Key Points
Question
Does a group-based lifestyle intervention (with or without trained volunteers with type 2 diabetes) reduce the risk of type 2 diabetes in people with current high-risk intermediate glycemic categories of impaired fasting glucose or nondiabetic hyperglycemia?
Finding
In this randomized clinical trial of 1028 participants with high-risk intermediate glycemic categories, the intervention significantly reduced the 2-year risk of type 2 diabetes by 40% to 47%, although lay volunteer support did not reduce the risk further. For every 11 participants treated, 1 diabetes diagnosis was prevented.
Meaning
Nearly half of the adult population has diabetes or a high-risk glycemic category, and this low-cost group-delivered intervention significantly reduced the risk of diabetes.
Abstract
Importance
Nearly half of the older adult population has diabetes or a high-risk intermediate glycemic category, but we still lack trial evidence for effective type 2 diabetes prevention interventions in most of the current high-risk glycemic categories.
Objective
To determine whether a group-based lifestyle intervention (with or without trained volunteers with type 2 diabetes) reduced the risk of progression to type 2 diabetes in populations with a high-risk glycemic category.
Design, Setting, and Participants
The Norfolk Diabetes Prevention Study was a parallel, 3-arm, group-based, randomized clinical trial conducted with up to 46 months of follow-up from August 2011 to January 2019 at 135 primary care practices and 8 intervention sites in the East of England. We identified 141 973 people at increased risk of type 2 diabetes, screened 12 778 (9.0%), and randomized those with a high-risk glycemic category, which was either an elevated fasting plasma glucose level alone (≥110 and <126 mg/dL [to convert to millimoles per liter, multiply by 0.0555]) or an elevated glycated hemoglobin level (≥6.0% to <6.5%; nondiabetic hyperglycemia) with an elevated fasting plasma glucose level (≥100 to <110 mg/dL).
Interventions
A control arm receiving usual care (CON), a theory-based lifestyle intervention arm of 6 core and up to 15 maintenance sessions (INT), or the same intervention with support from diabetes prevention mentors, trained volunteers with type 2 diabetes (INT-DPM).
Main Outcomes and Measures
Type 2 diabetes incidence between arms
Results
In this study, 1028 participants were randomized (INT, 424 [41.2%] [166 women (39.2%)]; INT-DPM, 426 [41.4%] [147 women (34.5%)]; CON, 178 [17.3%] [70 women (%39.3)]) between January 1, 2011, and February 24, 2017. The mean (SD) age was 65.3 (10.0) years, mean (SD) body mass index 31.2 (5) (calculated as weight in kilograms divided by height in meters squared), and mean (SD) follow-up 24.7 (13.4) months. A total of 156 participants progressed to type 2 diabetes, which comprised 39 of 171 receiving CON (22.8%), 55 of 403 receiving INT (13.7%), and 62 of 414 receiving INT-DPM (15.0%). There was no significant difference between the intervention arms in the primary outcome (odds ratio [OR], 1.14; 95% CI, 0.77-1.7; P = .51), but each intervention arm had significantly lower odds of type 2 diabetes (INT: OR, 0.54; 95% CI, 0.34-0.85; P = .01; INT-DPM: OR, 0.61; 95% CI, 0.39-0.96; P = .033; combined: OR, 0.57; 95% CI, 0.38-0.87; P = .01). The effect size was similar in all glycemic, age, and social deprivation groups, and intervention costs per participant were low at $153 (£122).
Conclusions and Relevance
The Norfolk Diabetes Prevention lifestyle intervention reduced the risk of type 2 diabetes in current high-risk glycemic categories. Enhancing the intervention with DPM did not further reduce diabetes risk. These translatable results are relevant for current diabetes prevention efforts.
Trial Registration
ISRCTN Registry Identifier: ISRCTN34805606
This randomized clinical trial examines the effect of a group-based lifestyle intervention with or without trained volunteers with type 2 diabetes on the risk of progression to type 2 diabetes in British populations with a high-risk glycemic category.
Introduction
The worldwide diabetes population quadrupled between 1980 and 2014 to 422 million,1 matched by what has been described as a worldwide epidemic of the intermediate glycemic categories that carry a high risk of type 2 diabetes.2,3 Nearly half of the older US and UK population now has type 2 diabetes or a high-risk intermediate glycemic category,4,5,6 as do a third of young adults with obesity.7 There is a need for effective and affordable diabetes prevention strategies,1,8,9 and national diabetes prevention programs are now operating in the US,10,11 UK,12 and elsewhere. These offer a lifestyle intervention to people with a high-risk score, or a high-risk intermediate glycemic category, with plasma glucose levels, glycated hemoglobin (HbA1c) levels, or both that are elevated but not within the diagnostic range for diabetes.10,11,12 In the UK, entry to the national program12 and general recommendations for lifestyle interventions13 are targeted toward people with a high-risk elevated HbA1c level of 6.0% or greater to less than 6.5% (nondiabetic hyperglycemia [NDH]) or an elevated fasting plasma glucose (FPG) level of 100 or greater to less than 126 mg/dL (impaired fasting glucose [IFG]; to convert to millimoles per liter, multiply by 0.0555).14,15,16 Early observational outcomes are encouraging,10,11,12 although clinicians’ understanding of diabetes prevention remains poor.17
One critically important issue is that the trial evidence for type 2 diabetes prevention in the now commonly used high-risk glycemic categories of IFG or NDH is limited, and this lack of evidence in the current high-risk phenotypes has been emphasized recently.18 The early landmark prevention trials (used as the evidence base for current prevention programs) were mostly in populations defined as high risk based on an impaired glucose tolerance (IGT) category in a 2-hour oral glucose tolerance test, rather than FPG and IFG.19,20,21,22 The shift to HbA1c criteria for categorizing risk and diagnosing type 2 diabetes14,15,16 then created new large at-risk NDH populations. To our knowledge, there is no substantial trial evidence for benefit from a lifestyle intervention in people with high-risk IFG and/or NDH.18,19,20,21,22,23 No prevention trial of more than 2 years’ duration has used HbA1c as the diabetes diagnostic primary end point, in line with modern diagnostic practice, so the prevention evidence base does not align with current diagnostic approaches.18,19,20,21,22 We cannot assume that the outcomes of earlier trials (in different high-risk glycemic populations with IGT) are translatable to populations with current high-risk intermediate glycemic categories, who differ in pathophysiology, progression rates, and vascular risk.24,25,26,27
The diabetes prevention benefit found in the earlier high-intensity landmark trials 19,20,21,22 has been less marked in real-world pragmatic interventions.28,29 This means that, although there is a need for lower-cost and more pragmatic intervention models, the current evidence to support their effectiveness is limited.10,11,12,18,19,20,21,22,23,28,29 One attractive option is to include volunteer lay workers, who can support a diabetes prevention intervention alongside health care professionals, to codeliver an intervention at potentially lower cost.30,31,32,33,34 People with type 2 diabetes are an appealing choice for this role, as they share similar lifestyle challenges with the target group. To our knowledge, no large clinical trial has tested a diabetes prevention intervention supported by trained lay volunteers with type 2 diabetes compared with a standard intervention.
