Summary
Non-alcoholic fatty liver disease (NAFLD), specifically its progressive form non-alcoholic steatohepatitis (NASH), represents the fastest growing indication for liver transplantation in Western countries. Diabetes mellitus, morbid obesity and cardiovascular disease are frequently present in patients with NAFLD who are candidates for liver transplantation. These factors require specific evaluation, including a detailed pre-surgical risk stratification, in order to improve outcomes after liver transplantation. Moreover, in the post-transplantation setting, the incidence of cardiovascular events and metabolic complications can be amplified by immunosuppressive therapy, which is a well-known driver of metabolic alterations. Indeed, patients with NASH are more prone to developing early post-transplant complications and, in the long-term, de novo malignancy and cardiovascular events, corresponding to higher mortality rates. Therefore, a tailored multidisciplinary approach is required for these patients, both before and after liver transplantation. Appropriate candidate selection, lifestyle modifications and specific assessment in the pre-transplant setting, as well as pharmacological strategies, adjustment of immunosuppression and a healthy lifestyle in the post-transplant setting, play a key role in correct management.
Keywords: Solid organ transplantation, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Hypertension, New-onset diabetes after transplantation, Obesity, Graft survival, Immunosuppressant, Patient survival, Metabolic complication
Abbreviations: CKD, chronic kidney disease; CNI, calcineurin inhibitors; DM, diabetes mellitus; DPP-4, dipeptidyl peptidase-4; ELTR, European Liver Transplant Registry; ESLD, end-stage liver disease; GLP1 RAs, glucagon-like peptide-1 receptor agonists; HCC, hepatocellular carcinoma; HR, hazard ratio; IRR, incidence rate ratio; LT, liver transplant; MAFLD, metabolic dysfunction-associated fatty liver disease; mTORi, mammalian target of rapamycin inhibitors; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OR, odds ratio; SGLT2, sodium-glucose co-transporter-2; UNOS, United Network for Organ Sharing
Key points.
-
•
Non-alcoholic steatohepatitis (NASH) is the fastest growing indication for liver transplantation with consequences for pre- and post-transplant management.
-
•
Diabetes mellitus, obesity and cardiovascular disease are frequent in patients with NAFLD/NASH; these comorbidities require specific assessment and treatment in the pre-liver transplant setting to avoid unacceptably risky surgery.
-
•
Patients with NAFLD/NASH are at higher risk of metabolic complications after liver transplantation, which can be exacerbated by immunosuppressive therapy, and require specialised management.
-
•
Lifestyle modifications, modulation of immunosuppressive therapy and specific medical treatment of these metabolic conditions represent the key points for post-liver transplant management.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a pathological condition, which ranges from simple steatosis (NAFL) to non-alcoholic steatohepatitis (NASH) and cirrhosis. The liver is primarily involved – however, systemic metabolic complications are often present in patients with NAFLD.1 Recently the term metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed to better describe liver disease associated with metabolic dysfunction,2 leading to discussions within the scientific community regarding potential consequent changes in diagnosis, clinical management and drug development.[3], [4], [5]
Until now, NAFLD has been diagnosed by the presence of hepatic steatosis on radiological imaging or biopsy, after ruling out all other reasonable causes (e.g. alcohol consumption). Conversely, the diagnosis of NASH requires identification of 3 pathognomonic features on liver biopsy: ballooning, lobular inflammation and steatosis.6 Differentiating NASH from NAFLD is paramount for determining the prognosis of liver disease. Patients affected by NASH are at risk of worsening outcomes, such as end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC). It is estimated that 20% of patients with NASH develop cirrhosis, whereas the risk of HCC in patients with NASH increased 7.7-fold between 2002–2016, from 2.1% to 16.2%.7 Since the prevalence rates of NAFLD in patients with diabetes mellitus (DM) and morbid obesity are nearly 58%8 and 30%,9 respectively, the correct assessment of these metabolic diseases and their complications is relevant in liver transplant (LT) candidates as well as in the post-LT period.
However, controversial data still exist regarding strategies for optimal candidate evaluation before LT and optimal management of metabolic complications after LT. Therefore, the aim of this manuscript is to highlight available scientific evidence on the management of patients with NAFLD before and after LT, focusing on the assessment and treatment of cardio-metabolic complications.
Epidemiology and natural history of NAFLD in the LT setting
Before liver transplantation
According to data from the European Liver Transplant Registry (ELTR) and the United Network for Organ Sharing (UNOS) databases, NAFLD/NASH has been the fastest growing indication for LT in the last 20 years.10,11 It is the second leading indication for LT among adults in the United States, with 1,321 (21.5%) LTs performed in 2018,10 and a similar increase has been seen in Europe, with NAFLD-related cirrhosis accounting for 1.2% of LTs in 2002 compared to 8.4% in 2016.11 However, despite the significant increase over recent years in Europe, the prevalence remains lower than in the United States.
Similarly, NAFLD/NASH is considered the fastest growing cause of HCC in both Europe and the United States.7 In a recent modelling study, France is predicted to have the largest increase in incident HCC cases (117%), and the UK the smallest (88%). Similarly, in the United States the incidence of NAFLD/NASH-related HCC has been estimated to increase by 137% between 2015 and 2030.12
Several factors favour the development of HCC in the context of NAFLD/NASH, including genetic polymorphisms (i.e. PNPLA3 and TM6SF2) and environmental modifiers such as sedentary lifestyle and high-caloric intake, which lead to obesity and insulin resistance.13
Usually patients with NASH and HCC are older, have large tumours, and are sometimes difficult to screen with ultrasound because of obesity.14 Moreover, non-cirrhotic patients with NAFLD/NASH are usually not included in screening programme15 and are therefore often diagnosed late. It is estimated that only 15% of patients with HCC and NAFLD/NASH are diagnosed at Barcelona Clinic for Liver Cancer (BCLC) stages 0 or A, which would enable a curative approach to the neoplastic disease.13
Considering indications for LT, in the European cohort of ELTR, among a total of 68,950 LT recipients, 4% were transplanted for NASH, of whom 39.1% had HCC.16 In the United States, this indication is currently identified as the most rapidly growing cause of HCC among patients on the waiting list, increasing from 2.1% in 2002 to 16.2% in 2016; p <0.0001.7 Indeed, while the proportions of HCC related to HCV or alcohol-related liver disease remained stable, the proportion of HCC related to NAFLD/NASH increased 7.7 fold.7 Although attention has been paid to the potentially higher risk of death or drop-out in these patients while they are on the waiting list for LT,17 recent data seem to suggest that they are not disadvantaged in terms of waiting list removal or lower transplantation rates.18
After liver transplantation
After LT, patients with NAFLD are more prone to develop peri-surgical complications such as infections,19 whereas in the long-term, they have a higher incidence of malignancies (33%) and cardiovascular events (24%) compared to patients transplanted for other reasons.20 However, the incidence of major complications was similar in patients with NAFLD/NASH-related or non-NAFLD/NASH indications, as were patient and graft survival,19 with 1- and 5-year survival rates of 85%–90% and 70%–80% reported, respectively.21 Despite these encouraging data, a recent American study reported a lower 1-year survival rate for NAFLD/NASH recipients compared to patients transplanted for HCV or alcohol-related liver disease, showing that the higher risk of death was mainly due to cardiovascular or cerebrovascular disease.22
Considering all the metabolic complications potentially affecting patients after LT, the risk of developing post-transplant NAFLD/NASH can be considered high, although studies published on this topic are based on small sample sizes, with heterogeneous definitions of disease recurrence.23 In a meta-analysis, the mean 1-, 3-, and 5-year incidence rates of recurrent and de novo NAFLD were 59%, 57%, 82% and 67%, 40%, 78%, respectively. Conversely, the incidence of post-transplant NASH was significantly higher when considering recurrent NASH (53%, 57.4%, and 38% at 1, 3, 5 years after LT, respectively) compared with de novo NASH (13%, 16%, and 17%, respectively).23 Regarding advanced liver disease, the reported rates of cirrhosis in patients with recurrent or de novo NAFLD are around 11%–14% and 1% at 5 years post-LT, respectively.23
Post-transplant high BMI, post-transplant hyperlipidaemia, and a history of alcohol abuse are considered predictors of post-LT graft steatosis.23 Nevertheless, although allograft steatosis is extremely frequent, it does not seem to impact severely on post-LT outcomes.24 Conversely, the effect of histologically proven NASH on post-LT outcomes might be different from simple steatosis, representing a strong predictor of lower long-term survival.25 Moreover, data on re-LT outcomes in patients with NASH seem to suggest lower survival rates at 5-years after re-LT when compared to other aetiologies.26
Despite these data highlighting the importance of the early diagnosis of NAFLD/NASH post-LT, it is still unclear if this population needs specific follow-up, in terms of frequency and type of screening, to detect recurrent and de novo NAFLD/NASH early. Standard biochemical liver tests and imaging techniques, such as transient elastography27 or magnetic resonance elastography,28 are used to monitor patients at risk after LT.
