal climatic variation in three vertebrate clades. Glob. Ecol. Biogeogr. 2013;22:422–432. [Google Scholar]
- Rae T.C., Koppe T., Stringer C.B. The Neanderthal face is not cold adapted. J. Hum. Evol. 2011;60:234–239. doi: 10.1016/j.jhevol.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Raia P., Boggioni M., Carotenuto F., Castiglione S., Di Febbraro M., Di Vincenzo F., Melchionna M., Mondanaro A., Papini A., Profico A. Unexpectedly rapid evolution of mandibular shape in hominins. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-018-25309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raia P., Mondanaro A., Melchionna M., Di Febbraro M., Diniz-Filho J.A.F., Rangel T.F., Holden P.B., Carotenuto F., Edwards N.R., Lima-Ribeiro M.S. Past extinctions of Homo species coincided with increased vulnerability to climatic change. One Earth. 2020 doi: 10.1016/j.oneear.2020.09.007. [DOI] [Google Scholar]
- Riede F. Niche construction theory and human biocultural evolution. In: Prentiss A., editor. Handbook of Evolutionary Research in Archaeology. Springer; 2019. pp. 337–358. [Google Scholar]
- Rivals F., Moncel M.-H., Patou-Mathis M. Seasonality and intra-site variation of neanderthal occupations in the middle palaeolithic locality of payre (ardèche, France) using dental wear analyses. J. Archaeol. Sci. 2009;36:1070–1078. [Google Scholar]
- Rizal Y., Westaway K.E., Zaim Y., van den Bergh G.D., Bettis E.A., Morwood M.J., Huffman O.F., n R.G.X., Joannes-Boyau R., Bailey R.M. Last appearance of Homo erectus at Ngandong, Java, 117,000–108,000 years ago. Nature. 2019;577:381–385. doi: 10.1038/s41586-019-1863-2. [DOI] [PubMed] [Google Scholar]
- Roberts P., Stewart B.A. Defining the “generalist specialist” niche for Pleistocene Homo sapiens. Nat. Hum. Behav. 2018;2:542–550. doi: 10.1038/s41562-018-0394-4. [DOI] [PubMed] [Google Scholar]
- Sánchez-Quinto F., Lalueza-Fox C. Almost 20 years of Neanderthal palaeogenetics: adaptation, admixture, diversity, demography and extinction. Philos. Trans. R. Soc. B. 2015;370:20130374. doi: 10.1098/rstb.2013.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimelmitz R., Kuhn S.L., Jelinek A.J., Ronen A., Clark A.E., Weinstein-Evron M. `Fire at will': the emergence of habitual fire use 350,000 years ago. J. Hum. Evol. 2014;77:196–203. doi: 10.1016/j.jhevol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Spikins P., Needham A., Wright B., Dytham C., Gatta M., Hitchens G. Living to fight another day: the ecological and evolutionary significance of Neanderthal healthcare. Quat. Sci. Rev. 2019;217:98–118. [Google Scholar]
- Thieme H. Lower Palaeolithic hunting spears from Germany. Nature. 1997;385:807–810. doi: 10.1038/385807a0. [DOI] [PubMed] [Google Scholar]
- Ulijaszek S.J., Strickland S.S. Cambridge University Press; 1993. Seasonality and Human Ecology. [Google Scholar]
- Villmoare B., Kimbel W.H., Seyoum C., Campisano C.J., DiMaggio E.N., Rowan J., Braun D.R., Arrowsmith J.R., Reed K.E. Paleoanthropology. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science. 2015;347:1352–1355. doi: 10.1126/science.aaa1343. [DOI] [PubMed] [Google Scholar]
- White T.D., Asfaw B., Beyene Y., Haile-Selassie Y., Lovejoy C.O., Suwa G., WoldeGabriel G. Ardipithecus ramidus and the paleobiology of early hominids. Science. 2009;326:64–86. [PubMed] [Google Scholar]
- Wroe S., Parr W.C.H., Ledogar J.A., Bourke J., Evans S.P., Fiorenza L., Benazzi S., Hublin J.-J., Stringer C., Kullmer O. Computer simulations show that Neanderthal facial morphology represents adaptation to cold and high energy demands, but not heavy biting. Proc. R. Soc. B. 2018;285:20180085. doi: 10.1098/rspb.2018.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L., Crow T., Roberts N. Cerebral torque is human specific and unrelated to brain size. Brain Struct. Funct. 2019;224:1141–1150. doi: 10.1007/s00429-018-01818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Kohler T.A., Lenton T.M., Svenning J.C., Scheffer M. Future of the human climate niche. Proc. Natl. Acad. Sci. U S A. 2020;117:11350–11355. doi: 10.1073/pnas.1910114117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The human fossil record and phylogenetic tree of hominins are available as supplemental data files. The functions used in this study are freely available as parts of the package RRphylo. Environmental niche limits (climatic variables) for each hominin species to generate estimates at the tree nodes (ancestors) are available in Table S1.