Abstract
Background
Cellular senescence, measured by expression of the cell cycle kinase inhibitor p16INK4a, may contribute to accelerated aging in survivors of childhood, adolescent, and young adult cancer. The authors measured peripheral blood T‐lymphocyte p16INK4a expression among pediatric and young adult cancer survivors, hypothesizing that p16INK4a expression is higher after chemotherapy and among frail survivors.
Methods
A cross‐sectional cohort of young adult survivors and age‐matched, cancer‐free controls were assessed for p16INK4a expression and frailty. Newly diagnosed pediatric patients underwent prospective measurements of p16INK4a expression before and after cancer therapy. Frailty was measured with a modified Fried frailty phenotype evaluating sarcopenia, weakness, slowness, energy expenditure, and exhaustion.
Results
The cross‐sectional cohort enrolled 60 survivors and 29 age‐matched controls with a median age of 21 years (range, 17‐29 years). The prospective cohort enrolled 9 newly diagnosed patients (age range, 1‐18 years). Expression of p16INK4a was higher among survivors compared with controls (9.6 vs 8.9 log2 p16 units; 2‐sided P = .005, representing a 25‐year age acceleration in survivors) and increased among newly diagnosed patients from matched pretreatment to posttreatment samples (7.3‐8.9 log2 p16 units; 2‐sided P = .002). Nine survivors (16%) were frail and had higher p16INK4a expression compared with robust survivors (10.5 [frail] vs 9.5 [robust] log2 p16 units; 2‐sided P = .055), representing a 35‐year age acceleration among frail survivors.
Conclusions
Chemotherapy is associated with increased cellular senescence and molecular age in pediatric and young adult cancer survivors. Frail survivors, compared with robust survivors, exhibit higher levels of p16INK4a, suggesting that cellular senescence may be associated with early aging in survivors.
Keywords: adolescents, aging, cancer survivorship, frailty, p16INK4a, pediatrics, young adults
Short abstract
The expression of p16INK4a, a biomarker of cellular aging and senescence, increases among childhood, adolescent, and young adult cancer survivors after chemotherapy and is higher among frail survivors, indicating advanced cellular aging related to cancer treatment.
Introduction
Childhood, adolescent, and young adult cancer survival has improved dramatically 1 ; however, many survivors experience accelerated aging, evidenced by a disproportionate loss of exercise capacity, 2 cognitive decline, 3 and early development of chronic medical morbidities, 4 , 5 leading to discordance between chronological and physiologic ages. Frailty, defined as diminished physiologic capacity, usually related to aging, occurs earlier among young adult survivors of cancers and at rates equivalent to individuals aged ≥65 years without a history of cancer. 6 , 7 Nearly 10% of young adult survivors exhibit a frailty phenotype, defined by having ≥3 of the following: low skeletal muscle mass, exhaustion, low energy expenditure, slowness, or weakness. 6 , 8 Over 20% of survivors are categorized as prefrail (having 2 of these factors). Understanding frailty status among survivors is important because frail individuals have greater risk for chronic illnesses and early death. 6 , 7
Accumulation of senescent cells is a hallmark of human aging. 9 , 10 In response to persistent DNA damage, cellular senescence occurs through the activation of the INK4a/ARF (CDKN2a) locus on chromosome 9p21.3, leading to expression of the cell‐cycle kinase inhibitor p16INK4a (hereafter referred to as p16). Peripheral blood T‐lymphocyte (PBTL) expression of p16 has been established as an indicator of organismal cellular senescence, increasing exponentially with chronological age. 11 In addition, exposure to cytotoxic chemotherapy increases PBTL p16 expression beyond that of chronological aging, suggesting that cellular senescence plays a role in the pathophysiology of chemotherapy‐related aging. 12 , 13 Higher levels of p16 have also been associated with increased risk for treatment‐related morbidities, such as fatigue. 14 As such, increased cellular senescence has been proposed as a mediator of early aging and frailty among cancer survivors (molecular changes [increased p16 expression] → physiologic dysregulation [frailty] → morbidity → early mortality). 6 , 15
By using p16 expression as a biomarker of molecular age, we measured PBTL p16 mRNA levels among young adult survivors of childhood cancers and compared these levels with frailty, a clinical biomarker of physiologic impairment. In addition, we measured p16 expression among young children and adolescents newly diagnosed with cancer to determine prospectively whether expression increases with cytotoxic chemotherapy exposure. We hypothesized that p16 expression is higher after chemotherapy exposure and among frail survivors.