In patients with HCC developed on NAFLD/NASH-related cirrhosis, post-LT patient and graft survival rates are comparable to those of other disease indications, as recently reported by a study based on the ELTR.16 Similarly, post-transplant tumour recurrence rates have been shown to be similar between NASH and non-NASH aetiologies.29
Metabolic complications
Diabetes mellitus
Pre-LT DM is often associated with liver diseases, such as HCV or NAFLD, with a prevalence of 33%–66% amongst patients with NAFLD.30 Patients with pre-LT DM are at higher risk of post-LT infections, cardiovascular complications and longer hospital stays,31 and present reduced long-term graft and overall survival.32 Two distinct mechanisms seem to be responsible for the development of DM in patients with chronic liver disease.33 The first is related to the impairment of insulin secretion or sensitivity due to a specific aetiological agent, such as HCV, alcohol or NAFLD. This condition can develop even before the development of cirrhosis, especially in patients presenting well-known risk factors for DM. The second, the so-called hepatogenous DM, is strictly related to pancreatic beta-cell dysfunction, which is proportional to liver disease severity and loss of liver function.33
Although the pathophysiological mechanisms underlying regression of hepatogenous diabetes are largely unknown, beta-cell restoration seems to play a central role.34 In this view, hepatogenous diabetes might benefit from LT. It is important to assess glycaemia in all potential LT candidates and to optimise the management of patients who show unsatisfactory glycaemic control.10
Oral glucose tolerance test is considered the gold standard for the diagnosis of type 2 DM; when glycated haemoglobin (HbA1c) is used it needs to be associated with continuous blood glucose monitoring, since HbA1c may be falsely low due to splenomegaly and anaemia, which are frequent conditions in patients with ESLD.33
Several issues need to be taken into account when considering pharmacological treatment of pre-LT DM, such as the development of side effects, the risk of hypoglycaemia, and potential limitations due to the presence of acute kidney injury.35 Moreover, specific guidelines for the use of anti-hyperglycaemic drugs are lacking, mainly because patients with cirrhosis are excluded from clinical trials,36 leaving insulin as the only completely safe treatment in cirrhotic patients regardless of the stage of liver disease. Oral agents can be used with caution in patients with Child-Pugh class A or B. In particular, metformin can be considered as first-line therapy, followed by any of the other therapeutic options, based on the type and extent of DM and the aetiology of liver disease. Patients with NAFLD-related cirrhosis may benefit from thiazolidinediones, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), and possibly dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose co-transporter-2 (SGLT2) inhibitors. However, caution should be taken and potential dose reductions evaluated, particularly for sulfonylureas or thiazolidinediones, as they are metabolised by the liver and their impact on disease progression is still unknown.35,37 Conversely, in patients with Child-Pugh class C, the use of non-insulin agents should be avoided and insulin remains the only safe treatment option (Table 1).35
Table 1.
Management of patients with NAFLD before liver transplantation.
| Condition | Recommendations |
|---|---|
| Diabetes mellitus |
|
| Nutritional status |
|
| Cardiovascular disease |
|
| Kidney dysfunction |
|
| Malignancies |
|
HbA1c, glycated haemoglobin; LT, liver transplant; NAFLD, non-alcoholic fatty liver disease; OGTT, oral glucose tolerance test.
After LT, the prevalence of DM ranges between 31% and 38%, whereas the prevalence of new-onset DM ranges between 13% and 28% in the first 3 years after surgery.38
DM severely affects the prognosis of LT recipients, with higher 10-year mortality,39 infection rates and cardiovascular events.40 Given this detrimental effect on post-transplant outcomes, euglycaemia is a primary goal in post-LT management.41
Male gender,42 ethnicity, family history41 and hepatitis C,43 are well-identified risk factors for the development of post-transplant DM. Once a diagnosis of DM is established, specific treatments need to be considered. Lifestyle changes represent the first-line treatment, but they are often not effective enough to achieve adequate glycaemic control. Among oral anti-diabetic drugs, metformin, rosiglitazone, pioglitazone and sulfonylureas have been studied and established as safe following solid organ transplantation, either alone or in association with insulin (to minimise its use).44,45 In the context of patients transplanted for NAFLD-related cirrhosis, therapeutic strategies effective against NASH, such as pioglitazone or liraglutide (GLP-1 RAs) may be preferred.35,46 Recently DPP-4 inhibitors have started to be routinely used in the setting of solid organ transplantation, with increasing attention to their adjunctive effect on weight loss. Nonetheless, the potential effect of DPP-4 inhibitors and GLP-1 RAs on the bioavailability of immunosuppressive drugs has not been completely clarified. In particular, specific interactions between sitagliptin and cyclosporine, as well as tacrolimus and vildagliptin, need to be investigated further.47,48 Lastly, no data are available for SGLT2 inhibitors (i.e. canagliflozin, dapagliflozin and empagliflozin) in the post-LT setting, although a recent trial has suggested that empagliflozin reduces liver fat and improves alanine aminotransferase levels in patients with type 2 DM and NAFLD.49 When therapeutic goals are not achieved or symptomatic metabolic decompensation is present, insulin remains the therapy of choice (Table 2).41
Table 2.
Management of patients with NAFLD after liver transplantation.
| Condition | Recommendations |
|---|---|
| Diabetes mellitus |
|
| Obesity |
|
| Dyslipidaemia |
|
| Arterial hypertension |
|
| Cardiovascular disease |
|
| Kidney dysfunction |
|
ACE, angiotensin-converting enzyme; CKD, chronic kidney disease; CNI, calcineurin inhibitors; DPP-4, dipeptidyl peptidase-4; GLP1 RAs, glucagon-like peptide-1 receptor agonists; LT, liver transplant; mTORi, mammalian target of rapamycin inhibitors.
Obesity
Obesity and being overweight are virtually constant in patients with NAFLD. Nearly 95% of patients with morbid obesity (BMI >40 kg/m2) present with NAFLD,50 and obese patients with NAFLD are often affected by sarcopenia and myosteatosis, which can negatively affect waiting list mortality and post-LT outcomes.51 Moreover, in patients with ESLD, a linear association has been described between high BMI and high rates of clinical decompensation, independently of the aetiology of liver disease.52 Obesity represents a relevant challenge in the LT setting, as it can make the procedure technically difficult, with potentially high rates of complications.53
Poorer outcomes in obese patients are mainly related to peri-transplant complications, such as infections and longer intensive care unit stays, whereas mid-term survival rates are similar to those of non-obese patients.54 These considerations led to several transplant centres applying specific BMI thresholds and weight loss requirements for LT candidates.
When dietary measures and physical exercise are insufficient for adequate weight loss, some pharmacological options can be considered. Among these, liraglutide, a GLP-1 RA, appears to be the most promising as it promotes weight loss as well as improving NASH. This drug was initially introduced only for its anti-diabetic properties, but it has recently been approved for the treatment of obesity in non-diabetic patients.55 In the LEAN trial, liraglutide was safe and led to resolution of NASH in 39% of patients (vs. 9% in the placebo group), although no data are available for patients with NAFLD/NASH-related cirrhosis and concerns remain as to whether this positive effect is due to weight loss rather than to the effect of the drug per se. Indeed, when the treatment arm was compared with the placebo arm, the reductions in absolute bodyweight (kg) and BMI (kg/m2) were −5.3 kg vs. −0.6 kg, p = 0.003 and −1.8 vs. −0.3, p = 0.005, respectively.56 Moreover, adverse gastrointestinal effects of GLP-1 analogues such as vomiting, diarrhoea and constipation, could represent an issue for patients with ESLD.