In the Norfolk Diabetes Prevention Study (NDPS), we tested the effectiveness of a pragmatic group-based lifestyle intervention that was supported by diabetes prevention mentors (trained volunteers with type 2 diabetes) in reducing the incidence of type 2 diabetes in people with current prediabetes glycemic categories.
Methods
Study Design
The NDPS was a 7-year research program (UK National Institute for Health Research RP PG 0109-10013). The NDPS protocol35 (Supplement 1) and baseline publications35,36,37,38 summarize NDPS sample sizes, recruitment plans, training materials, and screening data. The NDPS identified people with high-risk intermediate glycemic categories in the East of England,35 and eligible participants entered a randomized clinical 3-arm parallel group trial with up to 46 months of follow-up that tested a group-delivered, theory-based lifestyle intervention with or without the support of trained lay volunteers (diabetes prevention mentors [DPM]) with type 2 diabetes.35,37
Screening
Potential participants were screened with FPG levels, venous HbA1c levels, and biometric and clinical data collection.35 Participants with an eligible glycemic high-risk category on initial testing results had repeated testing a median of 40 days (interquartile range, 27-69 days) later.35,36 Trial randomization was offered if paired baseline tests were concordant for a high-risk intermediate glycemic category. The first screening appointment was August 22, 2011, and the last March 24, 2017. Protocol-driven screening was undertaken by NDPS program staff in 8 screening sites across the East of England.35
Inclusion Criteria
Nondiabetic hyperglycemia was defined as an HbA1c level of 6.0% or greater to less than 6.5%,14,15,16 and IFG was defined as an FPG level of 100 or greater to less than 126 mg/dL.14,15,16 We defined 2 study populations with a high-risk intermediate glycemic category based on then current glycemic definitions.14,15,16 We randomized participants if they had paired baseline isolated IFG range FPG measurements of 110 or greater to less than 126 mg/dL or if they had NDH HbA1c levels combined with an IFG FPG level of100 or greater to less than 110 mg/dL.14,15,16 Initial recruitment (beginning in 2011) into the trial was for participants with an isolated IFG level of 110 or greater to less than 126 mg/dL. In light of international changes in diabetes diagnostic criteria during the program, the new definition of high-risk NDH, and UK national policy changes,14,15,16 we also then randomized those with NDH and a lower-range IFG level (≥100 to <110 mg/dL)14,15,16 from May 6, 2014, and also accepted a paired HbA1c level of 6.5% or greater as a primary end point (as well as a paired FPG level of ≥126 mg/dL) for the diagnosis of type 2 diabetes.14,15,16,35 Impaired glucose tolerance during an oral glucose tolerance test was not used to randomize participants.14,15,16,35 To identify high-risk participants, we contacted 194 primary care practices in the East of England, and 135 (70%) collaborated. We invited all individuals without known diabetes in these practices who were (1) age ≥40 years with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of or greater; (2) age 40 years or older and a BMI of 25 or greater with a recorded first-degree family history of type 2 diabetes, a history of coronary artery disease, or gestational diabetes; or (3) any previous high-risk glycemic category diagnosis by recorded category or biochemical range.35
Ethnicity was participant defined, and these data were collected as important in type 2 diabetes risk assessment.
Randomization and Consent
We used rolling recruitment methods to randomize participants parallel to the screening program (Figure 1). Participant screening, recruitment, and randomization spanned the study duration and continued from August 2011 until March 24, 2017, and allowed each participant to reach at least the 6-month point follow-up appointment and up to 46 months. Eligible participants were randomized into a control arm (CON) who received no trial intervention, an intervention arm (INT) who received a lifestyle intervention, or an intervention arm who received the same intervention but with additional telephone support from lay DPMs (INT-DPM). Randomization was conducted automatically using a dedicated function in the trial data management system. The randomization mechanism consisted of a preprepared random list of codes (for the INT and CON groups) stored in the trial database. To reduce the risk of predicting the next allocation while maintaining a reasonable even spread of INT and CON patients, the list was constructed of blocks of 17 codes (3 CON, 7 INT, and 7 INT-DPM) to approximate the proportions of 170:390:390 participants, respectively. The randomization policies are described in eMethods in Supplement 2 and have been published previously.35 Randomization was asymmetric between groups to maximize sample size and power for comparisons between the intervention groups. Ethical approval was obtained from the National Research Ethics Service research ethics committee (10/H0301/55), and all participants gave written informed consent. There was no significant clinical trial evidence for diabetes prevention benefit with a structured lifestyle intervention in participants with IFG or NDH at NDPS inception and no UK national prevention program; it was ethical to have a control group who received then-standard best care.
Figure 1. Trial Consolidated Standards of Reporting Trials Profile.
Time not reached refers to the number of participants randomized as planned but not at a point during rolling recruitment that provided planned data collection at that later time. DPM indicates diabetes prevention mentors; IFG, impaired fasting glucose; NDH, nondiabetic hyperglycemia; T2DM, type 2 diabetes.
Interventions
The intervention was delivered by trained health care professionals alone (diabetes prevention facilitators [DPF]) or delivered jointly by DPFs and DPMs.35,37 The intervention theory aimed to support maintenance of changes in physical activity and diet using patient-centered counseling techniques to encourage decision-making about behavior changes; increase motivation to change; engage social support; aid individually tailored goal setting, action planning, and self-monitoring; and support problem solving.35,37 Behavior change targets were set by participants, who were encouraged to think about (and were presented with the health benefits of) a 7% weight loss if their BMI was greater than 30, achieving 150 minutes per week of moderate intensity physical activity over 5 days or more, 2 to 3 sessions of muscle-strengthening exercise per week, and reducing intake of total and saturated fat. The intervention comprised 6 2-hour educational group sessions of varying content for 12 weeks, followed by up to 15 maintenance sessions 8 weeks apart from month 4. Maintenance sessions were discussion based and followed the same format, including a 50-minute supervised physical activity/muscle-strengthening exercise session. Sessions contained no more than 15 participants. The maximum contact time per participant was 49.5 hours. Participants randomized to the INT-DPM arm received additional individual motivational telephone calls between sessions.35,37 The DPMs were assigned up to 7 participants, and telephone contacts were monthly for the first 3 months and then every 2 months. During these contacts, the DPM and participants discussed progress, goal achievement, action planning, and barriers to coping. The INT–DPM participants therefore received a contact from the study at least once every 4 weeks. The CON group received written information and discussion about the risk of diabetes and the effect of lifestyle modification on reducing this risk in line with then current local National Health Service (NHS) clinical policy. This was delivered in a single 2-hour session delivered by a DPF.35,37
Main Outcomes and Measures
The primary outcome was the development of type 2 diabetes based on paired HbA1c data both 6.5% or greater, or paired fasting glucose both 126 mg/dL or greater. Prespecified secondary outcomes were published previously35 and are described in the eMethods in Supplement 2.