Materials and Methods
Cross‐Sectional Survivor Cohort
Survivors of childhood, adolescent, or young adult cancers aged 18 to 29 years at the time of study enrollment whose treatment included an anthracycline, an anthracenedione, and/or an alkylating agent and who were returning for a routine survivorship appointment at either the University of North Carolina (UNC) Children's Oncology Clinic or the UNC Adolescent and Young Adult Survivors' Clinic were approached for enrollment. After obtaining informed consent, patients: 1) completed measures of wellness and physical function (the Medical Outcomes Study 36‐item Short‐Form Health Survey 16 and the National Health and Nutrition Examination Survey physical activity questionnaire) 17 as well as a report of 19 common symptoms, 2) completed evaluations for clinical frailty, and 3) provided a peripheral blood specimen. The presence of medical morbidities was determined by medical chart review. Age‐matched, cancer‐free controls were enrolled from either the UNC Children's Hematology Clinic (seen for benign conditions, such as iron deficiency anemia) or the UNC Platelet Donation Center.
Prospective New Diagnosis Cohort
Patients aged 1 to 18 years at the time of study enrollment with newly diagnosed cancer and an expected treatment regimen that included an anthracycline, an anthracenedione, and/or an alkylating agent were eligible. Patients were recruited from either the UNC Children's Oncology Clinic or the North Carolina Children's Hospital. After parental informed consent was obtained and before the start of chemotherapy, a peripheral blood sample was drawn. A second peripheral blood sample was obtained at least 3 months after the completion of treatment to allow for re‐equilibration of peripheral blood lymphocytes.
For both cohorts, participants' demographic, cancer, and treatment variables were abstracted from the medical record. The intensity of treatment was standardized using a validated measure, the Intensity of Treatment Rating (ITR) Scale, version 3. 18 Regimens were classified according to the ITR score from lowest (ITR‐1) to highest (ITR‐4) intensity. Two clinicians (J.B. and A.B.S.) who were blinded to patient outcomes independently assigned intensity scores with 100% agreement.
Measurement of PBTL p16 Expression
The analysis of PBTL p16 expression was performed by Sapere Bio using modified protocols of the previously described p16 assay. 11 , 12 , 13 Blood was drawn into an EDTA collection tube, and CD3‐positive T lymphocytes were isolated and stored frozen (UNC Virology, Immunology, Microbiology Core). Total RNA was isolated, and p16 expression was measured using a TaqMan quantitative reverse‐transcription polymerase chain reaction (RT‐PCR) (Sapere Bio). The assay modifications included automation of the assay, different housekeeping genes, and multiple quality controls of T‐cell purity and RNA quality. Every run included external and internal controls (including multiple positive donor controls) to monitor assay performance. Cycle threshold (Ct) values for individual genes >37 were considered below detection and were excluded from the analysis. Expression of p16 was determined by normalizing p16 Ct values to housekeeping Ct values for each sample. The same assay and quality‐control procedures were used for all samples in this study. The overall precision (reproducibility based on run‐to‐run and between operators' analytical validation) of p16INK4a measurement was 0.8 Ct. As previously demonstrated, p16 is generally undetectable in children and young adults. 11 The limit of detection of p16 expression was 7 (the assay yields value 95% of the time), and we assigned this value to samples in which p16 could not be detected for the purposes of data visualization.
Clinical Frailty Assessment
Frailty was assessed using a modification of the phenotype reported by Fried and colleagues 7 adapted by Ness et al. 6 The Fried frailty phenotype includes 5 clinically derived factors: weakness, slowness, decreased skeletal muscle mass, exhaustion, and low energy expenditure. Individuals with ≥3 factors were classified as frail, those with 2 factors were classified as prefrail, and those with ≤1 factor were classified as robust (see Supporting Table 1).
Weakness was measured with a hand‐held hydraulic dynamometer (model J00105; Lafayette Instrument) using the dominant hand while in a seated position with forearm flexed at the elbow to 90 degrees. 7 The average of 2 measures was used. Weakness was classified using cutoffs reported by Fried et al based on body mass index. 7
Slowness was assessed by a 15‐foot walk at the survivor's usual pace on a hard, noninclined surface. Women ≤159 cm and men ≤173 cm in height were considered slow with a time ≥7 seconds. Women >159 cm and men >173 cm in height were considered slow with a time ≥6 seconds. 7 No accommodations were made for individuals who had health concerns affecting gait, such as neuropathy, ataxia, or prosthesis.