Conversely, orlistat is considered safe and effective in cirrhotic patients, and may have positive effects on NASH fibrosis and inflammation.57 In addition to behavioural therapy and exercise, it was reported to induce an 8% reduction in weight compared to a 5% reduction with placebo and the same behavioural component. However, high rates of gastrointestinal intolerance have been reported.58 Other anti-obesity medications, such as lorcaserin, phentermine hydrochloric, phentermine/topiramate (approved only by the FDA) and naltrexone/bupropion, although effective in reducing weight, have not been investigated in patients with NAFLD, particularly those with NAFLD-related cirrhosis.59
When morbid obesity is present, and standard interventions are ineffective, bariatric surgery can be considered, as it has been shown to lead to weight loss and improve fibrosis.60,61 However, despite recent encouraging data, there are concerns about the high decompensation rates when bariatric surgery is performed on cirrhotic patients with elevated hepatic venous pressure gradient.62
Bariatric surgery – performed after or at the same time as LT – has been confined to isolated experiences and case series. Among these, in 2018, 3-year outcomes in 13 patients who underwent LT and simultaneous sleeve gastrectomy were reported and compared with 36 patients who underwent standard weight loss intervention. At 3 years after LT, patients treated with LT + sleeve gastrectomy maintained a significantly higher percentage of total body weight loss compared with the control group (34.8 ± 17.3% vs. 3.9 ± 13.3%; p <0.001), with a lower prevalence of hypertension, insulin resistance, and hepatic steatosis at last follow-up (Table 1).63
After LT, weight gain tends to increase progressively over time, with reported obesity rates of 33.7% and 40.3% at 1- and 5-years post-transplant, respectively.64 Identified risk factors for post-LT obesity include age >50 years, pre-LT obesity and NASH as the indication for LT.65
Appropriate counselling before and immediately after LT is important to prevent post-transplant obesity and its related complications, such as the development of DM, graft steatosis, and de novo malignancies. Lifestyle modifications and a low-calorie diet represent the cornerstone of weight gain prevention after LT. Moreover, supervised physical activity is considered safe after LT in stable patients66 and effective for glucose homeostasis,67 which could be particularly beneficial in patients transplanted for NAFLD/NASH-related liver disease.
Pharmacological therapy can also be considered, although no data are available in the post-LT setting for liraglutide and no significant weight loss has been shown for orlistat.68
When indicated, bariatric surgery can be a therapeutic option; however, only small and heterogeneous case series have been published to date. All the studies reported effective weight loss, ranging between 21% and 75%, but with a high rate of mild-severe complications (30%–40%) and a mortality rate of 20% when gastric by-pass was performed.69
In the setting of LT, sleeve gastrectomy is always preferable to Roux-en-Y gastric bypass, not only because of concerns related to potential malabsorption of immunosuppressive drugs, but also the inaccessibility of the gastric remnant in case of gastric bleeding or if endoscopic access to the biliary tree is required.69
More recently endoscopic bariatric therapy has emerged as a potential approach for obese patients with NAFLD. This includes gastric and duodenal devices and techniques such as intragastric balloons, endoscopic sleeve gastroplasty, endoscopic small bowel by-pass and duodenal mucosal resurfacing, although the latter could interfere with adsorption of immunosuppressive drugs. Available data suggest that intragastric balloons induce rapid weight loss, leading to improvements in NAFLD parameters at least in the short-term, whereas endoscopic sleeve gastroplasty leads to stable excess body weight loss, and can therefore represent a long-term therapeutic option (Table 2).70
To date, no data are available for cirrhotic and transplanted patients, however we believe this might represent an effective strategy, especially in the post-transplant setting, although further investigations are needed.
Arterial hypertension
Arterial hypertension is present in 30%–50% of patients after LT, with prevalence rates of nearly 70% over long-term follow-up.71 The aetiology of post-LT hypertension is related not only to post-LT haemodynamic changes, but also to the use of immunosuppressive therapy, in particular calcineurin inhibitors (CNIs). Reducing the use of immunosuppressive drugs, as well as lifestyle modifications such as reducing sodium intake and stopping smoking, represent the first-line therapy to decrease the risk of arterial hypertension. However, specific pharmacological therapy is often necessary to reach a blood pressure target of <130/80 mmHg.72 Dihydropyridine calcium channel blockers represent the first choice, when patients do not exhibit proteinuria,73 otherwise, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers can be considered.73 Beta-blockers are indicated when heart decompensation or arrhythmia are present,74 whereas diuretic therapy is specifically indicated when volume overload exists (Table 2).75
Dyslipidaemia
Dyslipidaemia is common in the post-LT setting, with a prevalence rate ranging between 45% and 71% according to criteria used for its diagnosis.76 Particularly, hypertriglyceridaemia is more frequent in the early period, whereas hypercholesterolemia seems to increase later, with 30% of patients presenting with elevated cholesterol levels 1 year after LT.76 Immunosuppressive therapy and recipient characteristics, such as DM, obesity and genetic predispositions, represent the principal determinants of post-LT dyslipidaemia.77
Compared to the pre-transplant setting, dyslipidaemia developing after LT is usually refractory to dietary interventions, so a pharmacological approach is used more frequently. The European Society of Cardiology recently recommend very restrictive goals for the management of dyslipidaemia in solid organ transplanted patients, based on the same targets recommended for patients at high and very high cardiovascular risk.78 When a pharmacological therapy is necessary, statins are considered the first-line option in liver transplant recipients, although possible interactions with immunosuppressive therapy must be carefully monitored.79 Specifically, systemic exposure to statins may be increased in patients treated with cyclosporin, increasing the risk of myopathy. Conversely, despite tacrolimus also being metabolised by CYP3A4, it seems to interact less with statins than cyclosporin. Among the statins, fluvastatin, pravastatin, pitavastatin and rosuvastatin are metabolised through different CYP enzymes than the others and are therefore associated with fewer pharmacological interactions.80
Ezetimibe may be considered in recipients who do not tolerate statins,81 whereas the use of fibrates, although well tolerated, should be strictly monitored based on the potential development of myopathy.82 Patients with isolated post-LT hypertriglyceridaemia can benefit from omega-3 fish oil, which has few drug-drug interactions and some pleiotropic effects, including anti-inflammatory properties, which might be particularly beneficial in patients transplanted for NASH-related liver disease (Table 2).83
Cardiovascular disease
Patients with NAFLD-related cirrhosis present specific metabolic and cardiovascular alterations that need to be properly assessed both during the LT evaluation, as they could represent an absolute or relative contraindication to LT, and after LT, as they are one of the major causes of death in the post-LT period.
Patients with NAFLD are at higher risk of cardiovascular events compared to those without NAFLD (odds ratio [OR] 1.64, 95% CI 1.26–2.13), and the risk doubles when considering more severe NAFLD or NASH (OR 2.58; 95% CI 1.78–3.75).84 In patients with ESLD, severe peripheral vasodilatation can mask myocardial dysfunction, so-called cirrhotic cardiomyopathy, rendering pre-LT cardiovascular risk stratification difficult. This population not only exhibits morphological or kinetic cardiac alterations, but frequently also QTc interval prolongation,85 increasing the risk of ventricular arrhythmia86 when DM is present.87 Obstructive sleep apnoea and its cardiopulmonary-related complications also need to be considered, since they can increase the surgical risk during LT.88
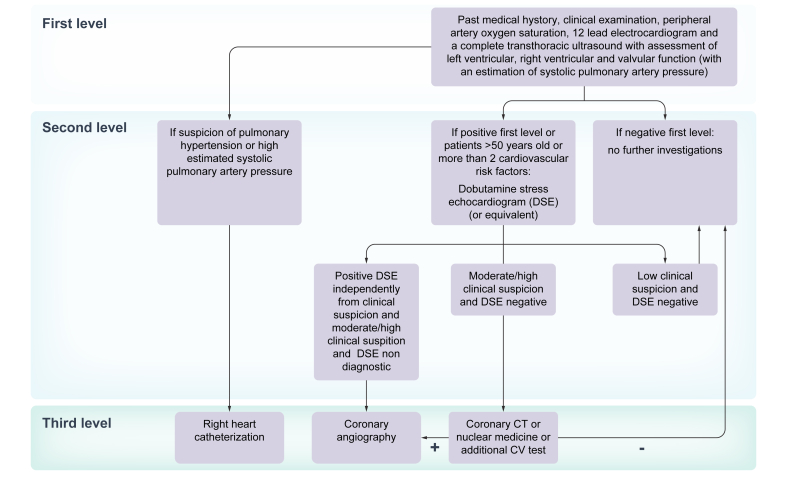
In patients undergoing evaluation for LT, the first level of cardiac assessments (i.e. history and clinical examination, electrocardiogram and a complete transthoracic ultrasound) should be followed by second-line examinations according to the specific cardiovascular risk stratification (Fig. 1). In patients >50 years old or with ≥2 cardiovascular risk factors (i.e. arterial hypertension, obesity, dyslipidaemia, diabetes), non-invasive stress testing (e.g. dobutamine stress echography or nuclear perfusion stress testing) should be performed.89,90 However, non-invasive functional testing has limited predictive value for obstructive coronary artery disease in patients with cirrhosis, in whom the sensitivity and specificity of dobutamine stress echocardiography are reduced.91,92
Fig. 1.
Cardiac work-up algorithm in the evaluation of patients with non-alcoholic fatty liver disease before liver transplantation.