Fasting plasma glucose was measured by a hexokinase/glucose-6-phosphate dehydrogenase method (Architect c8000; Abbott). Participants’ HbA1c levels were measured using Affinity high-performance liquid chromatography (Hb9210; Menarini Diagnostics Ltd). Additional detail on the study methods and materials38,39,40,41,42 were previously published 35 and are described in the eMethods in Supplement 2, which includes a fuller description of measurement of physical activity, homeostasis model assessment of insulin sensitivity and β cell function, and social deprivation scores.
Statistical Analysis Plan and Power Estimates
The assumed power calculations and sample size estimates are summarized in the eMethods in Supplement 2. The primary statistical analysis compared the proportions of participants in each group who progressed to type 2 diabetes independent of the duration of follow-up. We used an intention-to-treat approach for the analysis. We used the χ2 test for binary outcomes and logistic regression for adjustment for baseline imbalances. For continuous outcomes, we used the t test for comparison of 2 arms or analysis of covariance for comparison of all 3, and linear regression for adjustment for baseline imbalances. The primary outcome measure was progression to type 2 diabetes by study exit, analyzed using a logistic regression model, including a covariate to account for the different potential follow-up times at baseline. The full statistical analysis plan, power estimates, sample size, and attained power are published 35 and summarized in the eMethods in Supplement 2. We also analyzed and present the main outcome data using a proportional hazards model as a secondary analysis. Statistical analyses were conducted using Stata, version 16.1, and statistical significance was set at P < .05.
Health Economic Analysis
A within-trial analysis was conducted to estimate the cost-effectiveness of the intervention (with and without DPM) compared with usual care.35 These methods are summarized briefly in eMethods in Supplement 2, but the full analysis will be published separately.
Results
We invited 141 973 people at increased risk of developing type 2 diabetes to participate, and 12 778 (9.0%) were screened. Between October 1, 2011, and June 1, 2017, we randomized 424 eligible participants into the standard INT arm, 426 into the INT-DPM arm, and 178 into the CON arm. Baseline characteristics and flow through the trial are shown in Table 130,38,39,40,41,42,43,44 and Figure 1. Mean (SD) follow-up was 742 (403) days (24.7 months), and by arm was 727 (383) (CON), 744 (415) (INT), and 746 (402) days (INT-DPM). Between 75% and 78% were followed for at least 12 months (CON, n = 135; INT, n = 304; INT-DPM, n = 305) in a rolling recruitment until the end of the recruitment period (Figure 1). There were no significant differences between arms in baseline age (CON: 63.5 [11.2] years; INT: 64.7 [7.7] years; INT-DPM: 64.7 [10.5] years; P = .87) or BMI (CON: 32.6 [5.9]; INT: 31.8 [5.9]; INT-DPM: 31.5 [5.7]; P = .80) in those who withdrew from the intervention.
Table 1. Baseline Characteristics of CON, INT, and INT-DPM.
| Characteristic | CON | INT | INT-DPM |
|---|---|---|---|
| No. | 178 | 424 | 426 |
| Age, mean (SD), y | 65.3 (10.0) | 66.5 (8.6) | 66.7 (9.5) |
| Ethnicity, % | |||
| White | 96.0 | 97.1 | 97.1 |
| South Asian | 1.7 | 1.7 | 1.2 |
| Black | 0.6 | 0 | 0 |
| Other | 1.7 | 1.2 | 1.7 |
| Sex, No. (%) | |||
| Women | 70 (39.3) | 166 (39.2) | 147 (34.5) |
| Men | 108 (60.7) | 258 (60.8) | 279 (65.5) |
| Family history | |||
| Type 2 diabetes, No. (%) | 67 (37.6) | 173 (40.8) | 167 (39.2) |
| Cardiovascular disease, No. (%) | 22 (12.4) | 63 (14.9) | 57 (13.4) |
| Previous gestational diabetes, No. (%)a | 4 (5.7) | 12 (7.2) | 18 (12.2) |
| Social deprivation score, mean (SD)b | 15.5 (10.6) | 15.4 (10.2) | 16.2 (10.7) |
| Weight, mean (SD), kg | 90.5 (17.8) | 90.2 (18.2) | 89.8 (17.4) |
| BMI, mean (SD) | 31.2 (5.0) | 31.1 (5.6) | 30.9 (5.6) |
| Waist circumference, mean (SD), cm | 105.1 (13.1) | 105.1 (13.5) | 105.2 (13.0) |
| Body fat mass, mean (SD), kgc | 35.2 (8.8) | 34.0 (9.0) | 33.6 (8.9) |
| IFG, No. (%)d | 114 (64.0) | 261 (61.6) | 256 (60.1) |
| NDH, No. (%)d | 64 (36.0) | 163 (38.4) | 170 (39.9) |
| HbA1c, mean (SD), % | 6.1 (0.3) | 6.1 (0.3) | 6.1 (0.3) |
| Fasting, mean (SD) | |||
| Plasma glucose, mg/dL | 112 (7.2) | 112 (7.2) | 113 (7.2) |
| HDL cholesterol, mg/dL | 49.5 (13) | 38.7 (13) | 38.7 (13) |
| LDL cholesterol, mg/dL | 119.1 (35) | 117 (34) | 118 (35) |
| Plasma insulin, pmol/l | 108.3 (72.5) | 95.7 (54.4) | 91.0(57.1) |
| HOMA, mean (SD) | |||
| Insulin sensitivity, (%)e | 68.5 (41.9) | 73.2 (51.5) | 77.6 (47.2) |
| β cell function, (%)e | 98.1 (44.0) | 90.6 (35.6) | 88.2 (36.3) |
| Physical activity, mean (SD) | |||
| MET min per wkf | 2507 (2761) | 2701 (2640) | 2660 (2748) |
| Min sitting per wkf | 442 (269) | 463 (263) | 431 (241) |
| Low physical activity category, No. (%)f | 42 (32.3) | 91 (29.4) | 98 (32.3) |
| Dietary fat intake scale, mean (SD)g | 2.3 (0.3) | 2.3 (0.3) | 2.3 (0.3) |
| W-BQ12, mean (SD)h | 24.8 (6.1) | 25.1 (6.5) | 25.0 (6.1) |
| EQ-5D, mean (SD)h | 0. 8 (0.2) | 0.8 (0.2) | 0.8 (0.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CON, control arm; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; IFG, impaired fasting glucose; INT, standard intervention arm; INT-DPM, intervention arm with diabetes prevention mentors; LDL, low-density lipoprotein; MET, metabolic equivalent of task; NDH, nondiabetic hyperglycemia.
SI conversion factors: To convert total, HDL and LDL cholesterol to mmol/L, multiply by 0.0259; plasma glucose to mmol/L, multiply by 0.0555.
Female participants.
Indices of multiple deprivation social deprivation score.30
Body fat by Tanita body composition analyzer.30
Impaired fasting glucose–paired baseline fasting plasma glucose levels of 110 or greater to less than 126 mg/dL. Nondiabetic hyperglycemia–paired baseline HbA1c levels of 6.0% or greater to less than 6.5% with an IFG fasting plasma glucose level of 100 or greater to less than 110 mg/dL.