Skeletal muscle mass was measured using a bioelectrical impedance body composition analyzer (Quantum IV; RJL Systems) as described by the manufacturer. Resistance was measured at an operating frequency of 50 kHz at 800 µA. Skeletal muscle mass was calculated from bioelectrical impedance values using the equation derived by Janssen and colleagues using an RJL Systems body composition analyzer. 19 Skeletal muscle index (SMI) was determined by dividing the skeletal muscle mass by the total mass. Bioelectrical impedance was chosen for ease of use in the examination room and because it entails no radiation exposure. Sarcopenia was defined as an SMI ≤0.37 for men and ≤0.28 for women, as previously determined among a population of young adults. 20
Exhaustion was measured using the vitality subscale on the Medical Outcomes Study 36‐item Short‐Form Health Survey. 16 A score ≤40 defined exhaustion, which correlates to 1 standard deviation below the population mean and the lowest 15.9% of the population.
Leisure time energy expenditure was assessed using the National Health and Nutrition Examination Survey physical activity questionnaire. 17 Low energy expenditure was classified as <383 kcal per week for men and <270 kcal per week for women, as previously defined. 6 , 7
Calculating the Proaging Effects of Chemotherapy
As described above, for samples in which p16 expression was below the level of detection, a value of 7 was assigned as the lowest limit of detection. Although this value was used to demonstrate changes between pretreatment and posttreatment levels, this change is not precise; therefore, we did not convert the difference in p16 levels into chronological years. In other cases, differences in p16 levels were converted to chronological years to conceptualize the degree of age acceleration. The version of the assay used in this study has a p16 expression versus age correlation slope with a mean increase of 0.028 log2 units per year of chronological age. 21 Therefore, conversion of the change in log2 p16 units to years of chronological aging in this study was calculated using the following formula: ∆log2 p16/0.028, consistent with the assay version used.
Statistical Analysis
Expression of p16 was reported in log2 units and compared in bivariable analysis among subgroups with 2‐sided Student t tests or linear regression. In our analysis, we observed that treatment with dexrazoxane, a cardioprotective medication often administered concomitantly with anthracyclines, appeared to mitigate the increase in p16 expression. For this reason, we performed analyses including and excluding survivors who received dexrazoxane. Multivariable linear regression was used to model p16 expression by age at evaluation as a continuous variable, treatment intensity in 4 categories, and frailty status. A paired t test was used to compare pretreatment and posttreatment p16 levels in the prospective cohort. Analyses were performed using Stata 16 (StataCorp) and SAS version 9.4 (SAS Analytics). Both the UNC Institutional Review Board and the Lineberger Comprehensive Cancer Center Protocol Review Committee approved the study.
Results
Expression of p16 was cross‐sectionally measured in 60 cancer survivors and 29 controls with a mean age of 21 years in both groups. Survivors were a median 5.75 years posttherapy (range, 3 months to 25 years posttherapy). The survivor cohort is described in Table 1. On average, expression of p16 was 0.7 log2 p16 units higher in survivors than in controls (9.6 vs 8.9 log2 p16 units; P = .005) (Fig. 1). Three survivors had undetectable levels of p16 expression and, as described above (see Materials and Methods), were assigned a value of 7, the lower limit of detection for the assay.
Table 1.
Descriptive Characteristics of the Survivor and Newly Diagnosed Patient Cohorts
| Characteristic | No. of Patients (%) | |
|---|---|---|
| Survivor Cohort, n = 60 | New Diagnosis Cohort, n = 9 | |
| Age at evaluation: Median [range], y | 21 [18‐29] | 14 [1.5‐19] a |
| Female | 38 (63) | 3 (33) |
| Cancer type | ||
| Acute leukemia | 24 (40) | 0 (0) |
| Hodgkin lymphoma | 10 (17) | 4 (45) |
| Non‐Hodgkin lymphoma | 3 (5) | 1 (11) |
| Osteosarcoma | 3 (5) | 1 (11) |
| Germ cell tumor | 3 (5) | 2 (22) |
| Central nervous system | 4 (7) | 0 (0) |
| Neuroblastoma | 1 (2) | 0 (0) |
| Rhabdomyosarcoma | 3 (5) | 0 (0) |
| Ewing sarcoma | 2 (3) | 0 (0) |
| NRSTS | 4 (7) | 0 (0) |
| Wilms tumor | 3 (5) | 1 (11) |
| Time from treatment end at evaluation: Median [range], y | 5.75 [0.25‐25] | — |
Abbreviation: NRSTS, nonrhabdomyosarcoma soft tissue sarcoma.
The age indicated is at the time of diagnosis.
Figure 1.
