Abstract
This case series evaluates case investigation and contact tracing outcomes in San Francisco, California, during shelter-in-place restrictions during the COVID-19 pandemic.
Given the pressing need to reopen economic activity prior to the availability of a vaccine, the US and other nations are investing in contact tracing as a core component of the coronavirus disease 2019 (COVID-19) response.1 An estimated 75% of infected contacts need to be quarantined to contain COVID-19.2,3 We evaluated case investigation and contact tracing outcomes in San Francisco, California, during shelter-in-place restrictions.
Methods
San Francisco residents diagnosed with COVID-19 were routinely reported to the health department and assigned for case investigation and contact tracing.4 On May 5, 2020, universal testing for COVID-19 contacts was recommended, regardless of symptoms. This study included cases diagnosed during shelter-in-place from April 13 to June 8, 2020. Cases identified through outbreak investigations in long-term care facilities were excluded (10% of cases).5
To evaluate the outcomes of contact tracing, we calculated the proportion of people who were interviewed, identified close contacts, and had at least 1 contact notified, tested, and newly diagnosed with COVID-19. A deterministic match based on personal identifiers was performed between contact and testing databases to (1) exclude contacts who were known to have COVID-19, (2) deduplicate previously named household contacts, and (3) ascertain testing results. We report the median number of days (with interquartile range [IQR]) taken to process each step. Analyses were conducted in SAS, version 9.4 (SAS Institute). Bivariate tests including χ2 tests to compare categorical variables and t-tests for continuous variables were conducted, as appropriate, and a P < .05 identified statistical significance. This work was conducted as part of San Francisco Department of Public Health COVID-19 surveillance; institutional review board approval and informed consent were not required.
Results
Among 1633 cases reported, 1394 (85.4%) people were interviewed. Median (IQR) age was 37 (26-49) years; 972 (69.7%) were Latino (85% primarily Spanish-speaking), and 842 (60.3%) were male. Of the 603 (43.2%) interviewed people residing in a household with at least 5 persons, 510 (84.6%) were Latino. Half of interviewed people reported contact with someone diagnosed with COVID-19 (Table).
Table. Demographic, Clinical, and Exposure Characteristics of People With COVID-19, Stratified by Race, April 13 to June 8, 2020, San Francisco, California.
| Characteristic | No. (%) | P valueb | ||||
|---|---|---|---|---|---|---|
| All | Asian | Black or African American | Hispanic or Latino/all racesa | White | ||
| Study population, assigned | 1633 | 151 (9.3) | 65 (4.0) | 1044 (64.0) | 168 (10.3) | |
| Interviewed | 1394 (85.4) | 137 (90.7) | 47 (72.3) | 972 (93.1) | 148 (88.1) | <.001 |
| Not interviewed | 239 (14.6) | 14 (9.3) | 18 (27.7) | 72 (6.9) | 20 (11.9) | |
| Of those interviewed | ||||||
| Cases interviewedc | 1394 (100.0) | 137 (9.8) | 47 (3.4) | 972 (69.7) | 148 (10.6) | NA |
| Age, median (IQR), y | 37 (26-49) | 48 (30-65) | 52 (35-65) | 35 (24-45) | 43 (34-57) | NA |
| Gender | ||||||
| Female | 550 (39.6) | 59 (43.1) | 9 (19.1) | 399 (41.0) | 47 (31.8) | .003 |