Homeostasis model assessment of baseline insulin sensitivity and β cell function expressed as percentage of standard reference range, from fasting plasma insulin and glucose data.38
Physical activity scales, energy expenditure during physical activity (MET minutes per week), low physical activity category, and sedentary time derived from the international physical activity questionnaire.39,40
Dietary fat and fiber scores based on self-reported Diet Behavior Questionnaire.41
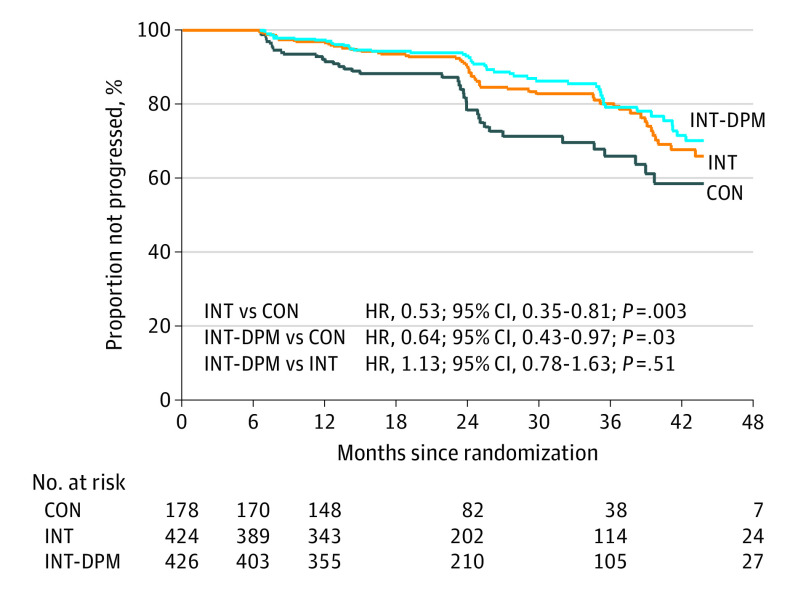
Of those who attended at least 1 intervention session, during follow-up, 156 participants progressed to type 2 diabetes, which comprised 39 of 171 in the CON arm (22.8%; estimated adjusted annual incidence, 11.0%), 55 of 403 in the INT arm (13.7%; estimated adjusted annual incidence, 6.4%), and 62 of 414 in the INT–DPM arm (15.0%; estimated adjusted annual incidence adjusted for follow-up, 7.1%; eTable 1 in Supplement 2). There was no significant difference between intervention arms (INT vs INT-DPM) in the primary outcome (odds ratio [OR], 1.14; 95% CI, 0.77-1.70; P = .51; Table 2 and Figure 2).
Table 2. Estimates of Difference Between Treatment Arms in Developing Type 2 Diabetes.
| Analysis | INT-DPM vs INT | P value | INT vs CON | P value | INT-DPM vs CON | P value | Combined intervention vs CON | P value |
|---|---|---|---|---|---|---|---|---|
| OR unadjusted (95% CI) | 1.11 (0.7-1.65) | .59 | 0.53 (0.34-0.84) | .01 | 0.60 (0.3-0.93) | .02 | 0.57 (0.38-0.85) | .01 |
| OR adjusted (95% CI)a | 1.12 (0.75-1.65) | .59 | 0.54 (0.34-0.85) | .01 | 0.60 (0.38-0.94) | .02 | 0.57 (0.38-0.85) | .01 |
| OR adjusted (95% CI)b | 1.14 (0.77-1.70) | .51 | 0.54 (0.34-0.85) | .01 | 0.61 (0.39-0.96) | .03 | 0.57 (0.38-0.87) | .01 |
| HR unadjusted (95% CI) | 1.09 (0.76-1.57) | .63 | 0.53 (0.35-0.80) | .003 | 0.62 (0.41-0.92) | .02 | 0.57 (0.40-0.82) | .002 |
| HR adjusted (95% CI)c | 1.13 (0.78-1.63) | .51 | 0.53 (0.35-0.81) | .003 | 0.64 (0.43-0.97) | .03 | 0.58 (0.41-0.84) | .004 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CON, control arm without trial intervention. HR, hazard ratio; INT, standard intervention group; INT-DPM, intervention group with diabetes prevention mentors; OR, odds ratio.
Adjusted for duration of follow-up.
Adjusted for follow-up length and age, BMI, and fasting plasma glucose levels at baseline.
Adjusted at baseline for age, BMI, and fasting plasma glucose levels. The primary analysis in the Norfolk Diabetes Prevention Study was a logistic regression model with data presented as ORs. A secondary analysis using a proportional hazard model is also shown with HRs.
Figure 2. Kaplan-Meier Estimate of Time to Progression by Treatment Trial Arm.
CON indicates control arm; DPM, diabetes prevention mentor; HR, hazard ratio; INT, standard intervention.
There were highly significant reductions in the primary end point between each intervention arm compared with CON and between a combined intervention group compared with CON (Table 2 and Figure 2) (INT: OR, 0.54; 95% CI, 0.34-0.85; P = .01; INT-DPM: OR, 0.61; 95% CI, 0.39-0.96; P = .03; combined INT and INT–DPM: OR, 0.57; 95% CI, 0.38-0.87; P = .01). The fully adjusted effect size was between a 36% and 42% reduction in the odds of type 2 diabetes (Table 2) depending on arm. These data are shown for the primary analysis using a logistic regression model (Table 2) as well as a proportional hazards model as a secondary analysis (Table 2)
Estimates of differences for the primary outcome of type 2 diabetes by subgroup showed no significant interactions with age band, sex, deprivation score, BMI, or initial diagnostic category (NDH or IFG) in the risk of developing type 2 diabetes in any arm or the combined group (Table 330). Broadly, 1 participant was prevented from developing diabetes for every 11 intervention participants.
Table 3. Estimated Odds Ratio Between Treatment Arms of Developing Type 2 Diabetes by Subgroups.