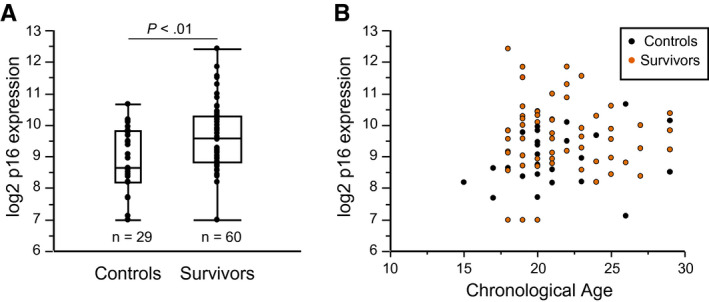
Expression of p16INK4a is higher among cancer survivors compared with age‐matched, cancer‐free young adults. (A) This is a cross‐sectional comparison of p16 expression between young adult controls (n = 29; mean p16 expression, 8.9 log2 units) and young adult cancer survivors (n = 60; mean p16 expression, 9.6 log2 units). The mean age for both groups was 21 years. All survivors received an anthracycline/anthracenedione, an alkylator, or both as part of their treatment. (B) Distribution demonstrates expression levels of p16 in the survivor (orange dots) and control (black dots) cohorts by chronological age.
Survivors who received higher intensity regimens had higher levels of p16 expression (Fig. 2A). A mean difference of 1.6 log2 p16 units was observed between the most intensely treated (ITR‐4) and least intensely treated (ITR‐1 and ITR‐2) survivors. Survivors who received concomitant dexrazoxane had lower posttherapy mean p16 levels compared with those who did not receive dexrazoxane (9.0 vs 9.7 log2 p16 units; P = .04). When stratified by treatment intensity level, mean p16 expression was lower among survivors who received dexrazoxane (ITR‐4: 10.1 vs 11.0 log2 p16 units; P = .36; ITR‐3: 8.8 vs 9.9 log2 p16 units; P = .01 with and without dexrazoxane, respectively). With the exclusion of patients who received dexrazoxane (Fig. 2B), the differences in p16 expression by treatment intensity were more pronounced.
Figure 2.
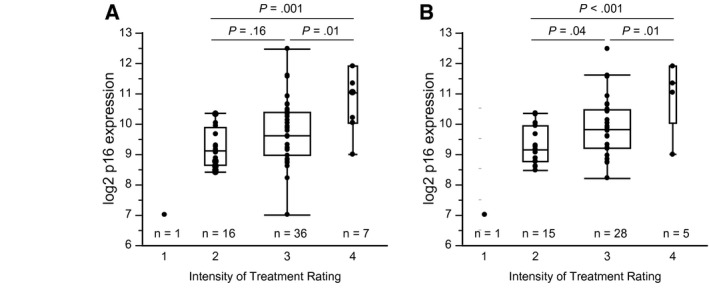
Expression of p16INK4a is higher among survivors who receive higher intensity chemotherapy regimens. The intensity of treatment is represented by the Intensity of Treatment Rating (ITR) Scale, with 1 (IRT‐1) indicating the lowest intensity and ITR‐4 indicating the highest intensity. (A) The analyses includes all patients in the survivor cohort (mean log2 p16 units: 7.0 [ITR‐1], 9.2 [ITR‐2], 9.6 [ITR‐3], 10.7 [ITR‐4]) and (B) exclude 11 patients who received treatment with dexrazoxane (mean log2 p16 units: 7.0 [ITR‐1], 9.3 [ITR‐2], 9.9 [ITR‐3], 11.0 [ITR‐4]).
Fifty‐five survivors had complete frailty phenotype data available. Nine patients (16%) were classified as frail, and 15 (27%) were classified as prefrail (Table 2). Low energy expenditure (47%) and sarcopenia (44%) were the factors most observed. Females (23%) were more commonly frail than males (5%). On average, frail survivors had higher p16 expression compared with robust survivors (10.0 [frail] vs 9.4 [robust] log2 p16 units; P = .19); this difference was greater with the exclusion of patients who received dexrazoxane (10.5 [frail] vs 9.5 [robust] log2 p16 units; P = .055) (Fig. 3A) and was equivalent to 35 years of age advancement among frail survivors. In addition, expression of p16 was higher among patients with sarcopenia (10.1 log2 p16 units) versus those with no sarcopenia (9.6 log2 p16 units; P = .06) (Fig. 3B).
Table 2.
Criteria and Final Frailty Status Classification for the Full Survivor Cohort and by Sex
| Frailty Criteria | Total, n = 55 | Female, n = 35 | Male, n = 20 | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sarcopenia | 24 | 44 | 18 | 51 | 6 | 30 |
| Exhaustion | 16 | 29 | 14 | 40 | 2 | 10 |
| Slowness | 2 | 4 | 2 | 6 | 0 | 0 |
| Weakness | 8 | 15 | 5 | 14 | 3 | 15 |
| Low energy expenditure | 26 | 47 | 19 | 54 | 7 | 35 |
| Robust (≤1 criterion) | 31 | 56 | 15 | 43 | 16 | 80 |
| Pre‐frail (2 criteria) | 15 | 27 | 12 | 34 | 3 | 15 |
| Frail (≥3 criteria) | 9 | 16 | 8 | 23 | 1 | 5 |
Figure 3.