| Male | 842 (60.3) | 78 (56.9) | 38 (80.9) | 571 (58.7) | 101 (68.2) | |
| Transmission category | ||||||
| Contact with known case | 704 (50.5) | 60 (43.8) | 18 (38.3) | 540 (55.6) | 46 (31.1) | <.001 |
| Community transmission | 690 (49.5) | 77 (56.2) | 29 (61.7) | 432 (44.4) | 102 (68.9) | |
| Clinical status | ||||||
| Ever hospitalized | 118 (8.5) | 28 (20.4) | 6 (12.8) | 62 (6.4) | 13 (8.8) | <.001 |
| Not hospitalized | 1256 (90.1) | 107 (78.1) | 40 (85.1) | 901 (92.7) | 135 (91.2) | |
| Unknown | 20 (1.4) | 2 (1.5) | 1 (2.1) | 9 (0.9) | 0 (0.0) | |
| Living situation | ||||||
| Homeless or shelter | 51 (3.7) | 2 (1.5) | 11 (23.4) | 20 (2.1) | 12 (8.1) | <.001 |
| SRO | 137 (9.8) | 10 (7.3) | 9 (19.1) | 96 (9.9) | 8 (5.4) | |
| Other housing situation | 1206 (86.5) | 125 (91.2) | 27 (57.4) | 856 (88.1) | 128 (86.5) | |
| Worked during infectious period | ||||||
| Went to work | 330 (23.7) | 35 (25.5) | 9 (19.1) | 234 (24.1) | 33 (22.3) | .68 |
| Did not work | 1035 (74.3) | 98 (71.5) | 37 (78.7) | 725 (74.6) | 113 (76.4) | |
| Unknown | 29 (2.1) | 4 (2.9) | 1 (2.1) | 13 (1.3) | 2 (1.4) | |
| Symptoms | ||||||
| Asymptomatic | 256 (18.4) | 25 (18.2) | 12 (25.5) | 176 (18.1) | 25 (16.9) | .33 |
| Any symptoms | 1123 (80.9) | 109 (79.6) | 35 (74.5) | 791 (81.4) | 122 (82.4) | |
| Unknown | 9 (0.6) | 3 (2.2) | 0 | 5 (0.5) | 1 (0.7) | |
| Preexisting medical conditions | ||||||
| Any preexisting condition | 520 (37.3) | 62 (45.3) | 23 (48.9) | 330 (34.0) | 67 (45.3) | .004 |
| No known medical conditions | 834 (59.8) | 69 (50.0) | 23 (48.9) | 621 (63.9) | 76 (51.4) | |
| Immune suppression | 83 (6.0) | 9 (6.6) | 4 (8.5) | 32 (3.3) | 30 (20.3) | <.001 |
| Household size | ||||||
| 1 | 138 (9.9) | 16 (11.7) | 16 (34.0) | 64 (6.6) | 29 (19.6) | <.001 |
| 2-4 | 610 (43.8) | 88 (64.2) | 17 (36.2) | 383 (39.4) | 87 (58.8) | |
| ≥5 | 603 (43.2) | 32 (23.4) | 7 (14.9) | 510 (52.5) | 22 (14.9) | |
| Unknown | 43 (3.1) | 1 (0.7) | 7 (14.9) | 15 (1.5) | 10 (6.8) | |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; NA, not applicable; SRO, single room occupancy building.
Demographic data not reliably available prior to interview.
Categorical variables compared with χ2 or Fisher exact test.
Not all races displayed as columns. Multiethnic, Native American, and Native Hawaiian or Other Pacific Islander people collectively account for 3% of cases and are not included in statistical comparisons.
Among 791 people interviewed after recommending universal testing for close contacts, 404 (51.1%) identified a contact not previously diagnosed with COVID-19, 356 (45.0%) had at least 1 contact notified, 206 (26.0%) had at least 1 contact tested, and 72 (9.1%) had at least 1 contact test positive for COVID-19. Of people residing in a household with at least 5 persons, 10.7% named a contact who was newly diagnosed with COVID-19. Among 1214 contacts traced, 1017 (83.8%) were successfully notified, 457 (37.6%) were tested, and 120 (9.9%) were newly diagnosed with COVID-19 (Figure). The secondary attack rate (calculated as the percentage of contacts who tested newly positive for COVID-19) was higher among household compared with nonhousehold contacts (111 of 983 [11.3%] and 9 of 231 [3.9%], respectively; P < .001), despite similar testing rates.
Figure. Contact Tracing Outcomes of 791 People With COVID-19 and Their 1214 Contacts, San Francisco, California, May 5 to June 8, 2020.