| Characteristic | INT-DPM vs INT | Interaction P valuea | INT vs CON | Interaction P valuea | INT-DPM vs CON | Interaction P valuea | Combined intervention vs CON | Interaction P valuea |
|---|---|---|---|---|---|---|---|---|
| Male | 1.07 (0.66-1.73) | .83 | 0.50 (0.28-0.87) | .68 | 0.53 (0.31-0.92) | .54 | 0.51 (0.31-0.85) | .58 |
| Female | 1.17 (0.59-2.34) | 0.61 (0.89-1.35) | 0.72 (0.32-1.57) | 0.66 (0.32-1.34) | ||||
| Age, y | ||||||||
| <65 | 1.34 (0.71-2.51) | .46 | 0.47 (0.23-0.96) | .63 | 0.63 (0.31-1.25) | .87 | 0.55 (0.29-1.03) | .88 |
| ≥65 | 0.99 (0.60-1.63) | 0.59 (0.33-1.06) | 0.58 (0.32-1.05) | 0.58 (0.34-1.00) | ||||
| Deprivation quartile | ||||||||
| 1 (Low)b | 1.49 (0.62-3.57) | .53 | 0.54 (0.20-1.47) | .13 | 0.80 (0.31-2.09) | .11 | 0.66 (0.28-1.59) | .08 |
| 2 | 0.71 (0.35-1.46) | 0.87 (0.34-2.21) | 0.62 (0.24-1.59) | 0.73 (0.31-1.73) | ||||
| 3 | 1.40 (0.63-3.09) | 0.80 (0.30-2.15) | 1.11 (0.42-2.93) | 0.94 (0.39-2.31) | ||||
| 4 (High) | 1.14 (0.51-2.50) | 0.23 (0.09-0.53) | 0.26 (0.11-0.59) | 0.24 (0.11-0.51) | ||||
| BMI quartile | ||||||||
| 1b | 1.63 (0.72-3.73) | .15 | 0.42 (0.15-1.14) | .94 | 0.67 (0.27-1.71) | .45 | 0.55 (0.23-1.32) | .89 |
| 2 | 1.03 (0.48-2.21) | 0.62 (0.25-1.49) | 0.63 (0.26-1.54) | 0.62 (0.28-1.39) | ||||
| 3 | 0.54 (0.24-1.22) | 0.59 (0.26-1.37) | 0.32 (0.13-0.80) | 0.45 (0.21-0.97) | ||||
| 4 (High) | 1.78 (0.79-3.99) | 0.50 (0.19-1.31) | 0.88 (0.36-2.17) | 0.69 (0.30-1.57) | ||||
| IFGc | 0.92 (0.53-1.61) | .35 | 0.55 (0.29-1.05) | .89 | 0.51 (0.26-0.97) | .50 | 0.53 (0.29-0.95) | .76 |
| NDHd | 1.34 (0.77-2.32) | 0.52 (0.27-0.98) | 0.69 (0.37-1.28) | 0.60 (0.34-1.06) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CON, control arm; IFG, impaired fasting glucose; INT, standard intervention; INT-DPM, intervention with diabetes prevention mentors; NDH, nondiabetic hyperglycemia.
SI conversion factors: To convert total plasma glucose to mmol/L, multiply by 0.0555.
P value for interaction within each subgroup by arm comparison.
Indices of multiple deprivation social deprivation scores.30 Body mass index quartile values are (1)19 to 27. (2) 27 to 30.37, (3) 30.38 to 33.83, and (4) 33.86 to 57.65.
Impaired fasting glucose–paired baseline fasting plasma glucose level of 110 or greater to less than 126 mg/dL.
Nondiabetic hyperglycemia–paired baseline hemoglobin A1c level of 6.0% or greater to less than 6.5% with an IFG fasting plasma glucose level of 100 or greater to less than 110 mg/dL.
At 12 months, the combined intervention group showed significantly lower baseline-adjusted weight (–1.76 kg ; 95% CI, –2.55 to – 0.97; P = .01), waist circumference (–2.48 cm, 95% CI, –3.67 to –1.29 ; P = .01), BMI (–0.59; 95% CI, –0.86 to –0.31; P = .01), and greater physical activity (metabolic equivalent of task minutes per week; P = .01) compared with controls (eTable 2 in Supplement 2), with no significant changes in self-reported dietary measures. These differences were apparent for each intervention arm compared with the CON arm. At 24 months, a lower mean adjusted weight loss in the combined intervention group was maintained (–1.47 kg; 95% CI, –2.64 to –0.30; P = .01), with highly significant differences in adjusted physical activity compared with controls (eTable 3 in Supplement 2). Within the intervention arms, weight loss was particularly marked in the intervention subgroup who attained a high dose of the intervention compared with those with a low dose at 24 months (INT: −3.29; 95% CI, −4.97 to −1.62; P < .001; INT-DPM: −3.65 kg ; 95% CI, −5.99 to −1.32; P = .002; eTable 4 in Supplement 2). The data on dose response effects, unadjusted data, and descriptive data at each point are further described the eResults and eTables 5-8 in Supplement 2). Mean intervention costs per participant were estimated as $153 ($71) (£122 [£55]) in the INT arm and $301 ($67) (£241 [£52]) in the INT-DPM arm. The full health economic analysis will be published separately.
Discussion
In this trial, people with a current high-risk intermediate glycemic category of IFG and/or NDH were 40% to 47% less likely to develop type 2 diabetes in the intervention groups compared with controls over an average 24 months. Broadly, 1 person was prevented from developing type 2 diabetes for every 11 who received the intervention. The enhanced intervention with trained DPMs did not further reduce the risk of type 2 diabetes. These findings are relevant to normal clinical practice, as nearly half of the older adult population now has a high-risk glycemic category or diabetes,4,5,6,36 as do one-third of young adults with obesity, with IFG constituting the largest element.4,5,6,7
To our knowledge, NDPS is the largest type 2 diabetes prevention trial since the US Diabetes Prevention Program more than 20 years ago19,20,21,22 and now extends the prevention evidence base to contemporary high-risk glycemic categories. Nearly all of the earlier landmark trial evidence on diabetes prevention is drawn from people categorized as having IGT using an oral glucose tolerance test.19,20,21,22 The assumption that this earlier evidence can simply be translated with similar expected benefit to IFG or NDH populations with a different phenotype may not be valid.18,19,20,21,22,23 The NDPS affirms that a low-cost group-based lifestyle intervention in these high-risk groups does have a substantial effect in preventing type 2 diabetes.19,20,21,22 The glycemic criteria we used are those now recognized as identifying individuals with a high risk of diabetes in UK prevention policy, in the NHS England diabetes prevention program, and in US prevention programs.10,11,12,13 Our results are therefore translatable to the current clinical and policy context.