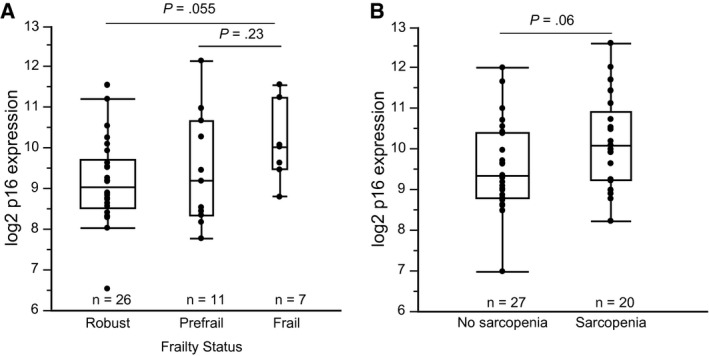
Expression of p16INK4a is associated with clinical biomarkers of physiologic impairment. (A) Expression of p16 is higher among frail survivors compared with robust survivors (mean log2 p16 units: 9.5 [robust], 9.8 [prefrail], 10.5 [frail]). (B) Expression of p16 is higher among patients who have sarcopenia compared with those who have normal skeletal muscle levels (mean log2 p16 units: 9.6 [no sarcopenia] vs 10.1 [sarcopenia]). Both plots exclude patients who received treatment with dexrazoxane.
In multivariate regression analysis of p16 expression including age at the time of evaluation, treatment intensity (ITR 1‐4), and frailty status (frail vs other) as covariables, higher treatment intensity was significantly associated with higher p16 expression. Frail status was also associated with higher levels of p16, although the difference did not reach statistical significance (P = .1) (Table 3). Seven survivors (12%) reported at least 1 Common Terminology Criteria for Adverse Events grade 3 toxicity, and 3 (5%) reported >1 grade 3 toxicity. Arthralgias were reported most in addition to cognitive changes, fatigue, insomnia, anxiety, gait disturbances, peripheral neuropathy, and generalized pain. Expression of p16 among survivors who reported at least 1 grade 3 toxicity versus those who did not was 9.9 versus 9.5 log2 p16 units (P = .39). Twenty survivors reported grade ≥2 toxicities, including cognitive difficulties (20%), anxiety (18%), and fatigue (17%). Expression of p16 was higher among survivors who reported grade ≥2 conditions compared with those who reported no such conditions (10.0 vs 9.2 log2 p16 units; P = .017). Only 1 survivor had a medical comorbidity (asthma).
Table 3.
Multiple Linear Regression Analysis Associating Expression of p16INK4a With Age at Study Evaluation, Treatment Intensity, and Frailty Status
| Parameter | β Coefficient | SE | P |
|---|---|---|---|
| Age (continuous) | −0.053 | 0.046 | .25 |
| Treatment intensity | |||
| ITR‐1 | −3.953 | 1.013 | <.001 |
| ITR‐2 | −1.54 | 0.501 | <.01 |
| ITR‐3 | −1.165 | 0.450 | .013 |
| ITR‐4 | Comparator | — | — |
| Frail (vs other) | 0.693 | 0.410 | .10 |
Abbreviation: ITR, Intensity of Treatment Rating Scale with scores from 1 (least intense) to 4 (most intense).
In the newly diagnosed cohort, expression of p16 increased dramatically from pretreatment to posttreatment (from 7.3 to 8.9 log2 p16 units; P = .002). Six patients had undetectable p16 levels before treatment and were assigned a value of 7 (the lowest limit of detection for the assay). As observed in the survivor cohort, the increase in p16 expression was positively associated with intensity of treatment (Fig. 4A,B). One child aged 4 years with Wilms tumor who received treatment with dactinomycin and vincristine had slightly detectable p16 levels that did not increase. A second patient, a child aged 18 months who received cisplatin, etoposide, and bleomycin for sacral germ cell tumor, had undetectable p16 levels before and after therapy.
Figure 4.