Although coronary angiography is considered the gold standard, studies performed in LT candidates are not completely conclusive,93,94 making it difficult to predict the real impact of pre-LT asymptomatic cardiovascular abnormalities on long-term outcomes. However, a recent study has shown that pre-transplant coronary artery disease, when adequately treated, may not affect post-LT survival.95 Moreover, the use of statins in the post-transplant period might confer a survival benefit (hazard ratio [HR] 0.25; 95% CI 0.12–0.49; p <0.001), independently of the use of aspirin.95
An adequate pre-LT cardiovascular risk assessment is therefore crucial to stratify patients according to their probability of developing cardiovascular events after LT, enabling rapid diagnosis and optimal management. For this purpose, the use of the Framingham Risk Score (an algorithm to predict cardiovascular risk at 10 years based on age, sex, smoking, DM, arterial hypertension, and dyslipidaemia) has been proposed to predict cardiovascular risk after LT, and to tailor diagnostic tests and therapeutic strategies according to the risk score.96 More recently the CAR-OLT risk score (Cardiovascular Risk in Orthotopic Liver Transplantation) has been validated in the United States population. This score includes preoperative recipient variables such as age, sex, race, employment status, education status, history of HCC, diabetes, heart failure, atrial fibrillation, pulmonary or systemic hypertension, and respiratory failure. While solid recommendations cannot be made, it might be routinely applied to identify candidates who deserve further cardiological investigations, based on its discriminative performance (C statistic = 0.78, bias-corrected C statistic = 0.77), which is better than published risk models, and its easy availability.97 Moreover, because of the possibility of misdiagnosing cirrhotic cardiomyopathy, which may affect post-LT outcome, 6-monthly echocardiography has been proposed for patients with any degree of systolic/diastolic dysfunction on the LT waiting list, irrespective of liver disease aetiology.98 Further strategies include coronary CT angiography or cardiac MR as functional non-invasive tests to diagnose cardiac abnormalities. A preoperative coronary calcium score of >400 was found to predict cardiovascular events 1 year after LT,99 suggesting a possible role of coronary calcium scores in pre-surgical assessment. Despite concerns related to radiation exposure (for CT), lack of validation in the LT setting and cost, these techniques represent a very promising alternative for the study of pre-LT cardiac dysfunction.
After LT, patients with NASH present a significantly higher risk of death from cardiovascular complications than patients transplanted for other aetiologies, as shown by a recent meta-analysis.98 Interestingly, the incidence of post-transplant cardiovascular events seems significantly increased in the first year after transplant, as reported by VanWagner et al.,100 who observed an increase in cardiovascular-related 1-year mortality after LT in patients with NAFLD vs. patients transplanted for alcohol-related liver disease (26% vs. 8%). This difference was also evident after controlling for potential confounding factors such as age, sex, smoking habit, pre-transplant diabetes, cardiovascular disease, and the presence of metabolic syndrome.
The most common cardiovascular events developing post-LT in patients with NAFLD/NASH are acute pulmonary oedema (18%), new onset atrial fibrillation (10%) and sudden cardiac arrest (8%).100 In particular, the development of atrial fibrillation after LT can increase the risk of thromboembolism, representing an independent risk factor for post-transplant mortality.101
In a recent retrospective study, the authors reported a cardiovascular event rate of approximately 40% at 5 years after LT in patients with underlying NASH compared to 5% to 10% in non-NASH recipients. In this cohort, cardiovascular disease was defined as coronary artery disease, myocardial infarction, cardiac arrest, systolic or diastolic heart failure, arrhythmia, stroke, transient ischaemic attack, and symptomatic peripheral vascular disease.24
Specific risk factors have been associated with post-LT cardiovascular mortality, such as age >55 years old, male sex, DM, and kidney failure100; therefore, a strict cardiovascular follow-up should be performed with echocardiography at 6, 12, and 24 months after LT in patients with any of these risk factors (Table 2).98
Other medical complications
Kidney dysfunction
Additional risk factors are often present in patients with NAFLD/NASH and represent additional issues in the evaluation for LT and management after LT. Amongst these, chronic kidney disease (CKD) is one of the most critical,102 as a recent study has estimated that pre-LT CKD is significantly and independently associated with post-LT mortality (HR 1.16, p <0.001).103 Moreover, it has been demonstrated that NASH is an independent predictor of CKD stage ≥3 after LT (Tables 1 and 2).104
A recent study based on the UNOS database showed that NAFLD/NASH-cirrhosis is the most rapidly growing indication for simultaneous liver-kidney transplant in the United States. Five-year liver graft survival rates were comparable between patients with NASH and patients transplanted for other non-viral indications (78% vs. 74%, p = 0.14), whereas kidney graft outcome was 1.5-fold inferior.105
While combined liver-kidney transplantation should be reserved for those patients who fulfil the internationally recognised criteria,106 specific strategies in post-LT management should be reserved for patients with NASH who present with CKD at LT. These should mainly focus on immunosuppressive minimisation strategies, as well as the prevention or treatment of metabolic complications, such as diabetes and hypertension, which could further worsen renal function in the post-transplant period.
Malignancies
NAFLD is also associated with an increase in the risk of developing gastrointestinal tumours (stomach: incidence rate ratio [IRR] = 2.3; 95% CI 1.3–4.1, colon: IRR = 1.8; 95% CI 1.1–2.8) and uterine cancer (IRR = 2.3; 95% CI 1.4–4.1), as demonstrated in a recent longitudinal-study on a large cohort of patients with NAFLD.107 Interestingly, when NAFLD is not present, obesity and cancer are less associated, suggesting that NAFLD could increase the carcinogenetic effect of obesity. Although these data alone do not allow for any systematic recommendations, they highlight the importance of a complete and thorough evaluation of patients with NAFLD/NASH, especially if they are potential candidates for LT (Table 1).
Nutrition and physical exercise
Nutritional status assessment needs to be considered as part of the standard pre-LT evaluation in patients with NAFLD-related cirrhosis. This should focus not only on the identification of sarcopenic and malnourished patients but also on obese patients for whom the effect of ≥10% weight loss on liver fat and inflammation is well-established (Table 1).108
Similarly after LT, given the high risk of developing metabolic complications, a multidisciplinary approach is mandatory to promote lifestyle modifications, such as a balanced diet and adequate physical activity, starting in the early post-LT period.109
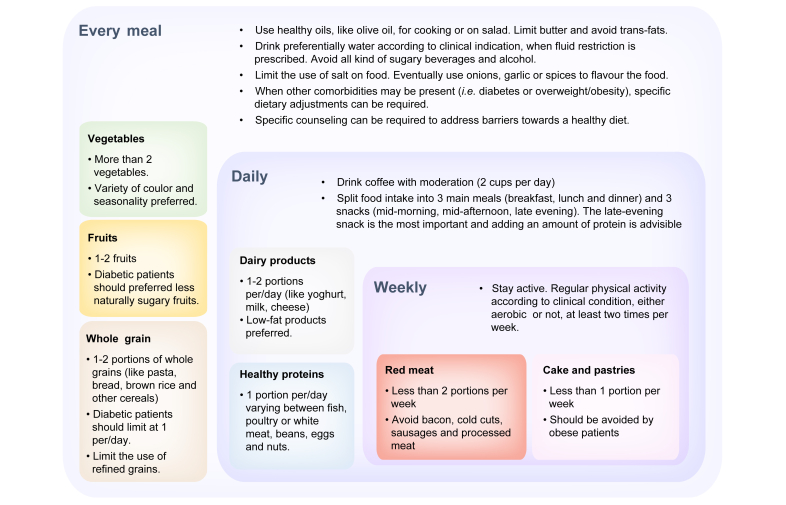
Dietary advice, based on large cohort-population studies and on patients with NAFLD/NASH,110 consists of the choice of a Mediterranean diet, preferring extra virgin olive oil111 to trans-fat foods, avoiding processed meats and increasing the consumption of fish rich in omega 3-fatty acids.112 Coffee consumption above 2 cups per day has proven to be effective in decreasing fibrosis in patients with NASH,113 although data on ESLD and in the post-LT setting are scarce (Fig. 2).
Fig. 2.
Practical dietary advice for patients with non-alcoholic steatohepatitis-related advanced liver disease according to the Mediterranean diet.
Considering physical activity, it has been shown that in patients listed for LT, maximal exercise capacity predicts 90-day post-transplant mortality114; it is therefore paramount to try to preserve as much residual motility as possible.
Physical activity has been proven to be beneficial and safe even in patients with moderately advanced portal hypertension,115 although LT candidates are often too sick to safely perform physical activities.
A pilot-study of 8 patients awaiting LT (mean Child Pugh score, 7 ± 3) showed that an adapted physical activity programme (cycloergometry and muscle strengthening) for 12 weeks might be feasible and effective. The authors recorded an increase in VO2 peak values (21.5 ± 5.9 m/kg per min vs. 23.2 ± 5.9 ml/kg; p <0.008), with an improvement in all parameters evaluated: maximum power (p = 0.02), 6-min walk distance (p <0.02), muscle strength (p = 0.008), respiratory threshold power (p = 0.02), and quality of life scale (36% ± 4% to 39% ± 3%) compared to baseline.116
In a recent review and meta-analysis117 based on 4 randomised controlled trials, including 81 patients, physical activity was not associated with severe adverse events, or worsening of liver function (measured by Child-Pugh and model for end-stage liver disease scores). Moreover, a significant reduction in HVPG in the treatment group was observed in a single study (mean difference −2.5 mmHg; 95% CI −4.76 to −0.24; p = 0.03).