A meta-analysis of 11 similar trials with a diet and physical activity intervention of more than 2 years in high-risk glycemic categories20 described a similar composite effect size of a risk ratio of 0.57 (95% CI, 0.5-.64; P > .001). In that analysis of 9 trials20 exclusively randomized based on oral glucose tolerance test data, 1 included IFG or IGT, and 1 included people with a fasting glucose level of 95-124 mg/dL.20,23 None used NDH-IFG as the primary entry criteria to trial, or HbA1c levels as a primary end point, in line with current international practice, although the US Diabetes Prevention Program did analyze HbA1c levels as a secondary outcome.43 The NDPS effect size did not differ significantly in subgroups defined by glycemic category, BMI, age, or social deprivation. The only other comparable UK clinical trial used oral glucose tolerance testing as entry criteria and the primary end point and found no overall diabetes prevention benefit other than in a subgroup attaining a higher intervention dose.44,45 This study’s full within-trial economic analysis will be published separately, and the high costs of the intense interventions in the early landmark research trials are well recognized,46 although intervention models translated into clinical settings may be deliverable at a lower cost.45,47,48,49
The combined intervention group at 12 months had a significantly lower mean weight (−1.76 kg), waist circumference (−2.48 cm), and BMI. Despite relatively low levels of weight loss, compared with the landmark studies in the field, the maintenance of behavior changes or area under the curve generated may be partly responsible for the marked effect on diabetes incidence. For the subgroup who attained a high intervention dose, weight loss was significant even at 2 years into the program (−3.47 kg) compared with those attaining a low dose. These weight changes are similar to that seen in a systematic analysis of weight loss in intervention arms in translational and controlled trial prevention studies.28 It is also similar to the observed mean weight loss in high attenders in the NHS England diabetes prevention program.12
The longer-term legacy effect of the NDPS intervention on type 2 diabetes incidence and maintained weight loss is unknown, but some short-term regain of lost weight after an intensive lifestyle intervention is a common observation in people with obesity, type 2 diabetes, or high-risk glycemic categories, particularly in those with the least initial weight loss.49,50,51,52 We also observed a significant increase in energy expenditure in the intervention groups (eTables 3-7 in Supplement 2). There is a direct consistent association between reduced type 2 diabetes risk and an increase in almost all type of physical activity and energy expenditure that is only partially mediated through changes in adiposity.53
The DPM-supplemented intervention group (INT- DPM) did not differ significantly from the INT group in the risk of type 2 diabetes, any secondary outcome, or in participant adherence to the intervention. The use of lay volunteer health workers to deliver lifestyle modification interventions for people at high risk of type 2 diabetes, or with established type 2 diabetes, is well recognized 30,31,32 but this study’s model did not add value.30,31,32,37 To our knowledge, only 1 other study has used people with type 2 diabetes in this role to prevent diabetes,53 with significant improvement in risk markers, although it is unknown if this translated into a lower type 2 diabetes incidence. The effect of lay or peer volunteers on type 2 diabetes prevention in high-risk groups has been reviewed, with 30 studies (including 10 randomized clinical trials) largely delivered in high-income countries to largely minority populations of color and studies of between 20 and 2369 participants.30 None of these reported a diabetes prevention benefit with diabetes as an end point or were powered to detect such an outcome, although there were commonly improvements in surrogate markers for diabetes risk.30 Cluster randomized clinical trials in high-risk groups using generic lay trainer programs to support or deliver the intervention have also shown no significant effect in diabetes prevention in community or primary care settings.32 The NDPS DPM training, levels of retention in the program, responsibilities, and level of contact would be regarded as moderate to high compared with other models.30,37 A telephone-delivered intervention as used in this study is as acceptable to participants with NDH as more complex digitally enabled health coaching54 and as effective in risk marker reduction as face-to-face interventions in people with intermediate high risk of diabetes categories.33,34,55 There is evidence that more frequent contact by telephone peer contact has a greater value in reducing type 2 diabetes risk.56 In established type 2 diabetes, the frequency of peer contact is a key feature of effectiveness in terms of glycemic change.57 The framework in which the NDPS DPM operated was also highly supportive and structured within a multidisciplinary diabetes prevention team, one of the more effective ways to use lay volunteers.30,36 Therefore, we do not think that the lack of effect of this study’s DPM model was because of low-intensity DPM training, a short duration of intervention, or an unsupportive framework.30,36 It is possible that more intensive contact from the DPM and higher frequency telephone contact may have been more effective.32,33,55,56 It is also quite possible that the lack of DPM effect could be because of the already large prevention effect size attained with the standard intervention alone. The use of lay trainers (with or without type 2 diabetes) in diabetes prevention remains an attractive model, but the most effective model remains to be determined, and future trials should test different levels of contact intensity, compare the efficacy of different lay groups, and use DPM as the primary intervention team.
The progression incidence to type 2 diabetes in the control arm was an adjusted annual 11.9%. This is a high incidence for these glycemic categories, in which an annual rate of 5% to 11% has been described over 5 years.21 In the US Diabetes Prevention Program, the mean follow-up was 2.8 years, and crude incidence rates were 11.0 cases per 100 participant years in controls and 4.8 in the lifestyle intervention group.22 This high rate in NDPS reflects our inclusion criteria, which were designed to identify and randomize those at highest risk.35 We also excluded lower-risk participants with an NDH-range HbA1c level and a normal FPG level of less than 100 mg/dL.58 We also randomized only those with paired abnormal baseline data at lower risk of regression to normal glycemic status,35,56,59,60,61 and we used HbA1c and fasting glucose–based definitions of diabetes for the end point in line with normal clinical practice. The high progression incidence when high risk is categorized this way validates the use of this approach in clinical guidelines and in the NHS England NDPP and confirms the importance of taking action in these high-risk groups.12,13
At the start of NDPS, there was no substantial trial evidence of an outcomes benefit from an active lifestyle intervention in diabetes prevention in IFG or NDH populations, and NDPS antedated the UK national diabetes prevention program12 and UK national guidance on best practice in preventing type 2 diabetes.13 During NDPS, the control participants received what was (and still is, for much of the population) standard best practice UK NHS care for people with a higher-risk intermediate glycemic risk category, with a 2-hour education session with a diabetes prevention facilitator to discuss their risk of diabetes and then 6-month and annual review and monitoring. Lifestyle educators are not generally available in practices, and arguably the control group received a higher level of support than in normal clinical practice. The original age and BMI criteria for screening in NDPS were designed to comply with the UK National Cardiovascular Risk Assessment primary care program in England.62,63 This program started in 2009, is one of the largest cardiovascular risk screening programs worldwide, and aims to screen more than 3 million high-risk individuals in primary care. There is a substantial glycemic element to the screening, and in 2011, we wished any positive outcomes from NDPS to be translatable to the large populations detected in this national program and to access participants selected in this way as part of normal clinical care.62,63
Limitations
There are limitations in NDPS. The participants come from a largely White population, and the results may not be translatable to more ethnically diverse populations or other ethnicities with different patterns of glycemic risk.11,12,51,52 This would also apply to adolescent and young adults with a high prevalence of high-risk glycemic categories, but for whom there is no trial evidence for efficacy.6 The attained power and effect size support the view that our intervention is effective in diabetes prevention, and while the power attained between intervention groups analysis was lower, rates of progression were very similar, and any difference is unlikely to be meaningful. More than 75% of participants were followed for at least 12 months, with prolonged follow-up of participants recruited earliest, which added power to the study, and missing data levels were very low.64 There is a more general observation, common to all similar trials, that wider population-level approaches to type 2 diabetes prevention are needed.65 However, NDPS now extends the diabetes prevention evidence base to modern populations with an at-risk glycemic category,18 for whom trial evidence has been lacking, and with an effect on diabetes incidence close to that seen in high-intensity clinical trials.18,19,20
Conclusions
The NDPS confirms that prevention efforts in these current high-risk populations are effective, and aligns the evidence base with current practice. The glycemic criteria we used are those now recognized as identifying individuals with a high risk of diabetes in UK prevention policy, NHS England diabetes prevention program, UK national vascular screening program,62 and US prevention programs.10,11,12,13 Our intervention materials and model are translatable and available to clinicians in practice, and suggest that a pragmatic group-based lifestyle intervention reduces the risk of type 2 diabetes in these large populations currently being detected in primary care.