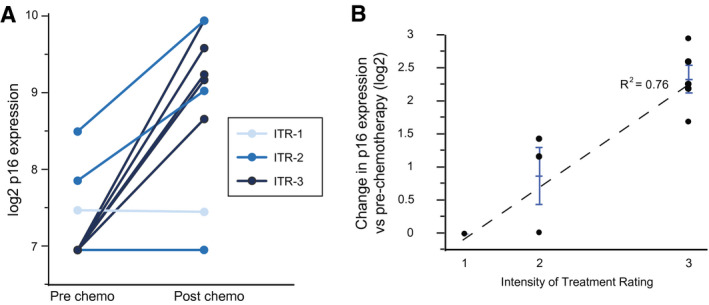
Expression levels of p16INK4a are compared between matched pretreatment and posttreatment specimens from children and adolescents who were treated for a first cancer. (A) Changes in p16 expression in the current prospective cohort are categorized according to the intensity of treatment regimen (Intensity of Treatment Rating [ITR] Scale scores are indicated from 1 [lowest] to 4 [highest]). (B) Changes in p16 expression from pretreatment to posttreatment are strongly correlated with intensity of the chemotherapy regimen. Error bars represent standard error of the mean.
Discussion
Expression of the cell‐cycle kinase inhibitor p16, a biomarker of cellular senescence and molecular aging, is commonly detectable in peripheral blood T cells among healthy young adults (aged 18‐29 years). Strikingly, exposure to cytotoxic chemotherapy significantly increases PBTL p16 expression. In the current study, among the cross‐sectional cohort of young adult survivors who were treated for a broad range of cancers, the mean p16 level (9.6 log2 p16 units) was 0.7 log2 units higher than that of age‐matched controls. On the basis of analyses of p16 expression by age in a cohort of 536 donors either without a history of cancer or without a history of chemotherapy exposure, 21 a 0.7‐log2‐unit increase in p16 expression is equivalent to an advancement of 25 years in chronological age. Increased senescence related to cytotoxic chemotherapy has been observed previously in multiple human tumor cell lines, in mouse models (for reviews, see Spallarossa et al 22 and Marcoux et al 22 , 23 ), and among older adults 12 , 13 ; however, to our knowledge, this is the first report of such findings among young adult survivors of childhood, adolescent, and young adult cancers. These findings demonstrate our ability to measure p16 expression in young patients with cancer and survivors, and these data will be used to design and power larger, more homogenous studies in this patient population.
Among younger patients with newly diagnosed cancer (aged 1‐17 years), levels of p16 before treatment were frequently undetectable, as expected; however, after chemotherapy exposure, these levels increased dramatically (median Δ 1.7 log2 p16 units; range, −0.02 to 2.9 log2 p16 units). Our observed pretherapy‐to‐posttherapy increase in p16 expression was higher than an estimate previously reported among adult women (median age, 49 years) who received adjuvant chemotherapy for breast cancer (0.89 log2 p16 units). 12 Because our cohort was significantly younger than the adult cohort in that study, and their baseline p16 value was lower, we hypothesize that our younger patient population had greater capacity for inducing changes in p16 levels starting from a lower baseline.
In both the young adult survivor and newly diagnosed pediatric cohorts, the PBTL p16 levels were higher with increased treatment intensity. These results support prior findings associating treatment intensity with the degree of increased p16 expression. Women who received dose‐dense (every 2 weeks) adjuvant doxorubicin and cyclophosphamide for breast cancer had higher p16 levels than women who received a standard regimen (every 3 weeks). 12 In addition, patients undergoing autologous bone marrow transplantation were observed to have a 3‐fold increase in p16 levels from pretreatment to posttreatment, which is equivalent to 30 years of age acceleration. 13 In adults, it was observed that increased p16 expression in T cells after chemotherapy persisted at 6‐month and 12‐month follow‐up evaluations. 12 , 13 Further work is needed to determine whether the increased PBTL p16 expression persists after cancer treatment among childhood, adolescent, and young adult survivors.
Concurrent treatment with dexrazoxane may mitigate increased PBTL p16 expression among young adult survivors. Including all survivors and with stratification by treatment intensity, survivors who received concomitant dexrazoxane had lower levels of PBTL p16. These results should be interpreted with caution because our patient sample included only 11 survivors who had received dexrazoxane. However, these early findings are provocative and consistent with a previous report demonstrating that dexrazoxane decreased the senescence‐inducing effects of the anthracycline epirubicin on rat cardiomyocytes. Expression of the senescence markers p16 and β‐galactosidase were lower in cell lines treated with both dexrazoxane and epirubicin versus those treated with epirubicin alone. The authors of that report proposed that dexrazoxane is capable of protecting cells by not only scavenging for reactive oxygen species but also through noniron‐chelating pathways, such as activation of the PI3K/Akt and erbB2 survival pathways.