Although data on patients with compensated cirrhosis are reassuring in confirming the effectiveness and safety of a combination of hypocaloric-normoproteic diet and moderate supervised physical activity,115 only few data exist in decompensated patients.
Moreover, it is important to note that exercise performed by malnourished and sarcopenic patients may be deleterious, leading to increased catabolism and muscle loss.118 The European Clinical Practice Guidelines on nutrition in chronic liver disease suggests adopting screening tools like the “Royal Free Hospital-nutritional prioritizing tool” in patients with normal BMI. When malnutrition is diagnosed, a thorough evaluation and consequent interventions need to be put in place.119
Three randomised controlled trials demonstrated that structured exercise training initiated early after LT improves recipients' exercise capacity and physical function,[120], [121], [122] although it is not yet known if the identified improvements are maintained in the long-term after completing the exercise programme.123
Moreover, despite the lack of well-designed randomised controlled trials, it is well-known that better quality of life is reported in patients who are physically active in the post-transplant period.120,124,125
Pharmacological therapy for NASH
To date, several new pharmacological therapies have been tested for NASH, although the majority have not received regulatory approved nor been evaluated in advanced liver disease. In patients without DM, the use of vitamin E can be considered. In patients with NASH-related cirrhosis, Vilar-Gomez et al.126 reported higher transplant-free survival rates (78% vs. 49%, p <0.01) and lower rates of hepatic decompensation (37% vs. 62%, p = 0.04) in patients treated with vitamin E compared to those who did not receive this therapy. However, concerns exist about the safety of vitamin E supplementation, based on reports of an increased risk of haemorrhagic stroke and prostate cancer, as well as controversial data on increased overall mortality.[127], [128], [129], [130]
In the setting of LT, where advanced or decompensated liver disease is prevalent, the principal goal of these therapies should be to counteract the natural history of the disease, rather than the regression of inflammation and fibrosis. However, most of these therapies are being tested in phase IIb/III trials in patients with NASH-related mild to moderate liver disease. Moreover, the endpoints are not homogeneously defined, making it difficult to perform comparisons in terms of effectiveness. Recently, emricasan was tested in a multicentre randomised placebo-controlled trial in patients with NASH-related ESLD and severe portal hypertension; unfortunately the results were negative.131 Conversely, obeticholic acid has been shown to be effective in improving fibrosis in patients with NASH and fibrosis stages F2-F3 or F1, although concerns still exist in patients with advanced liver disease.132
Management of immunosuppressive therapy
In patients transplanted for NAFLD/NASH-related cirrhosis, immunosuppressive protocols need to be optimised in order to minimise the risk of metabolic complications. This is essential not only to reduce the cardiovascular risk, but also to decrease the risk of developing graft steatosis, and consequently NAFLD/NASH recurrence (Table 2).133
DM is mainly affected by corticosteroid therapy, which is usually used early post-LT, and by tacrolimus. Although tacrolimus and cyclosporine share the same mechanism of damage, acting directly on pancreatic islets, the risk of developing or worsening DM is significantly higher with tacrolimus than with cyclosporine (relative risk 1.38, 95% CI 1.01–1.86).134 Therefore, in patients with DM, steroids should be withdrawn early after LT and immunosuppressive protocols which include minimising the tacrolimus dose should be preferred.
Concerning obesity, besides applying the same concepts as for patients with DM, a post hoc analysis of a previous trial designed to investigate the effect of everolimus on post-LT renal function135 showed that the early use of everolimus plus lower dose tacrolimus was associated with a weight reduction (although modest) at 1 and 2 years after LT.136
Arterial hypertension, hyperlipidaemia and hypertrigly-ceridaemia are mainly affected by the use of CNIs, with cyclosporine conferring a higher risk of these side effects than tacrolimus.137 Similarly, mammalian target of rapamycin inhibitors (mTORi) act on dyslipidaemia, particularly hypertriglyceridaemia.38 Therefore, patients with arterial hypertension and/or dyslipidaemia should be treated with immunosuppressive minimisation protocols and mTORi should be avoided.137,138
Although the relationship between cardiovascular events and immunosuppression has not been well established, tailoring immunosuppression to the patient's specific metabolic risk profile can be used to prevent post-LT cardiovascular morbidity. Moreover, since major cardiac events can be affected by post-LT renal impairment135 minimising CNI exposure is paramount, not only to spare kidney function, but also to indirectly reduce cardiovascular morbidity. Therefore, immunosuppression protocols based on mTORi or antimetabolites in association with reduced doses of tacrolimus, or tacrolimus withdrawal when feasible, can be considered an effective immunosuppressive strategy for patients transplanted for NASH-related cirrhosis.139
Conclusion
NAFLD/NASH-related cirrhosis is becoming one of the leading indications for LT in Western countries. Special issues in the pre-transplant work-up and post-transplant management need to be addressed to improve outcomes in these patients. Although short- and mid-term survival rates are comparable to those of patients transplanted for other indications, NAFLD/NASH recipients present higher post-transplant incidence of infections and cardiovascular events. Lifestyle modifications, which can counteract obesity and metabolic complications in both pre- and post-LT, as well as tailored use of immunosuppressive therapy remain the cornerstone of clinical management. Further prospective, longitudinal studies are needed to adequately highlight specific recommendations in order to achieve the best results for patients with NAFLD/NASH before and after LT.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
C.B. contributed to this paper with conception, literature review and writing the manuscript. G.G. and P.B. participated in drafting, critical revision and editing. All the authors approved the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100192.
Supplementary data
References
- 1.Kim C.H., Younossi Z.M. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75(10):721–728. doi: 10.3949/ccjm.75.10.721. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Kuchay M.S., Misra A. From non-alcoholic fatty liver disease (NAFLD) to metabolic-associated fatty liver disease (MAFLD): a journey over 40 years. Diabetes Metab Syndr. 2020;14(4):695–696. doi: 10.1016/j.dsx.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Fouad Y., Waked I., Bollipo S., Gomaa A., Ajlouni Y., Attia D. What's in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40(6):1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 5.Lin S., Huang J., Wang M., Kumar R., Liu Y., Liu S. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 6.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):85–89. doi: 10.1111/liv.13301. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z., Stepanova M., Ong J.P., Jacobson I.M., Bugianesi E., Duseja A. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755 e3. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Ong J.P., Elariny H., Collantes R., Younoszai A., Chandhoke V., Reines H.D. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15(3):310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 10.Cotter T.G., Charlton M. Nonalcoholic steatohepatitis after liver transplantation. Liver Transpl. 2020;26(1):141–159. doi: 10.1002/lt.25657. [DOI] [PubMed] [Google Scholar]
- 11.Adam R., Karam V., Cailliez V., Grady J.G.O., Mirza D., Cherqui D. 2018 Annual report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31(12):1293–1317. doi: 10.1111/tri.13358. [DOI] [PubMed] [Google Scholar]
- 12.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 14.Calzadilla Bertot L., Adams L.A. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolly P., Dufour J.F. Surveillance for hepatocellular carcinoma in patients with NASH. Diagnostics (Basel) 2016;6(2):22. doi: 10.3390/diagnostics6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldar D., Kern B., Hodson J., Armstrong M.J., Adam R., Berlakovich G. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71(2):313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young K., Aguilar M., Gish R., Younossi Z., Saab S., Bhuket T. Lower rates of receiving model for end-stage liver disease exception and longer time to transplant among nonalcoholic steatohepatitis hepatocellular carcinoma. Liver Transpl. 2016;22(10):1356–1366. doi: 10.1002/lt.24507. [DOI] [PubMed] [Google Scholar]