Trial Protocol
eMethods. Summary of the methods, secondary analyses, statistical analysis plan, and intervention, health economic analysis, and randomization methodology
eResults. Results for additional subgroup analyses by dose adherence
eTable 1. Percentage of individuals who progressed to Type 2 diabetes in each arm
eTable 2. Adjusted mean differences (95% CI) between trial arms at 12 months: linear regression models
eTable 3. Adjusted mean differences (95% CI) between arms at 24 months: linear regression models
eTable 4. Changes at 12 and 24 months by participants achieving higher ‘doses’ of intervention (moderate, or high)
eTable 5. Descriptive data for outcomes at 12 months by trial arm
eTable 6. Descriptive data for outcomes at 24 months by trial arm
eTable 7. Unadjusted mean differences arms at 12 months: linear regression models
eTable 8. Unadjusted mean differences arms at 24 months: linear regression models
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513-1530. doi: 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards CM, Cusi K. Prediabetes: a worldwide epidemic. Endocrinol Metab Clin North Am. 2016;45(4):751-764. doi: 10.1016/j.ecl.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 3.Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ. 2014;349:g4485. doi: 10.1136/bmj.g4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 5.Mainous AG III, Tanner RJ, Baker R, Zayas CE, Harle CA. Prevalence of prediabetes in England from 2003 to 2011: population-based, cross-sectional study. BMJ Open. 2014;4(6):e005002. doi: 10.1136/bmjopen-2014-005002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 7.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr. 2019;174(2):e194498. doi: 10.1001/jamapediatrics.2019.4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Global action plan for the prevention and control of non-communicable diseases 2013–2020. Accessed January 6, 2020. http://apps.who.int/iris/bitstream/10665/94384 /1/9789241506236_eng.pdf?ua=1
- 9.The Lancet Beat diabetes: an urgent call for global action. Lancet. 2016;387(10027):1483. doi: 10.1016/S0140-6736(16)30185-4 [DOI] [PubMed] [Google Scholar]
- 10.Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and use of diabetes prevention services in the United States, 2016-2017. JAMA Netw Open. 2019;2(5):e193160. doi: 10.1001/jamanetworkopen.2019.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331-1341. doi: 10.2337/dc16-2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valabhji J, Barron E, Bradley D, et al. Early outcomes from the English National Health Service Diabetes Prevention Programme. Diabetes Care. 2020;43(1):152-160. doi: 10.2337/dc19-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Clinical Excellence Public health draft guidance: preventing type 2 diabetes: risk identification and interventions for individuals at high risk. Accessed January 6, 2020. https://www.nice.org.uk/guidance/ph38.
- 14.International Expert Committee International expert committee report on the role of the HbA1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327-1334. doi: 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO /IDF consultation. Accessed January 6, 2020. http://whqlibdoc.who.int/diabetes/publications/diagnosis-diabetes2006/en
- 16.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80. doi: 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 17.Voelker R Study identifies primary care knowledge gaps and barriers in type 2 diabetes prevention. JAMA. 2019. doi: 10.1001/jama.2019.18024 [DOI] [PubMed] [Google Scholar]
- 18.Campbell MD, Sathish T, Zimmet PZ, et al. Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype. Nat Rev Endocrinol. 2020;16(7):395-400. doi: 10.1038/s41574-019-0316-1 [DOI] [PubMed] [Google Scholar]
- 19.Schwarz PE, Greaves CJ, Lindström J, Yates T, Davies MJ. Nonpharmacological interventions for the prevention of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(6):363-373. doi: 10.1038/nrendo.2011.232 [DOI] [PubMed] [Google Scholar]
- 20.Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12(12):CD003054. doi: 10.1002/14651858.CD003054.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. doi: 10.1002/14651858.CD012661.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito T, Watanabe M, Nishida J, et al. ; Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med. 2011;171(15):1352-1360. doi: 10.1001/archinternmed.2011.275 [DOI] [PubMed] [Google Scholar]
- 24.Faerch K, Vaag A, Witte DR, Jørgensen T, Pedersen O, Borch-Johnsen K. Predictors of future fasting and 2-h post-OGTT plasma glucose levels in middle-aged men and women-the Inter99 study. Diabet Med. 2009;26(4):377-383. doi: 10.1111/j.1464-5491.2009.02688.x [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo C, Hartnett S, Hanley AJ, et al. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2013;98(4):1622-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engberg S, Glümer C, Witte DR, Jørgensen T, Borch-Johnsen K. Differential relationship between physical activity and progression to diabetes by glucose tolerance status: the Inter99 Study. Diabetologia. 2010;53(1):70-78. [DOI] [PubMed] [Google Scholar]
- 27.O’Donoghue G, Kennedy A, Andersen GS, et al. Phenotypic responses to a lifestyle Intervention do not account for inter-individual variability in glucose tolerance for individuals at high risk of type 2 Diabetes. Front Physiol. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922-933. [DOI] [PubMed] [Google Scholar]
- 29.Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. 2018;41(7):1526-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill J, Peer N, Oldenburg B, Kengne AP. Roles, responsibilities and characteristics of lay community health workers involved in diabetes prevention programmes: A systematic review. PLoS One. 2017;12(12):e0189069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Yang S, Sun K, Fisher EB, Sun X. How to achieve better effect of peer support among adults with type 2 diabetes: a meta-analysis of randomized clinical trials. Patient Educ Couns. 2016;99(2):186-197. [DOI] [PubMed] [Google Scholar]
- 32.Thankappan KR, Sathish T, Tapp RJ, et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: a cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018;15(6):e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakane N, Kotani K, Takahashi K, et al. Effects of telephone-delivered lifestyle support on the development of diabetes in participants at high risk of type 2 diabetes: J-DOIT1, a pragmatic cluster randomised trial. BMJ Open. 2015;5(8):e007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascale M, Murray N, Bachmann M, et al. Study protocol: the Norfolk Diabetes Prevention Study [NDPS]: a 46 month multi-centre, randomised, controlled parallel group trial of a lifestyle intervention [with or without additional support from lay lifestyle mentors with Type 2 diabetes] to prevent transition to type 2 diabetes in high risk groups with non-diabetic hyperglycaemia, or impaired fasting glucose. BMC Public Health. 2017;17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampson M, Elwell-Sutton T, Bachmann MO, et al. Discordance in glycemic categories and regression to normality at baseline in 10,000 people in a Type 2 diabetes prevention trial. Sci Rep. 2018;8(1):6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garner NJ, Pascale M, France K, et al. ; NDPS Group . Recruitment, retention, and training of people with type 2 diabetes as diabetes prevention mentors (DPM) to support a healthcare professional-delivered diabetes prevention program: the Norfolk Diabetes Prevention Study (NDPS). BMJ Open Diabetes Res Care. 2019;7(1):e000619. doi: 10.1136/bmjdrc-2018-000619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachmann MO, Lewis G, John WG, et al. ; Norfolk Diabetes Prevention Study . Determinants of diagnostic discordance for non-diabetic hyperglycemia and type 2 diabetes using paired glycated hemoglobin measurements in a large English primary care population: cross-sectional study. Diabet. Med. 2019;36(11):1478-1486. doi: 10.1111/dme.14111 [DOI] [PubMed] [Google Scholar]