By using a clinically derived frailty phenotype, we assessed physiologic impairment among survivors to determine whether accelerated expression of p16 is associated with frailty in young adult cancer survivors. We observed a high prevalence of frailty (16%) in this cohort of survivors who, on average, were aged 21 years and 5.75 years posttreatment. This prevalence is higher than that observed among cancer‐free older adults (aged >65 years; 10%) 7 and in a prior study of more than 1900 long‐term childhood cancer survivors who were approximately 20 years posttreatment (8%). 6 Consistent with the prior study of young adult cancer survivors, females were much more commonly frail than males, and the most commonly observed frailty characteristics were low muscle mass and low energy expenditure.
Mean PBTL expression of p16 was higher among frail survivors versus robust survivors. Our data suggest that physiologic impairment such as frailty may not be directly equivalent to senescence and p16 expression. Expression of p16 is not a marker of frailty but, rather, a marker of molecular aging that may prove to be an early indicator of an increased risk for developing subsequent frailty and physiologic impairment. Although a few robust survivors had higher than average p16 expression, a far greater proportion of frail survivors had higher than average p16 expression. This speaks to the interindividual difference of p16 expression but is also consistent with the theory that aging‐related molecular changes such as increasing senescence precede the development of organ system and organismal physiologic dysfunction: (molecular changes [increased p16 expression] → organ level dysfunction [sarcopenia] → physiological dysregulation [frailty] → morbidity → early mortality). Further research is needed to determine whether baseline elevations in p16 expression are a harbinger for impending physiologic impairment.
Our findings should be interpreted with the consideration of study limitations. Our study populations are small and heterogeneous with regard to cancer types and, for the survivor cohort, the time from treatment. Although all patients were treated with either an alkylating agent or an anthracycline, because of the variety of cancer types included within the cross‐sectional survivor cohort, there were various treatment exposures. By using the validated ITR Scale, we were able to standardize treatment exposures. In addition, the prospective cohort of newly diagnosed patients did not include any patients who were treated for acute leukemia, the most common childhood cancer. Because acute leukemia is treated for years, it was beyond the scope of the current study to obtain pretreatment and posttreatment measurements of p16 for these patients. Many patients, particularly among the prospective cohort, had undetectable p16 levels. We believe this limitation does not significantly affect our findings. Undetectable levels were more commonly observed among the younger, newly diagnosed patients. Most of these patients had detectable and quite higher p16 levels after treatment. If anything, using 7 log2 units as the level for undetectable specimens would underestimate the increase in p16 expression from pretreatment to posttreatment should the actual level be even lower than 7. Only 2 survivors and 1 control had undetectable levels, limiting the effect of these undetermined levels on the comparison between these groups.
Expression of p16 was measured in PBTLs. Although PBTL p16 expression has been correlated with host chronological age and aging behaviors such as smoking and a sedentary lifestyle, the extent to which p16 expression in T lymphocytes represents organismal senescence is not known. 11 Some limited data exist regarding the association of cancer treatment with expression in other tissues among patients with childhood cancer. A previous study examined p16 protein levels in skin biopsies from patients with childhood cancer who received cranial radiation therapy. Compared with paired buttock‐skin biopsies, p16 levels were higher in the skin biopsies from irradiated areas, suggesting that cellular damage from radiation therapy increases p16 expression and thus cellular senescence. 23 Including a broad range of cancer types, both hematologic and solid, our data demonstrate that, in an intensity‐dependent fashion, exposure to cytotoxic chemotherapy is associated with increased cellular senescence and accelerated molecular aging in the hematopoietic compartment among young adult cancer survivors.
A prior study demonstrated changes in p16 expression with age in PBTLs, 11 established a methodology for measuring p16 expression, and reported that p16 expression increased by 0.055 log2 units for every year of chronological age. Although the version of the assay used in our study also used real‐time RT‐PCR reaction technology to measure p16 gene expression, the assay was redesigned to increase measurement precision and reliability and to eliminate batch effects. This version uses a different set of housekeeping genes, normalization strategy, and has established levels of detection, precision, and data quality control based on positive and negative samples included with every run. Therefore, the slope derived from using this version of the assay was used for age conversion throughout the study.
Finally, we used a clinical frailty measure that was created and validated among older adults (aged >65 years). We intentionally used a frailty phenotype similar to that used by Ness and colleagues. 6 In their study, frailty was associated with adverse outcomes, such as the development of subsequent disability and early mortality. These initial results suggest that a modified Fried frailty phenotype is applicable for use among young adult cancer survivors, although additional studies are needed to confirm the clinical relevance of frailty in this population.
In summary, we report that childhood, adolescent, and young adult cancer survivors exhibit evidence of increased cellular senescence and accelerated molecular aging equivalent to more than 2 decades of advanced chronological age. In addition, many survivors demonstrate evidence of physiologic impairment, as reflected in clinically defined frailty. Although increased p16 expression was observed among frail survivors, these 2 factors are not direct equivalents, suggesting that the development of molecular aging‐related derangements may precede the onset of frailty.