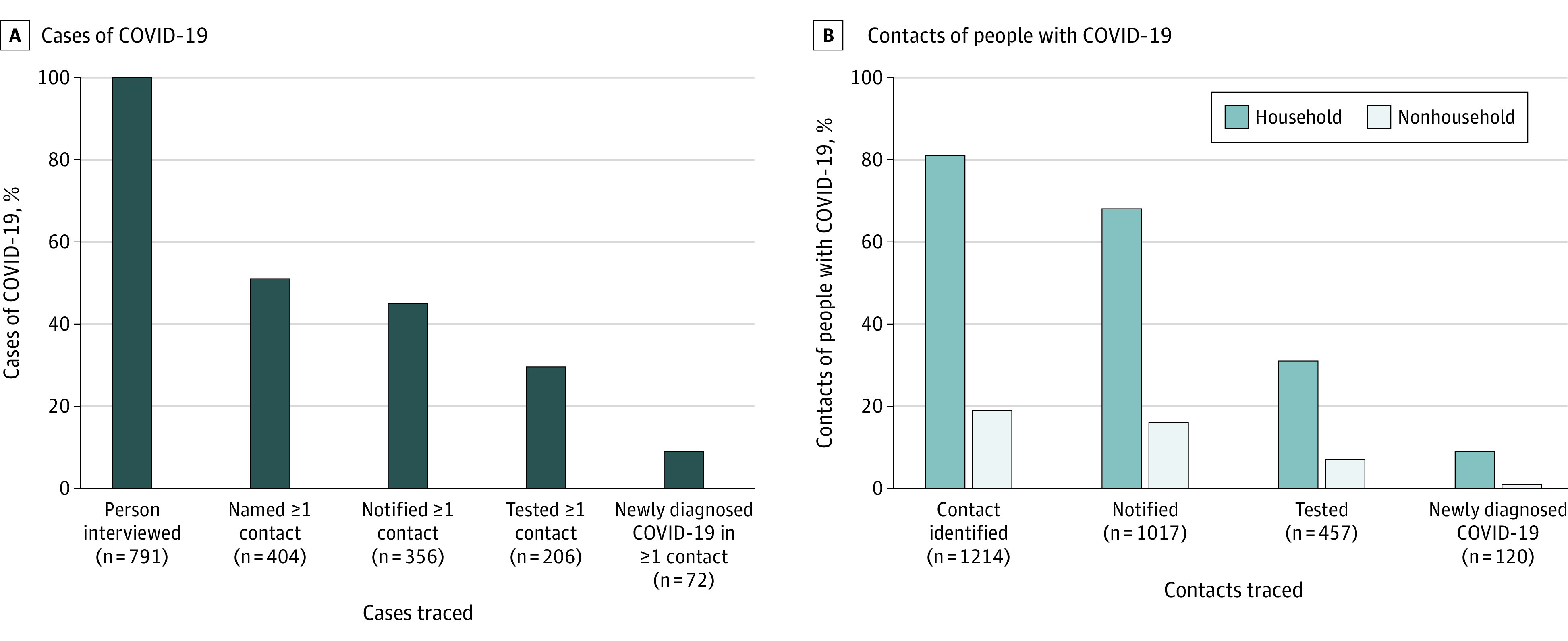
Percentages are shown for people with coronavirus disease 2019 (COVID-19) (A) and their contacts (B) at selected stages of contact tracing implementation.
The median (IQR) number of days was 3 (1-6) from symptom onset (or specimen collection date in asymptomatic cases) to testing, 2 (1-2) from testing to test result, 1 (0-1) from test result to case interview, and 1 (0-1) from case interview to notification of the first contact. Overall, median (IQR) time from the case’s symptoms to contact notification and testing was 6 (4-9) days and 6 (4-10) days, respectively.
Discussion
San Francisco’s COVID-19 contact tracing program reached more than 80% of cases and contacts and identified secondary cases within a median time frame of 6 days from the case’s symptom onset. While Latinos comprise 15% of San Francisco residents, they represented 70% of interviewed cases. Ten percent of contacts were newly diagnosed with COVID-19 compared with citywide testing positivity of 2.2% during this period. Although the majority of people with COVID-19 were interviewed within 1 day of test result, the 5-day delay between case symptom onset and positive test result raises concern regarding the timeliness of tracing in preventing onward transmission. Because 90% of secondary cases were household contacts, presymptomatic transmission could have occurred such that infected contacts may have already transmitted the virus by the time they were notified.6 Limitations include underreporting of close contacts during shelter-in-place and inability to ascertain adherence to quarantine. Addressing testing delays and improving contact identification will be necessary to optimize COVID-19 contact tracing efforts.
References
- 1.Rubin R. Building an “army of disease detectives” to trace COVID-19 contacts. JAMA. 2020;323(23):2357-2360. doi: 10.1001/jama.2020.8880 [DOI] [PubMed] [Google Scholar]
- 2.Peak CM, Kahn R, Grad YH, et al. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study. Lancet Infect Dis. 2020;20(9):1025-1033. doi: 10.1016/S1473-3099(20)30361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellewell J, Abbott S, Gimma A, et al. ; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group . Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8(4):e488-e496. doi: 10.1016/S2214-109X(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Prevention and Control . Contact tracing resources. Accessed July 14, 2020. https://www.cdc.gov/coronavirus/2019-ncov/php/open-america/contact-tracing-resources.html
- 5.Louie JK, Scott HM, DuBois A, et al. ; San Francisco Department of Public Health COVID-19 Skilled Nursing Facility Outbreak Response Team . Lessons from mass-testing for COVID-19 in long term care facilities for the elderly in San Francisco. Clin Infect Dis. Published online July 20, 2020. doi: 10.1093/cid/ciaa1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing QL, Liu MJ, Zhang ZB, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141-1150. doi: 10.1016/S1473-3099(20)30471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