- 18.Thuluvath P.J., Hanish S., Savva Y. Waiting list mortality and transplant rates for NASH cirrhosis when compared with cryptogenic, alcoholic, or AIH cirrhosis. Transplantation. 2019;103(1):113–121. doi: 10.1097/TP.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg E.H., Douwes R.M., de Meijer V.E., Schreuder T., Blokzijl H. Liver transplantation for NASH cirrhosis is not performed at the expense of major post-operative morbidity. Dig Liver Dis. 2018;50(1):68–75. doi: 10.1016/j.dld.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Adam R., McMaster P., O'Grady J.G., Castaing D., Klempnauer J.L., Jamieson N. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9(12):1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Charlton M.R., Burns J.M., Pedersen R.A., Watt K.D., Heimbach J.K., Dierkhising R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 22.Nagai S., Collins K., Chau L.C., Safwan M., Rizzari M., Yoshida A. Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Clin Gastroenterol Hepatol. 2019;17(13):2759–2768.e5. doi: 10.1016/j.cgh.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Saeed N., Glass L., Sharma P., Shannon C., Sonnenday C.J., Tincopa M.A. Incidence and risks for nonalcoholic fatty liver disease and steatohepatitis post-liver transplant: systematic review and meta-analysis. Transplantation. 2019;103(11):e345–e354. doi: 10.1097/TP.0000000000002916. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan P., Mara K., Izzy M., Dierkhising R., Heimbach J., Allen A.M. Recurrent or de novo allograft steatosis and long-term outcomes after liver transplantation. Transplantation. 2019;103(1):e14–e21. doi: 10.1097/TP.0000000000002317. [DOI] [PubMed] [Google Scholar]
- 25.Gitto S., de Maria N., di Benedetto F., Tarantino G., Serra V., Maroni L. De-novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol. 2018;30(7):766–773. doi: 10.1097/MEG.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 26.Thuluvath A.J., Chen P.H., Thuluvath P.J., Kantsevoy S., Savva Y. Poor survival after retransplantation in NASH cirrhosis. Transplantation. 2019;103(1):101–108. doi: 10.1097/TP.0000000000002135. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffenberger J., Hornuss D., Houben P., Wehling C., Von Haken R., Loszanowski V. Routine liver elastography could predict actuarial survival after liver transplantation. J Gastrointestin Liver Dis. 2019;28(3):271–277. doi: 10.15403/jgld-218. [DOI] [PubMed] [Google Scholar]
- 28.Singh S., Venkatesh S.K., Keaveny A., Adam S., Miller F.H., Asbach P. Diagnostic accuracy of magnetic resonance elastography in liver transplant recipients: a pooled analysis. Ann Hepatol. 2016;15(3):363–376. doi: 10.5604/16652681.1198808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadler E.M., Mehta N., Bhat M., Ghanekar A., Greig P.D., Grant D.R. Liver transplantation for NASH-related hepatocellular carcinoma versus non-NASH etiologies of hepatocellular carcinoma. Transplantation. 2018;102(4):640–647. doi: 10.1097/TP.0000000000002043. [DOI] [PubMed] [Google Scholar]
- 30.Leite N.C., Salles G.F., Araujo A.L., Villela-Nogueira C.A., Cardoso C.R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29(1):113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 31.John P.R., Thuluvath P.J. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002;8(8):708–713. doi: 10.1053/jlts.2002.34638. [DOI] [PubMed] [Google Scholar]
- 32.Younossi Z.M., Stepanova M., Saab S., Kalwaney S., Clement S., Henry L. The impact of type 2 diabetes and obesity on the long-term outcomes of more than 85 000 liver transplant recipients in the US. Aliment Pharmacol Ther. 2014;40(6):686–694. doi: 10.1111/apt.12881. [DOI] [PubMed] [Google Scholar]
- 33.Orsi E., Grancini V., Menini S., Aghemo A., Pugliese G. Hepatogenous diabetes: is it time to separate it from type 2 diabetes? Liver Int. 2017;37(7):950–962. doi: 10.1111/liv.13337. [DOI] [PubMed] [Google Scholar]
- 34.Grancini V., Trombetta M., Lunati M.E., Boselli M.L., Gatti S., Donato M.F. Central role of the beta-cell in driving regression of diabetes after liver transplantation in cirrhotic patients. J Hepatol. 2019;70(5):954–962. doi: 10.1016/j.jhep.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Grancini V., Resi V., Palmieri E., Pugliese G., Orsi E. Management of diabetes mellitus in patients undergoing liver transplantation. Pharmacol Res. 2019;141:556–573. doi: 10.1016/j.phrs.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Gangopadhyay K.K., Singh P. Consensus statement on dose modifications of antidiabetic agents in patients with hepatic impairment. Indian J Endocrinol Metab. 2017;21(2):341–354. doi: 10.4103/ijem.IJEM_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 38.Watt K.D. Metabolic syndrome: is immunosuppression to blame? Liver Transpl. 2011;17(Suppl 3):S38–S42. doi: 10.1002/lt.22386. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Prol A., Hervas-Marin D., Garcia-Castell A., Merino-Torres J.F. Outcomes in patients with diabetes 10 years after liver transplantation. J Diabetes. 2017;9(11):1033–1039. doi: 10.1111/1753-0407.12520. [DOI] [PubMed] [Google Scholar]
- 40.Bhat V., Tazari M., Watt K.D., Bhat M. New-onset diabetes and preexisting diabetes are associated with comparable reduction in long-term survival after liver transplant: a machine learning approach. Mayo Clin Proc. 2018;93(12):1794–1802. doi: 10.1016/j.mayocp.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Davidson J.A., Wilkinson A. International expert panel on new-onset diabetes after T. New-onset diabetes after transplantation 2003 international consensus guidelines: an endocrinologist's view. Diabetes Care. 2004;27(3):805–812. doi: 10.2337/diacare.27.3.805. [DOI] [PubMed] [Google Scholar]
- 42.Saab S., Shpaner A., Zhao Y., Brito I., Durazo F., Han S. Prevalence and risk factors for diabetes mellitus in moderate term survivors of liver transplantation. Am J Transplant. 2006;6(8):1890–1895. doi: 10.1111/j.1600-6143.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 43.Delgado-Borrego A., Casson D., Schoenfeld D., Somsouk M., Terella A., Jordan S.H. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77(5):703–710. doi: 10.1097/01.tp.0000114283.04840.3a. [DOI] [PubMed] [Google Scholar]
- 44.Luther P., Baldwin J.r., D. Pioglitazone in the management of diabetes mellitus after transplantation. Am J Transplant. 2004;4(12):2135–2138. doi: 10.1111/j.1600-6143.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- 45.Villanueva G., Baldwin D. Rosiglitazone therapy of posttransplant diabetes mellitus. Transplantation. 2005;80(10):1402–1405. doi: 10.1097/01.tp.0000181165.19788.95. [DOI] [PubMed] [Google Scholar]
- 46.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 47.Bae J., Lee M.J., Choe E.Y., Jung C.H., Wang H.J., Kim M.S. Effects of dipeptidyl peptidase-4 inhibitors on hyperglycemia and blood cyclosporine levels in renal transplant patients with diabetes: a pilot study. Endocrinol Metab (Seoul) 2016;31(1):161–167. doi: 10.3803/EnM.2016.31.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanyal D., Gupta S., Das P. A retrospective study evaluating efficacy and safety of linagliptin in treatment of NODAT (in renal transplant recipients) in a real world setting. Indian J Endocrinol Metab. 2013;17(Suppl 1):S203–S205. doi: 10.4103/2230-8210.119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuchay M.S., Krishan S., Mishra S.K., Farooqui K.J., Singh M.K., Wasir J.S. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial) Diabetes Care. 2018;41(8):1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki A., Nitta H., Otsuka K., Umemura A., Baba S., Obuchi T. Bariatric surgery and non-alcoholic fatty liver disease: current and potential future treatments. Front Endocrinol (Lausanne) 2014;5:164. doi: 10.3389/fendo.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czigany Z., Kramp W., Bednarsch J., van der Kroft G., Boecker J., Strnad P. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am J Transplant. 2020;20(2):493–503. doi: 10.1111/ajt.15577. [DOI] [PubMed] [Google Scholar]
- 52.Berzigotti A., Garcia-Tsao G., Bosch J., Grace N.D., Burroughs A.K., Morillas R. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54(2):555–561. doi: 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundaram V., Kaung A., Rajaram A., Lu S.C., Tran T.T., Nissen N.N. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther. 2015;42(11–12):1271–1280. doi: 10.1111/apt.13426. [DOI] [PubMed] [Google Scholar]