Funding Support
This work was supported by grants from the National Institutes of Health (KL2‐TR002490 to Andrew B. Smitherman, R01‐CA203023 to Hyman B. Muss).
Conflict of Interest Disclosures
William A. Wood reports grants from Genentech and Pfizer Inc, outside the submitted work. Natalia Mitin is a cofounder of Sapere Bio and holds shares in the company. Ian J. Davis serves on the Triangle Biotechnology advisory board, outside the submitted work. The remaining authors made no disclosures.
Author Contributions
Andrew B. Smitherman: Conceptualization, study design, data acquisition, analysis, and writing–original draft. William A. Wood: Conceptualization, study design, data interpretation, and writing–review and editing. Natalia Mitin: Conceptualization, data interpretation, and writing–review and editing. Vanessa L. Ayer Miller: Data acquisition, data interpretation, and writing–review and editing. Allison M. Deal: Data analyses, writing–original draft, and writing–review and editing. Ian J. Davis: Data interpretation and writing–review and editing. Julie Blatt: Data acquisition, data interpretation, and writing–review and editing. Stuart H. Gold: Data acquisition, data interpretation, and writing–review and editing. Hyman B. Muss: Conceptualization, data interpretation, and writing–review and editing.
Supporting information
Table S1
Smitherman AB, Wood WA, Mitin N, Ayer Miller VL, Deal AM, Davis IJ, Blatt J, Gold SH, Muss HB. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16INK4a and frailty. Cancer. 2020. 10.1002/cncr.33112
We thank Dr. Anne Kazak and colleagues for granting us permission to use the Intensity of Treatment Rating Scale and Dr. Lena Randhawa for her editing expertise.
References
- 1. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life‐long risks and responsibilities. Nat Rev Cancer. 2014;14:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Brussel M, Takken T, Lucia A, van der Net J, Helders PJ. Is physical fitness decreased in survivors of childhood leukemia? A systematic review. Leukemia. 2005;19:13‐17. [DOI] [PubMed] [Google Scholar]
- 3. Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood Hodgkin lymphoma. J Clin Oncol. 2012;30:3618‐3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life‐threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572‐1582. [DOI] [PubMed] [Google Scholar]
- 6. Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496‐4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146‐M156. [DOI] [PubMed] [Google Scholar]
- 8. Smitherman AB, Anderson C, Lund JL, Bensen JT, Rosenstein DL, Nichols HB. Frailty and comorbidities among survivors of adolescent and young adult cancer: a cross‐sectional examination of a hospital‐based survivorship cohort. J Adolesc Young Adult Oncol. 2018;7:374‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703‐713. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T‐cells is a biomarker of human aging. Aging Cell. 2009;8:439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106:dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood WA, Krishnamurthy J, Mitin N, et al. Chemotherapy and stem cell transplantation increase p16(INK4a) expression, a biomarker of T‐cell aging. EBioMedicine. 2016;11:227‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demaria M, O'Leary MN, Chang J, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7:165‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ness KK, Kirkland JL, Gramatges MM, et al. Premature physiologic aging as a paradigm for understanding increased risk of adverse health across the lifespan of survivors of childhood cancer. J Clin Oncol. 2018;36:2206‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ware JE Jr, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30:473‐483. [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC) , National Center for Health Statistics (NCHS) . National Health and Nutrition Examination Survey questionnaire In: US Department of Health and Human Services, CDC , eds. NCHS; 2009. [Google Scholar]
- 18. Kazak AE, Hocking MC, Ittenbach RF, et al. A revision of the Intensity of Treatment Rating Scale: classifying the intensity of pediatric cancer treatment. Pediatr Blood Cancer. 2012;59:96‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985). 2000;89:465‐471. [DOI] [PubMed] [Google Scholar]
- 20. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889‐896. [DOI] [PubMed] [Google Scholar]
- 21. Muss HB, Smitherman A, Wood WA, et al. p16 a biomarker of aging and tolerance for cancer therapy. Transl Cancer Res. 2020. doi: 10.21037/tcr.2020.03.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spallarossa P, Altieri P, Pronzato P, et al. Sublethal doses of an anti‐erbB2 antibody lead to death by apoptosis in cardiomyocytes sensitized by low prosenescent doses of epirubicin: the protective role of dexrazoxane. J Pharmacol Exp Ther. 2010;332:87‐96. [DOI] [PubMed] [Google Scholar]
- 23. Marcoux S, Le ON, Langlois‐Pelletier C, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat Oncol. 2013;8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