- 39.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495. Review. doi: 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 40.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 41.Booth M Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2)(suppl):S114-S120. doi: 10.1080/02701367.2000.11082794 [DOI] [PubMed] [Google Scholar]
- 42.Shannon J, Kristal AR, Curry SJ, Beresford SA. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiol Biomarkers Prev. 1997;6(5):355-361. [PubMed] [Google Scholar]
- 43.Diabetes Prevention Program Research Group HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care. 2015;38(1):51-58. doi: 10.2337/dc14-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray LJ, Yates T, Troughton J, Khunti K, Davies MJ; Let’s Prevent Diabetes Team . Engagement, retention, and progression to type 2 diabetes: a retrospective analysis of the cluster-randomized “Let’s Prevent Diabetes” trial. PLoS Med. 2016;13(7):e1002078. doi: 10.1371/journal.pmed.1002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leal J, Ahrabian D, Davies MJ, et al. Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let’s Prevent Diabetes cluster-randomised controlled trial. BMJ Open. 2017;7(1):e013592. doi: 10.1136/bmjopen-2016-013592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26(9):2518-2523. doi: 10.2337/diacare.26.9.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damschroder LJ, Reardon CM, AuYoung M, et al. Implementation findings from a hybrid III implementation-effectiveness trial of the Diabetes Prevention Program (DPP) in the Veterans Health Administration (VHA). Implement Sci. 2017;12(1):94. doi: 10.1186/s13012-017-0619-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alva ML, Hoerger TJ, Jeyaraman R, Amico P, Rojas-Smith L. Impact of the YMCA Of The USA Diabetes Prevention Program on Medicare spending and utilization. Health Aff (Millwood). 2017;36(3):417-424. doi: 10.1377/hlthaff.2016.1307 [DOI] [PubMed] [Google Scholar]
- 49.Berger SE, Huggins GS, McCaffery JM, Lichtenstein AH. Comparison among criteria to define successful weight-loss maintainers and regainers in the Action for Health in Diabetes (Look AHEAD) and Diabetes Prevention Program trials. Am J Clin Nutr. 2017;106(6):1337-1346. doi: 10.3945/ajcn.117.157446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chao AM, Wadden TA, Berkowitz RI, et al. ; Look AHEAD Research Group . Weight change 2 years after termination of the intensive lifestyle intervention in the Look AHEAD Study. Obesity (Silver Spring). 2020;28(5):893-901. doi: 10.1002/oby.22769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitolins MZ, Blackwell CS, Katula JA, Isom SP, Case LD. Long-term weight loss maintenance in the continuation of a randomized diabetes prevention translational study: the Healthy Living Partnerships to Prevent Diabetes (HELP PD) continuation trial. Diabetes Care. 2019;42(9):1653-1660. doi: 10.2337/dc19-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apolzan JW, Venditti EM, Edelstein SL, et al. ; Diabetes Prevention Program Research Group . Long-term weight loss with metformin or lifestyle intervention in the Diabetes Prevention Program Outcomes Study. Ann Intern Med. 2019;170(10):682-690. doi: 10.7326/M18-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529-542. doi: 10.1007/s10654-015-0056-z [DOI] [PubMed] [Google Scholar]
- 54.Pedley CF, Case LD, Blackwell CS, Katula JA, Vitolins MZ. The 24-month metabolic benefits of the healthy living partnerships to prevent diabetes: a community-based translational study. Diabetes Metab Syndr. 2018;12(3):215-220. doi: 10.1016/j.dsx.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coventry PA, Bower P, Blakemore A, et al. Comparison of active treatments for impaired glucose regulation: a Salford Royal Foundation Trust and Hitachi collaboration (CATFISH): study protocol for a randomized controlled trial. Trials. 2016;17(1):424. doi: 10.1186/s13063-016-1519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Betzlbacher AF, Grady K, Savas L, et al. Behaviour change among people with impaired glucose tolerance: comparison of telephone-based and face-to-face advice. J Health Serv Res Policy. 2013;18(1)(suppl):2-6. doi: 10.1177/1355819612473582 [DOI] [PubMed] [Google Scholar]
- 57.Wijesuriya M, Fountoulakis N, Guess N, et al. A pragmatic lifestyle modification programme reduces the incidence of predictors of cardio-metabolic disease and dysglycaemia in a young healthy urban South Asian population: a randomised controlled trial. BMC Med. 2017;15(1):146. doi: 10.1186/s12916-017-0905-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi L, Liu Q, Qi X, Wu N, Tang W, Xiong H. Effectiveness of peer support for improving glycaemic control in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Public Health. 2015;15:471. doi: 10.1186/s12889-015-1798-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipska KJ, Inzucchi SE, Van Ness PH, et al. ; Health ABC Study . Elevated HbA1c and fasting plasma glucose in predicting diabetes incidence among older adults: are two better than one? Diabetes Care. 2013;36(12):3923-3929. doi: 10.2337/dc12-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bodicoat DH, Khunti K, Srinivasan BT, et al. Incident Type 2 diabetes and the effect of early regression to normoglycaemia in a population with impaired glucose regulation. Diabet Med. 2017;34(3):396-404. doi: 10.1111/dme.13091 [DOI] [PubMed] [Google Scholar]
- 61.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243-2251. doi: 10.1016/S0140-6736(12)60525-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palladino R, Vamos EP, Chang KC, Khunti K, Majeed A, Millett C. Evaluation of the diabetes screening component of a national cardiovascular risk assessment programme in England: a retrospective cohort study. Sci Rep. 2020;10(1):1231. doi: 10.1038/s41598-020-58033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robson J, Dostal I, Sheikh A, et al. The NHS Health Check in England: an evaluation of the first 4 years. BMJ Open. 2016;6(1):e008840. doi: 10.1136/bmjopen-2015-008840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carpenter JR, Kenward MG Missing data in randomised controlled trials: a practical guide. Accessed January 6, 2020. https://researchonline.lshtm.ac.uk/id/eprint/4018500
- 65.Roberts S, Pilard L, Chen J, Hirst J, Rutter H, Greenhalgh T. Efficacy of population-wide diabetes and obesity prevention programs: an overview of systematic reviews on proximal, intermediate, and distal outcomes and a meta-analysis of impact on BMI. Obes Rev. 2019;20(7):947-963. doi: 10.1111/obr.12821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Summary of the methods, secondary analyses, statistical analysis plan, and intervention, health economic analysis, and randomization methodology
eResults. Results for additional subgroup analyses by dose adherence
eTable 1. Percentage of individuals who progressed to Type 2 diabetes in each arm
eTable 2. Adjusted mean differences (95% CI) between trial arms at 12 months: linear regression models
eTable 3. Adjusted mean differences (95% CI) between arms at 24 months: linear regression models
eTable 4. Changes at 12 and 24 months by participants achieving higher ‘doses’ of intervention (moderate, or high)
eTable 5. Descriptive data for outcomes at 12 months by trial arm
eTable 6. Descriptive data for outcomes at 24 months by trial arm
eTable 7. Unadjusted mean differences arms at 12 months: linear regression models
eTable 8. Unadjusted mean differences arms at 24 months: linear regression models