- 54.LaMattina J.C., Foley D.P., Fernandez L.A., Pirsch J.D., Musat A.I., D'Alessandro A.M. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26(6):910–918. doi: 10.1111/j.1399-0012.2012.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nuffer W.A., Trujillo J.M. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy. 2015;35(10):926–934. doi: 10.1002/phar.1639. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 57.Assy N., Hussein O., Abassi Z. Weight loss induced by orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with non-alcoholic steatohepatitis. Gut. 2007;56(3):443–444. doi: 10.1136/gut.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leblanc E.S., O'Connor E., Whitlock E.P., Patnode C.D., Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 59.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Weiner R.A. Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):274–279. doi: 10.1159/000282102. [DOI] [PubMed] [Google Scholar]
- 61.Furuya J.r., C.K., de Oliveira C.P., de Mello E.S., Faintuch J., Raskovski A., Matsuda M. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22(4):510–514. doi: 10.1111/j.1440-1746.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- 62.Reverter E., Cirera I., Albillos A., Debernardi-Venon W., Abraldes J.G., Llop E. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J Hepatol. 2019;71(5):942–950. doi: 10.1016/j.jhep.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Zamora-Valdes D., Watt K.D., Kellogg T.A., Poterucha J.J., Di Cecco S.R., Francisco-Ziller N.M. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology. 2018;68(2):485–495. doi: 10.1002/hep.29848. [DOI] [PubMed] [Google Scholar]
- 64.Richards J., Gunson B., Johnson J., Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18(4):461–466. doi: 10.1111/j.1432-2277.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 65.Everhart J.E., Lombardero M., Lake J.R., Wiesner R.H., Zetterman R.K., Hoofnagle J.H. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4(4):285–296. doi: 10.1002/lt.500040402. [DOI] [PubMed] [Google Scholar]
- 66.Neuberger J., Armstrong M.J., Fisher J., Mark P., Schmidtke K., Sharif A. Sport and exercise in improving outcomes after solid organ transplantation: overview from a UK meeting. Transplantation. 2019;103(7S1 Suppl 1):S1–S11. doi: 10.1097/TP.0000000000002710. [DOI] [PubMed] [Google Scholar]
- 67.Totti V., Tame M., Burra P., Mosconi G., Roi G.S., Sella G. Physical condition, glycemia, liver function, and quality of life in liver transplant recipients after a 12-month supervised exercise program. Transplant Proc. 2019;51(9):2952–2957. doi: 10.1016/j.transproceed.2019.03.087. [DOI] [PubMed] [Google Scholar]
- 68.Cassiman D., Roelants M., Vandenplas G., Van der Merwe S.W., Mertens A., Libbrecht L. Orlistat treatment is safe in overweight and obese liver transplant recipients: a prospective, open label trial. Transpl Int. 2006;19(12):1000–1005. doi: 10.1111/j.1432-2277.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 69.Diwan T.S., Rice T.C., Heimbach J.K., Schauer D.P. Liver transplantation and bariatric surgery: timing and outcomes. Liver Transpl. 2018;24(9):1280–1287. doi: 10.1002/lt.25303. [DOI] [PubMed] [Google Scholar]
- 70.Salomone F., Sharaiha R.Z., Boskoski I. Endoscopic bariatric and metabolic therapies for non-alcoholic fatty liver disease: evidence and perspectives. Liver Int. 2020;40(6):1262–1268. doi: 10.1111/liv.14441. [DOI] [PubMed] [Google Scholar]
- 71.Gojowy D., Adamczak M., Dudzicz S., Gazda M., Karkoszka H., Wiecek A. High frequency of arterial hypertension in patients after liver transplantation. Transplant Proc. 2016;48(5):1721–1724. doi: 10.1016/j.transproceed.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Issa D.H., Alkhouri N. Long-term management of liver transplant recipients: a review for the internist. Cleve Clin J Med. 2015;82(6):361–372. doi: 10.3949/ccjm.82a.14072. [DOI] [PubMed] [Google Scholar]
- 73.Guckelberger O. Long-term medical comorbidities and their management: hypertension/cardiovascular disease. Liver Transpl. 2009;15(Suppl 2):S75–S78. doi: 10.1002/lt.21903. [DOI] [PubMed] [Google Scholar]
- 74.Galioto A., Semplicini A., Zanus G., Fasolato S., Sticca A., Boccagni P. Nifedipine versus carvedilol in the treatment of de novo arterial hypertension after liver transplantation: results of a controlled clinical trial. Liver Transpl. 2008;14(7):1020–1028. doi: 10.1002/lt.21442. [DOI] [PubMed] [Google Scholar]
- 75.Fussner L.A., Heimbach J.K., Fan C., Dierkhising R., Coss E., Leise M.D. Cardiovascular disease after liver transplantation: when, what, and who is at risk. Liver Transpl. 2015;21(7):889–896. doi: 10.1002/lt.24137. [DOI] [PubMed] [Google Scholar]
- 76.Sheiner P.A., Magliocca J.F., Bodian C.A., Kim-Schluger L., Altaca G., Guarrera J.V. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000;69(5):781–789. doi: 10.1097/00007890-200003150-00018. [DOI] [PubMed] [Google Scholar]
- 77.Ling Q., Wang K., Lu D., Guo H.J., Jiang W.S., He X.X. Major influence of renal function on hyperlipidemia after living donor liver transplantation. World J Gastroenterol. 2012;18(47):7033–7039. doi: 10.3748/wjg.v18.i47.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 79.Watt K.D., Charlton M.R. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol. 2010;53(1):199–206. doi: 10.1016/j.jhep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 80.Page R.L., 2nd, Miller G.G., Lindenfeld J. Drug therapy in the heart transplant recipient: part IV: drug-drug interactions. Circulation. 2005;111(2):230–239. doi: 10.1161/01.CIR.0000151805.86933.35. [DOI] [PubMed] [Google Scholar]
- 81.Almutairi F., Peterson T.C., Molinari M., Walsh M.J., Alwayn I., Peltekian K.M. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009;15(5):504–508. doi: 10.1002/lt.21710. [DOI] [PubMed] [Google Scholar]
- 82.Barnard A., Konyn P., Saab S. Medical management of metabolic complications of liver transplant recipients. Gastroenterol Hepatol (N Y) 2016;12(10):601–608. [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S., Gura K.M., Puder M. Omega-3 fatty acids and liver disease. Hepatology. 2007;45(4):841–845. doi: 10.1002/hep.21645. [DOI] [PubMed] [Google Scholar]
- 84.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 85.Hung C.S., Tseng P.H., Tu C.H., Chen C.C., Liao W.C., Lee Y.C. Nonalcoholic fatty liver disease is associated with QT prolongation in the general population. J Am Heart Assoc. 2015;4(7):e001820. doi: 10.1161/JAHA.115.001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mantovani A., Rigamonti A., Bonapace S., Bolzan B., Pernigo M., Morani G. Nonalcoholic fatty liver disease is associated with ventricular arrhythmias in patients with type 2 diabetes referred for clinically indicated 24-hour holter monitoring. Diabetes Care. 2016;39(8):1416–1423. doi: 10.2337/dc16-0091. [DOI] [PubMed] [Google Scholar]
- 87.Targher G., Valbusa F., Bonapace S., Bertolini L., Zenari L., Pichiri I. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;24(6):663–669. doi: 10.1016/j.numecd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Pereira H., Xara D., Mendonca J., Santos A., Abelha F.J. Patients with a high risk for obstructive sleep apnea syndrome: postoperative respiratory complications. Rev Port Pneumol. 2013;19(4):144–151. doi: 10.1016/j.rppneu.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Sanchez-Torrijos Y., Ampuero J., Romero-Gomez M. Cardiovascular assessment in liver transplant for non-alcoholic steatohepatitis patients: what we do, what we should do. World J Hepatol. 2017;9(15):697–703. doi: 10.4254/wjh.v9.i15.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Ripoll C., Yotti R., Bermejo J., Banares R. The heart in liver transplantation. J Hepatol. 2011;54(4):810–822. doi: 10.1016/j.jhep.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Tsochatzis E., Coilly A., Nadalin S., Levistky J., Tokat Y., Ghobrial M. International liver transplantation consensus statement on end-stage liver disease due to nonalcoholic steatohepatitis and liver transplantation. Transplantation. 2019;103(1):45–56. doi: 10.1097/TP.0000000000002433. [DOI] [PubMed] [Google Scholar]
- 93.Patel S.S., Nabi E., Guzman L., Abbate A., Bhati C., Stravitz R.T. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl. 2018;24(3):333–342. doi: 10.1002/lt.25012. [DOI] [PubMed] [Google Scholar]
- 94.Romero-Cristobal M., Mombiela T., Caballero A., Clemente A., Fernandez-Yunquera A., Diaz-Fontenla F. Clinical utility of a risk-adapted protocol for the evaluation of coronary artery disease in liver transplant recipients. Liver Transpl. 2019;25(8):1177–1186. doi: 10.1002/lt.25493. [DOI] [PubMed] [Google Scholar]
- 95.Patel S.S., Rodriguez V.A., Siddiqui M.B., Faridnia M., Lin F.P., Chandrakumaran A. The impact of coronary artery disease and statins on survival after liver transplantation. Liver Transpl. 2019;25(10):1514–1523. doi: 10.1002/lt.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Maira T., Rubin A., Puchades L., Aguilera V., Vinaixa C., Garcia M. Framingham score, renal dysfunction, and cardiovascular risk in liver transplant patients. Liver Transpl. 2015;21(6):812–822. doi: 10.1002/lt.24128. [DOI] [PubMed] [Google Scholar]
- 97.VanWagner L.B., Ning H., Whitsett M., Levitsky J., Uttal S., Wilkins J.T. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: the CAR-OLT score. Hepatology. 2017;66(6):1968–1979. doi: 10.1002/hep.29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Izzy M., VanWagner L.B., Lin G., Altieri M., Findlay J.Y., Oh J.K. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology. 2020;71(1):334–345. doi: 10.1002/hep.30875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kong Y.G., Kang J.W., Kim Y.K., Seo H., Lim T.H., Hwang S. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br J Anaesth. 2015;114(3):437–443. doi: 10.1093/bja/aeu384. [DOI] [PubMed] [Google Scholar]
- 100.Vanwagner L.B., Bhave M., Te H.S., Feinglass J., Alvarez L., Rinella M.E. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56(5):1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 101.Rachwan R.J., Kutkut I., Hathaway T.J., Timsina L.R., Kubal C.A., Lacerda M.A. Postoperative atrial fibrillation and flutter in liver transplantation: an important predictor of early and late morbidity and mortality. Liver Transpl. 2020;26(1):34–44. doi: 10.1002/lt.25631. [DOI] [PubMed] [Google Scholar]
- 102.Yasui K., Sumida Y., Mori Y., Mitsuyoshi H., Minami M., Itoh Y. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60(5):735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 103.Musso G., Gambino R., Tabibian J.H., Ekstedt M., Kechagias S., Hamaguchi M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fussner L.A., Charlton M.R., Heimbach J.K., Fan C., Dierkhising R., Coss E. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34(8):1259–1266. doi: 10.1111/liv.12381. [DOI] [PubMed] [Google Scholar]
- 105.Singal A.K., Hasanin M., Kaif M., Wiesner R., Kuo Y.F. Nonalcoholic steatohepatitis is the most rapidly growing indication for simultaneous liver kidney transplantation in the United States. Transplantation. 2016;100(3):607–612. doi: 10.1097/TP.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 106.Bunnapradist S., Danovitch G.M. Evaluation of adult kidney transplant candidates. Am J Kidney Dis. 2007;50(5):890–898. doi: 10.1053/j.ajkd.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Allen A.M., Hicks S.B., Mara K.C., Larson J.J., Therneau T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - a longitudinal cohort study. J Hepatol. 2019;71(6):1229–1236. doi: 10.1016/j.jhep.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378.e5. doi: 10.1053/j.gastro.2015.04.005. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 109.Lunati M.E., Grancini V., Agnelli F., Gatti S., Masserini B., Zimbalatti D. Metabolic syndrome after liver transplantation: short-term prevalence and pre- and post-operative risk factors. Dig Liver Dis. 2013;45(10):833–839. doi: 10.1016/j.dld.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 110.Della Corte C., Mosca A., Vania A., Alterio A., Iasevoli S., Nobili V. Good adherence to the mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: the results of an Italian study. Nutrition. 2017;39-40:8–14. doi: 10.1016/j.nut.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 111.Buettner R., Ascher M., Gabele E., Hellerbrand C., Kob R., Bertsch T. Olive oil attenuates the cholesterol-induced development of nonalcoholic steatohepatitis despite increased insulin resistance in a rodent model. Horm Metab Res. 2013;45(11):795–801. doi: 10.1055/s-0033-1353209. [DOI] [PubMed] [Google Scholar]
- 112.Suarez M., Boque N., Del Bas J.M., Mayneris-Perxachs J., Arola L., Caimari A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients. 2017;9(10):1052. doi: 10.3390/nu9101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molloy J.W., Calcagno C.J., Williams C.D., Jones F.J., Torres D.M., Harrison S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55(2):429–436. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 114.Epstein S.K., Freeman R.B., Khayat A., Unterborn J.N., Pratt D.S., Kaplan M.M. Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transpl. 2004;10(3):418–424. doi: 10.1002/lt.20088. [DOI] [PubMed] [Google Scholar]
- 115.Berzigotti A., Albillos A., Villanueva C., Genesca J., Ardevol A., Augustin S. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65(4):1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 116.Debette-Gratien M., Tabouret T., Antonini M.T., Dalmay F., Carrier P., Legros R. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99(1):145–150. doi: 10.1097/TP.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 117.Brustia R., Savier E., Scatton O. Physical exercise in cirrhotic patients: towards prehabilitation on waiting list for liver transplantation. A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42(3):205–215. doi: 10.1016/j.clinre.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Berzigotti A., Saran U., Dufour J.F. Physical activity and liver diseases. Hepatology. 2016;63(3):1026–1040. doi: 10.1002/hep.28132. [DOI] [PubMed] [Google Scholar]
- 119.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the Liver EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krasnoff J.B., Vintro A.Q., Ascher N.L., Bass N.M., Paul S.M., Dodd M.J. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6(8):1896–1905. doi: 10.1111/j.1600-6143.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 121.Garcia A.M., Veneroso C.E., Soares D.D., Lima A.S., Correia M.I. Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplant Proc. 2014;46(6):1807–1808. doi: 10.1016/j.transproceed.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 122.Moya-Najera D., Moya-Herraiz A., Compte-Torrero L., Hervas D., Borreani S., Calatayud J. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl. 2017;23(10):1273–1281. doi: 10.1002/lt.24827. [DOI] [PubMed] [Google Scholar]
- 123.Dunn M.A., Rogal S.S., Duarte-Rojo A., Lai J.C. Physical function, physical activity, and quality of life after liver transplantation. Liver Transpl. 2020;26(5):702–708. doi: 10.1002/lt.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rongies W., Stepniewska S., Lewandowska M., Smolis-Bak E., Dolecki W., Sierdzinski J. Physical activity long-term after liver transplantation yields better quality of life. Ann Transplant. 2011;16(3):126–131. doi: 10.12659/aot.882005. [DOI] [PubMed] [Google Scholar]
- 125.Painter P., Krasnoff J., Paul S.M., Ascher N.L. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. 2001;7(3):213–219. doi: 10.1053/jlts.2001.22184. [DOI] [PubMed] [Google Scholar]
- 126.Vilar-Gomez E., Vuppalanchi R., Gawrieh S., Ghabril M., Saxena R., Cummings O.W. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology. 2020;71(2):495–509. doi: 10.1002/hep.30368. [DOI] [PubMed] [Google Scholar]
- 127.Abner E.L., Schmitt F.A., Mendiondo M.S., Marcum J.L., Kryscio R.J. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4(2):158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ballon-Landa E., Parsons J.K. Nutrition, physical activity, and lifestyle factors in prostate cancer prevention. Curr Opin Urol. 2018;28(1):55–61. doi: 10.1097/MOU.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 129.Schurks M., Glynn R.J., Rist P.M., Tzourio C., Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klein E.A., Thompson I.M., Jr., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garcia-Tsao G., Bosch J., Kayali Z., Harrison S.A., Abdelmalek M.F., Lawitz E. Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72(5):885–895. doi: 10.1016/j.jhep.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 132.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 133.Germani G., Laryea M., Rubbia-Brandt L., Egawa H., Burra P., O'Grady J. Management of recurrent and de novo NAFLD/NASH after liver transplantation. Transplantation. 2019;103(1):57–67. doi: 10.1097/TP.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 134.Haddad E.M., McAlister V.C., Renouf E., Malthaner R., Kjaer M.S., Gluud L.L. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;(4):CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saliba F., De Simone P., Nevens F., De Carlis L., Metselaar H.J., Beckebaum S. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13(7):1734–1745. doi: 10.1111/ajt.12280. [DOI] [PubMed] [Google Scholar]
- 136.Charlton M., Rinella M., Patel D., McCague K., Heimbach J., Watt K. Everolimus is associated with less weight gain than tacrolimus 2 Years after liver transplantation: results of a randomized multicenter study. Transplantation. 2017;101(12):2873–2882. doi: 10.1097/TP.0000000000001913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hernandez D., Alvarez A., Torres A., Oppenheimer F., Cobo M., Gonzalez-Posada J. Cardiovascular risk profile in nondiabetic renal transplant patients: cyclosporine versus tacrolimus. Transplant Proc. 2003;35(5):1727–1729. doi: 10.1016/s0041-1345(03)00630-4. [DOI] [PubMed] [Google Scholar]
- 138.Trotter J.F., Wachs M.E., Trouillot T.E., Bak T., Kugelmas M., Kam I. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transpl. 2001;7(5):401–408. doi: 10.1053/jlts.2001.23916. [DOI] [PubMed] [Google Scholar]
- 139.De Simone P., Fagiuoli S., Cescon M., De Carlis L., Tisone G., Volpes R. Use of everolimus in liver transplantation: recommendations from a working group. Transplantation. 2017;101(2):239–251. doi: 10.1097/TP.0000000000001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.